EGFR Mutations in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. The EGFR Structure
3. EGFR Mutations and Drug Resistance
3.1. Extracellular Domain Mutations
3.2. Tyrosine Kinase Domain Mutations
4. EGFR Alterations and Radiation Response
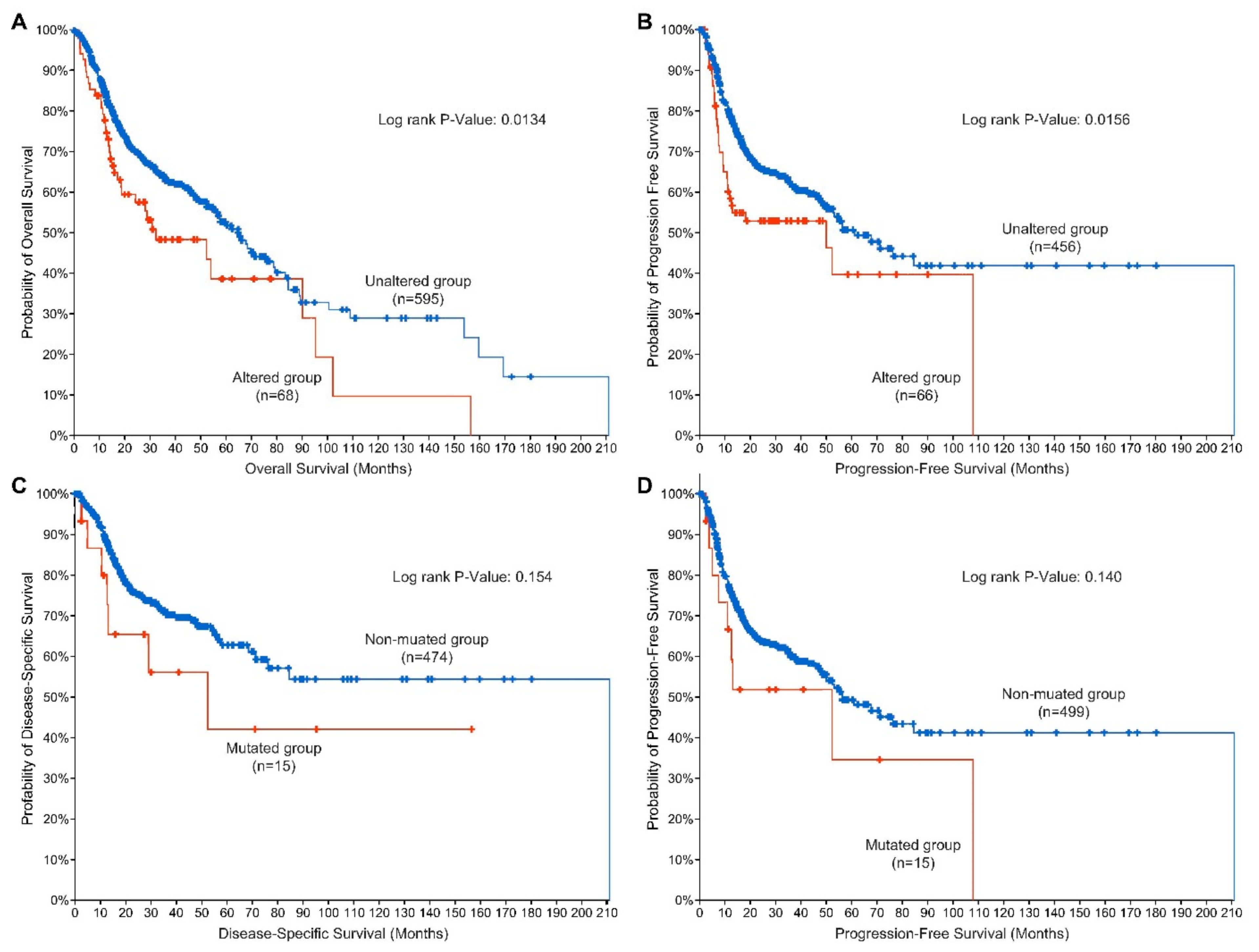
5. EGFR Alterations as Prognostic Indicators for Disease and Therapeutic Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59 (Suppl. 2), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharm. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, B.; Sun, Z. Spectrum of EGFR aberrations and potential clinical implications: Insights from integrative pan-cancer analysis. Cancer Commun. 2020, 40, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Paragliola, F.; Sparano, F.; Iacovino, M.L.; Castrichino, A.; Doria, F.; Sica, A.; et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol. 2021, 13, 1758835920949418. [Google Scholar] [CrossRef]
- Sundvall, M.; Karrila, A.; Nordberg, J.; Grenman, R.; Elenius, K. EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma. Expert Opin. Emerg. Drugs 2010, 15, 185–201. [Google Scholar] [CrossRef]
- Cooper, J.B.; Cohen, E.E. Mechanisms of resistance to EGFR inhibitors in head and neck cancer. Head Neck 2009, 31, 1086–1094. [Google Scholar] [CrossRef]
- Byeon, H.K.; Ku, M.; Yang, J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Purba, E.R.; Saita, E.I.; Maruyama, I.N. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells 2017, 6, 13. [Google Scholar] [CrossRef]
- Bessman, N.J.; Bagchi, A.; Ferguson, K.M.; Lemmon, M.A. Complex relationship between ligand binding and dimerization in the epidermal growth factor receptor. Cell Rep. 2014, 9, 1306–1317. [Google Scholar] [CrossRef]
- Ferguson, K.M. Structure-based view of epidermal growth factor receptor regulation. Annu. Rev. Biophys. 2008, 37, 53–73. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J.; Ferguson, K.M. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect Biol. 2014, 6, a020768. [Google Scholar] [CrossRef]
- Deric, L.; Wheeler, Y.Y. Receptor Tyrosine Kinases: Structure, Functions and Role in Human Disease; Humana Press: New York, NY, USA, 2015. [Google Scholar]
- Ortiz-Cuaran, S.; Bouaoud, J.; Karabajakian, A.; Fayette, J.; Saintigny, P. Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers. Front. Oncol. 2021, 11, 14332. [Google Scholar] [CrossRef]
- Nair, S.; Trummell, H.Q.; Rajbhandari, R.; Thudi, N.K.; Nozell, S.E.; Warram, J.M.; Willey, C.D.; Yang, E.S.; Placzek, W.J.; Bonner, J.A.; et al. Novel EGFR ectodomain mutations associated with ligand-independent activation and cetuximab resistance in head and neck cancer. PLoS ONE 2020, 15, e0229077. [Google Scholar] [CrossRef]
- Elferink, L.A.; Resto, V.A. Receptor-tyrosine-kinase-targeted therapies for head and neck cancer. J. Signal Transduct. 2011, 2011, 982879. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Dis. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Khattri, A.; Sheikh, N.; Acharya, R.; Tan, Y.-H.C.; Kochanny, S.; Lingen, M.W.; Vokes, E.E.; Seiwert, T.Y. Mechanism of acquired resistance to cetuximab in head and neck cancer. J. Clin. Oncol. 2018, 36 (Suppl. 15), e18061. [Google Scholar] [CrossRef]
- Orellana, L.; Thorne, A.H.; Lema, R.; Gustavsson, J.; Parisian, A.D.; Hospital, A.; Cordeiro, T.N.; Bernado, P.; Scott, A.M.; Brun-Heath, I.; et al. Oncogenic mutations at the EGFR ectodomain structurally converge to remove a steric hindrance on a kinase-coupled cryptic epitope. Proc. Natl. Acad. Sci. USA 2019, 116, 10009–10018. [Google Scholar] [CrossRef]
- Consortium, A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Dis. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- McGovern, S.L.; Rojas, M.L.; Gururaj, A.; Chiu, W.; Bogler, O.; Weinstein, J.N. Extracellular Domain Mutations in EGFR Occur Uniquely in Glioblastoma and Favor Ligand-independent Formation of the Active State. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, S177. [Google Scholar] [CrossRef]
- Braig, F.; Kriegs, M.; Voigtlaender, M.; Habel, B.; Grob, T.; Biskup, K.; Blanchard, V.; Sack, M.; Thalhammer, A.; Ben Batalla, I.; et al. Cetuximab Resistance in Head and Neck Cancer Is Mediated by EGFR-K521 Polymorphism. Cancer Res. 2017, 77, 1188–1199. [Google Scholar] [CrossRef]
- Nelhubel, G.A.; Cserepes, M.; Szabo, B.; Turk, D.; Karpati, A.; Kenessey, I.; Raso, E.; Barbai, T.; Hegedus, Z.; Laszlo, V.; et al. EGFR Alterations Influence the Cetuximab Treatment Response and c-MET Tyrosine-Kinase Inhibitor Sensitivity in Experimental Head and Neck Squamous Cell Carcinomas. Pathol. Oncol. Res. 2021, 27, 20256. [Google Scholar] [CrossRef]
- Naruse, T.; Tokuhisa, M.; Yanamoto, S.; Sakamoto, Y.; Okuyama, K.; Tsuchihashi, H.; Umeda, M. Lower gingival squamous cell carcinoma with brain metastasis during long-term cetuximab treatment: A case report. Oncol. Lett. 2018, 15, 7158–7162. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug. Dis. 2018, 17, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Chaikuad, A.; Gray, N.S.; Knapp, S. The ins and outs of selective kinase inhibitor development. Nat. Chem. Biol. 2015, 11, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.M.; Johnson, D.E.; Grandis, J.R. Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin. Investig. Drugs 2019, 28, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Vatte, C.; Al Amri, A.M.; Cyrus, C.; Chathoth, S.; Acharya, S.; Hashim, T.M.; Ali, Z.L.; Alshreadah, S.T.; Alsayyah, A.; Al-Ali, A.K.; et al. Tyrosine kinase domain mutations of EGFR gene in head and neck squamous cell carcinoma. Oncol. Targets Ther. 2017, 10, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Boeckx, C.; Weyn, C.; Vanden Bempt, I.; Deschoolmeester, V.; Wouters, A.; Specenier, P.; Laer, C.V.; Vanden Weyngaert, D.; Kockx, M.; Vermorken, J.B.; et al. Mutation analysis of genes in the EGFR pathway in Head and Neck cancer patients: Implications for anti-EGFR treatment response. BMC Res. Notes 2014, 7, 337. [Google Scholar] [CrossRef]
- Perisanidis, C. Prevalence of EGFR Tyrosine Kinase Domain Mutations in Head and Neck Squamous Cell Carcinoma: Cohort Study and Systematic Review. In Vivo 2017, 31, 23–34. [Google Scholar] [CrossRef]
- Wagener-Ryczek, S.; Heydt, C.; Suptitz, J.; Michels, S.; Falk, M.; Alidousty, C.; Fassunke, J.; Ihle, M.A.; Tiemann, M.; Heukamp, L.; et al. Mutational spectrum of acquired resistance to reversible versus irreversible EGFR tyrosine kinase inhibitors. BMC Cancer 2020, 20, 408. [Google Scholar] [CrossRef]
- Vaclova, T.; Grazini, U.; Ward, L.; O’Neill, D.; Markovets, A.; Huang, X.; Chmielecki, J.; Hartmaier, R.; Thress, K.S.; Smith, P.D.; et al. Clinical impact of subclonal EGFR T790M mutations in advanced-stage EGFR-mutant non-small-cell lung cancers. Nat. Commun. 2021, 12, 1780. [Google Scholar] [CrossRef]
- Du, Z.; Brown, B.P.; Kim, S.; Ferguson, D.; Pavlick, D.C.; Jayakumaran, G.; Benayed, R.; Gallant, J.N.; Zhang, Y.K.; Yan, Y.; et al. Structure-function analysis of oncogenic EGFR Kinase Domain Duplication reveals insights into activation and a potential approach for therapeutic targeting. Nat. Commun. 2021, 12, 1382. [Google Scholar] [CrossRef]
- Matar, P.; Rojo, F.; Cassia, R.; Moreno-Bueno, G.; Di Cosimo, S.; Tabernero, J.; Guzman, M.; Rodriguez, S.; Arribas, J.; Palacios, J.; et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): Superiority over single-agent receptor targeting. Clin. Cancer Res. 2004, 10, 6487–6501. [Google Scholar] [CrossRef]
- Subbiah, V.; Dumbrava, E.I.; Jiang, Y.; Thein, K.Z.; Naing, A.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Tsimberidou, A.M.; Janku, F.; et al. Dual EGFR blockade with cetuximab and erlotinib combined with anti-VEGF antibody bevacizumab in advanced solid tumors: A phase 1 dose escalation triplet combination trial. Exp. Hematol. Oncol. 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, P.; Zhao, S.; Yuan, L.; Wang, X.; Li, X. Gefitinib Combined with Cetuximab for the Treatment of Lung Adenocarcinoma Harboring the EGFR-Intergenic Region (SEC61G) Fusion and EGFR Amplification. Oncologist 2021, 26, e1898–e1902. [Google Scholar] [CrossRef]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Bossi, P.; Platini, F. Radiotherapy plus EGFR inhibitors: Synergistic modalities. Cancers Head Neck 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Kwok, T.T.; Sutherland, R.M. Differences in EGF related radiosensitisation of human squamous carcinoma cells with high and low numbers of EGF receptors. Br. J. Cancer 1991, 64, 251–254. [Google Scholar] [CrossRef][Green Version]
- Bonner, J.A.; Maihle, N.J.; Folven, B.R.; Christianson, T.J.; Spain, K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 243–247. [Google Scholar] [CrossRef]
- Bonner, J.A.; Raisch, K.P.; Trummell, H.Q.; Robert, F.; Meredith, R.F.; Spencer, S.A.; Buchsbaum, D.J.; Saleh, M.N.; Stackhouse, M.A.; LoBuglio, A.F.; et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J. Clin. Oncol. 2000, 18 (Suppl. 21), 47S–53S. [Google Scholar]
- Huang, S.M.; Bock, J.M.; Harari, P.M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999, 59, 1935–1940. [Google Scholar]
- Saleh, M.N.; Raisch, K.P.; Stackhouse, M.A.; Grizzle, W.E.; Bonner, J.A.; Mayo, M.S.; Kim, H.G.; Meredith, R.F.; Wheeler, R.H.; Buchsbaum, D.J. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother. Radiopharm. 1999, 14, 451–463. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Ang, K.K.; Berkey, B.A.; Tu, X.; Zhang, H.Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar]
- Hutchinson, M.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Fehrenbacher, B.; Schaller, M.; Raju, U.; Milas, L.; Chen, D.J.; Kehlback, R.; Rodemann, H.R. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 2005, 280, 31182–31189. [Google Scholar] [CrossRef]
- Taberna, M.; Oliva, M.; Mesia, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 83. [Google Scholar] [CrossRef]
- Okuyama, K.; Suzuki, K.; Naruse, T.; Tsuchihashi, H.; Yanamoto, S.; Kaida, A.; Miura, M.; Umeda, M.; Yamashita, S. Prolonged cetuximab treatment promotes p27(Kip1)-mediated G1 arrest and autophagy in head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 5259. [Google Scholar] [CrossRef] [PubMed]
- Bos, T.; Ratti, J.A.; Harada, H. Targeting Stress-Response Pathways and Therapeutic Resistance in Head and Neck Cancer. Front. Oral Health 2021, 26, 76643. [Google Scholar] [CrossRef]
- Lee, M.; Nam, H.Y.; Kang, H.B.; Lee, W.H.; Lee, G.H.; Sung, G.J.; Han, M.W.; Cho, K.J.; Chang, E.J.; Choi, K.C.; et al. Epigenetic regulation of p62/SQSTM1 overcomes the radioresistance of head and neck cancer cells via autophagy-dependent senescence induction. Cell Death Dis. 2021, 12, 250. [Google Scholar] [CrossRef]
- Wang, M.; Kern, A.M.; Hulskotter, M.; Greninger, P.; Singh, A.; Pan, Y.; Chowdhuy, D.; Krause, M.; Baumann, M.; Benes, C.H.; et al. EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation. Cancer Res. 2014, 74, 2825–2834. [Google Scholar] [CrossRef]
- Alsahafi, E.N.; Thavaraj, S.; Sarvestani, N.; Novoplansky, O.; Elkabets, M.; Ayaz, B.; Tavassoli, M. EGFR overexpression increases radiotherapy response in HPV-positive head and neck cancer through inhibition of DNA damage repair and HPV E6 downregulation. Cancer Lett. 2021, 4988, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Tang, D.G. Cancer stem cells and radioresistance. Int. J. Radiat. Biol. 2014, 90, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, G.; Miao, X.B.; Deng, X.B.; Wu, Y.; Liu, Y.; Jin, Z.R.; Li, X.Q.; Liu, Q.Z.; Sun, D.X.; et al. Cancer stem-like cell properties are regulated by EGFR/AKT/beta-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J. 2013, 280, 2027–2041. [Google Scholar] [CrossRef]
- Macha, M.A.; Rachagani, S.; Qazi, A.K.; Jahan, R.; Gupta, S.; Patel, A.; Seshacharyulu, P.; Lin, C.; Li, S.; Wang, S.; et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017, 8, 20961–20973. [Google Scholar] [CrossRef]
- Wang, X.K.; He, J.H.; Xu, J.H.; Ye, S.; Wang, F.; Zhang, H.; Huang, Z.C.; To, K.K.W.; Fu, L.W. Afatinib enhances the efficacy of conventional chemotherapeutic agents by eradicating cancer stem-like cells. Cancer Res. 2014, 74, 4431–4445. [Google Scholar] [CrossRef]
- Coliat, P.; Ramolu, L.; Jegu, J.; Gaiddon, C.; Jung, A.C.; Pencreach, E. Constitutive or Induced HIF-2 Addiction is Involved in Resistance to Anti-EGFR Treatment and Radiation Therapy in HNSCC. Cancers 2019, 11, 1607. [Google Scholar] [CrossRef]
- Cappuzzo, F. Guide to Targeted Therapies: EGFR Mutations in NSCLC; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Angulo, B.; Conde, E.; Suarez-Gauthier, A.; Plaza, C.; Martinez, R.; Redondo, P.; Izquierdo, E.; Rubio-Viqueira, B.; Paz-Ares, L.; Hidalgo, M.; et al. A comparison of EGFR mutation testing methods in lung carcinoma: Direct sequencing, real-time PCR and immunohistochemistry. PLoS ONE 2012, 7, e43842. [Google Scholar] [CrossRef]
- Normanno, N.; Denis, M.G.; Thress, K.S.; Ratcliffe, M.; Reck, M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget 2017, 8, 12501–12516. [Google Scholar] [CrossRef]
- Chung, C.H.; Ely, K.; McGavran, L.; Varella-Garcia, M.; Parker, J.; Parker, N.; Jarrett, C.; Carter, J.; Murphy, B.A.; Netterville, J.; et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J. Clin. Oncol. 2006, 24, 4170–4176. [Google Scholar] [CrossRef]
- Chung, C.H.; Zhang, Q.; Hammond, E.M.; Trotti, A.M., III; Wang, H.; Spencer, S.; Zhang, H.Z.; Cooper, J.; Jordan, R.; Rotman, M.H.; et al. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 331–338. [Google Scholar] [CrossRef]
- Temam, S.; Kawaguchi, H.; El-Naggar, A.K.; Jelinek, J.; Tang, H.; Liu, D.D.; Lang, W.; Issa, J.P.; Lee, J.J.; Mao, L. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 2007, 25, 2164–2170. [Google Scholar] [CrossRef]
- Alterio, D.; Marvaso, G.; Maffini, F.; Gandini, S.; Chiocca, S.; Ferrari, A.; Preda, L.; Rocca, M.C.; Lepanto, D.; Fodor, C.; et al. Role of EGFR as prognostic factor in head and neck cancer patients treated with surgery and postoperative radiotherapy: Proposal of a new approach behind the EGFR overexpression. Med. Oncol. 2017, 34, 107. [Google Scholar] [CrossRef]
- Chen, X.; Liang, R.; Lai, L.; Chen, K.; Zhu, X. Prognostic Role of EGFR/p-EGFR in Patients With Nasopharyngeal Carcinoma: A Meta-Analysis. Front. Oncol. 2021, 11, 97369. [Google Scholar] [CrossRef]
- Keren, S.; Shoude, Z.; Lu, Z.; Beibei, Y. Role of EGFR as a prognostic factor for survival in head and neck cancer: A meta-analysis. Tumour Biol. 2014, 35, 2285–2295. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Zhang, W.; He, J.; Zhao, Y.; Chen, X. Prognostic role of epidermal growth factor receptor in head and neck cancer: A meta-analysis. J. Surg. Oncol. 2013, 108, 387–397. [Google Scholar] [CrossRef]
- Licitra, L.; Mesia, R.; Rivera, F.; Remenar, E.; Hitt, R.; Erfan, J.; Rottey, S.; Kawecki, A.; Zabolotnny, D.; Benasso, M.; et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann. Oncol. 2011, 22, 1078–1087. [Google Scholar] [CrossRef]
- Wang, Y.; Zha, L.; Liao, D.; Li, X. A Meta-Analysis on the Relations between EGFR R521K Polymorphism and Risk of Cancer. Int. J. Genom. 2014, 2014, 312102. [Google Scholar] [CrossRef]
- Fung, C.; Zhou, P.; Joyce, S.; Trent, K.; Yuan, J.M.; Grandis, J.R.; Weissfeld, J.L.; Romkes, M.; Weeks, D.E.; Egloff, A.M. Identification of epidermal growth factor receptor (EGFR) genetic variants that modify risk for head and neck squamous cell carcinoma. Cancer Lett. 2015, 357, 549–556. [Google Scholar] [CrossRef]
- Stoehlmacher-Williams, J.; Obermann, L.; Ehninger, G.; Goekkurt, E. Polymorphisms of the epidermal growth factor receptor (EGFR) and survival in patients with advanced cancer of the head and neck (HNSCC). Anticancer Res. 2012, 32, 421–425. [Google Scholar]
- Bandres, E.; Barricarte, R.; Cantero, C.; Honorato, B.; Malumbres, R.; Zarate, R.; Alcade, J.; Garcia-Foncillas, J. Epidermal growth factor receptor (EGFR) polymorphisms and survival in head and neck cancer patients. Oral Oncol. 2007, 43, 713–719. [Google Scholar] [CrossRef]
- Krohn, V.; Wiegand, S.; Werner, J.A.; Mandic, R. EGFR codon 497 polymorphism—Implications for receptor sensitivity to inhibitors in HNSCC cell lines. Anticancer Res. 2011, 31, 59–65. [Google Scholar]
- Robichaux, J.P.; Le, X.; Vijayan, R.S.K.; Hicks, J.K.; Heeke, S.; Elamin, Y.Y.; Lin, H.Y.; Udagawa, H.; Skoulidis, F.; Tran, H.; et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021, 597, 732–737. [Google Scholar] [CrossRef]
- Russo, A.; Franchina, T.; Ricciardi, G.; Battaglia, A.; Picciotto, M.; Adamo, V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients with Uncommon EGFR Mutations: New Insights and Future Perspectives in this Complex Clinical Scenario. Int. J. Mol. Sci. 2019, 20, 1431. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Kukimoto-Niino, M.; Parker, L.; Handa, N.; Terada, T.; Fujimoto, T.; Terazawa, Y.; Wakiyama, M.; Sato, M.; Sano, S.; et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene 2013, 32, 27–38. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, B.; Zhao, Y.; Li, C.; Liu, H.; Lv, T.; Zhan, P.; Song, Y. Treatment of uncommon EGFR mutations in non-small cell lung cancer: New evidence and treatment. Transl. Lung Cancer Res. 2019, 8, 302–316. [Google Scholar] [CrossRef]
- Hirose, T.; Ikegami, M.; Endo, M.; Matsumoto, Y.; Nakashima, Y.; Mano, H.; Kohsaka, S. Extensive functional evaluation of exon 20 insertion mutations of EGFR. Lung Cancer 2021, 152, 135–142. [Google Scholar] [CrossRef]
- Passaro, A.; Mok, T.; Peters, S.; Popat, S.; Ahn, M.J.; de Marinis, F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021, 16, 764–773. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.L.; Cardona, A.F.; Besse, B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev. 2020, 90, 102105. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Vyse, S.; Huang, P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wu, Q.; Zhang, J.; Zhou, Y. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol. Lett. 2019, 18, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Yang, J.C.; Mok, T.S.; Loong, H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018, 29 (Suppl. 1), i3–i9. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.L.; Tan, S.Z.; Liu, G.; Tsao, M.S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations—A review. Transl. Lung Cancer Res. 2015, 4, 67–81. [Google Scholar]
- Van Assche, K.; Ferdinande, L.; Lievens, Y.; Vandecasteele, K.; Surmont, V. EGFR Mutation Positive Stage IV Non-Small-Cell Lung Cancer: Treatment Beyond Progression. Front. Oncol. 2014, 4, 50. [Google Scholar] [CrossRef]
- Ahn, M.J.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Janne, P.A.; et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol. Res. 2021, 1671, 05583. [Google Scholar] [CrossRef]
- Nagasaka, M.; Zhu, V.W.; Lim, S.M.; Greco, M.; Wu, F.; Ou, S.I. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors for Advanced EGFR+ NSCLC. J. Thorac. Oncol. 2021, 16, 740–763. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, S.; Hu, X.; Feng, J.; Ma, Z.; Zhou, J.; Yang, N.; Wu, L.; Liao, W.; Zhong, D.; et al. Safety, Clinical Activity, and Pharmacokinetics of Alflutinib (AST2818) in Patients with Advanced NSCLC with EGFR T790M Mutation. J. Thorac. Oncol. 2020, 15, 1015–1026. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Wang, Z.; Duan, J.; Bai, H.; Wang, J. The Status of the EGFR T790M Mutation is associated with the Clinical Benefits of Osimertinib Treatment in Non-small Cell Lung Cancer Patients: A Meta-Analysis. J. Cancer 2020, 11, 3106–3113. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, S.; Bonner, J.A.; Bredel, M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. https://doi.org/10.3390/ijms23073818
Nair S, Bonner JA, Bredel M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2022; 23(7):3818. https://doi.org/10.3390/ijms23073818
Chicago/Turabian StyleNair, Sindhu, James A. Bonner, and Markus Bredel. 2022. "EGFR Mutations in Head and Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 23, no. 7: 3818. https://doi.org/10.3390/ijms23073818
APA StyleNair, S., Bonner, J. A., & Bredel, M. (2022). EGFR Mutations in Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences, 23(7), 3818. https://doi.org/10.3390/ijms23073818





