Molecular Advances in Preeclampsia and HELLP Syndrome
Abstract
:1. Introduction
2. Results
2.1. ADAMTS13 and vWF in Preeclampsia/HELLP Syndrome
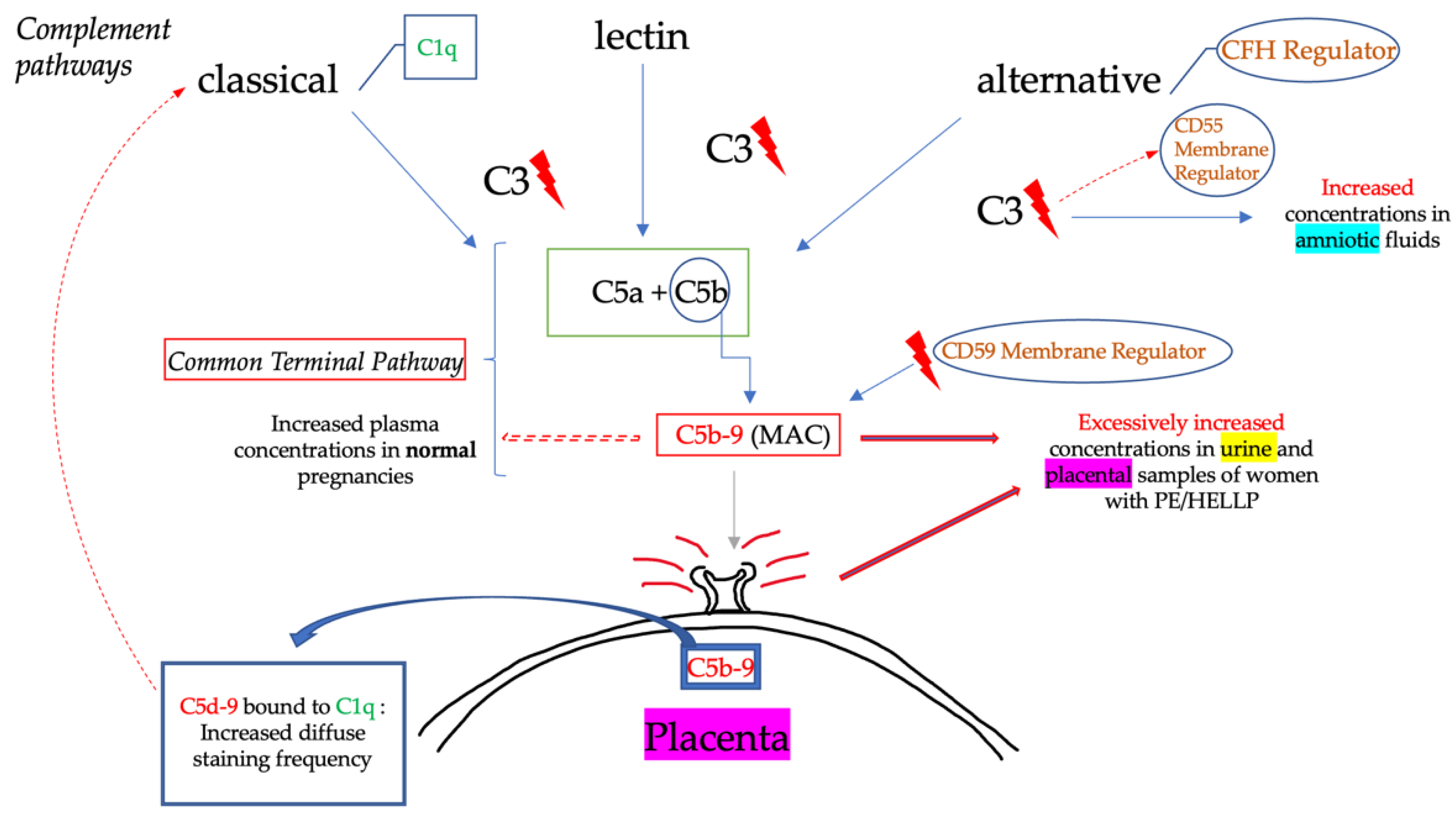
2.2. Complement in Preeclampsia/HELLP Syndrome
2.3. Complement and von Willebrand Factor Interaction
3. Discussion
3.1. Differential Diagnosis of TMAs
3.2. Role of ADAMTS13 and vWF in the Differential Diagnosis
3.3. Proposed Algorithm for the Differential Diagnosis and Management of Pregnant Women with TMA
3.3.1. Diagnosis
3.3.2. Management
4. Materials and Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duley, L. The Global Impact of Pre-Eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Aref, S.; Goda, H. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematology 2013, 18, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burwick, R.M.; Feinberg, B.B. Complement activation and regulation in preeclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Am. J. Obstet. Gynecol. 2020, 226, S1059–S1070. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, R.G.; Ogunbodede, A.C. Hypertensive Disorders of Pregnancy. Emerg. Med. Clin. N. Am. 2019, 37, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Demircan, K.; Cömertoglu, I.; Akyol, S.; Yigitoglu, B.N.; Sarikaya, E. A new biological marker candidate in female reproductive system diseases: Matrix metalloproteinase with thrombospondin motifs (ADAMTS). J. Turk. Gynecol. Assoc. 2014, 15, 250–255. [Google Scholar] [CrossRef]
- Abildgaard, U.; Heimdal, K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 117–123. [Google Scholar] [CrossRef]
- Zander, C.B.; Cao, W.; Zheng, X.L. ADAMTS13 and von Willebrand factor interactions. Curr. Opin. Hematol. 2015, 22, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Franchini, M.; Montagnana, M.; Targher, G.; Lippi, G. Reduced von Willebrand Factor-Cleaving Protease Levels in Secondary Thrombotic Microangiopathies and Other Diseases. Semin. Thromb. Hemost. 2007, 33, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Burwick, R.M.; Fleming, S.D. The Complement System and Preeclampsia. Curr. Hypertens. Rep. 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalem, M.N.; Kalem, Z.; Yüce, T.; Soylemez, F. ADAMTS 1, 4, 12, and 13 levels in maternal blood, cord blood, and placenta in preeclampsia. Hypertens. Pregnancy 2017, 37, 9–17. [Google Scholar] [CrossRef]
- Stepanian, A.; Cohen-Moatti, M.; Sanglier, T.; Legendre, P.; Ameziane, N.; Tsatsaris, V.; Mandelbrot, L.; de Prost, D.; Veyradier, A. Von Willebrand Factor and ADAMTS13. Arter. Thromb. Vasc. Biol. 2011, 31, 1703–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpoim, P.N.; Gomes, K.B.; Godoi, L.C.; Rios, D.R.; Carvalho, M.G.; Fernandes, A.P.; Dusse, L.M. ADAMTS13, FVIII, von Willebrand factor, ABO blood group assessment in preeclampsia. Clin. Chim. Acta 2011, 412, 2162–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabau, L.; Terriou, L.; Provot, F.; Fourrier, F.; Roumier, C.; Caron, C.; Susen, S.; Ducloy-Bouthors, A. Are there any additional mechanisms for haemolysis in HELLP syndrome? Thromb. Res. 2016, 142, 40–43. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, Y.; Li, X.; Li, W.; Fan, L.; Liu, J.; Zeng, X.; Chen, K.; Chen, X.; Zhou, X.; et al. Expression of ADAMTS13 in Normal and Abnormal Placentae and Its Potential Role in Angiogenesis and Placenta Development. Arter. Thromb. Vasc. Biol. 2017, 37, 1748–1756. [Google Scholar] [CrossRef] [Green Version]
- Hulstein, J.J.J.; Heimel, P.J.V.R.; Franx, A.; Lenting, P.J.; Bruinse, H.W.; Silence, K.; De Groot, P.G.; Fijnheer, R. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J. Thromb. Haemost. 2006, 4, 2569–2575. [Google Scholar] [CrossRef]
- Molvarec, A.; Rigó, J.; Bõze, T.; Derzsy, Z.; Cervenak, L.; Makó, V.; Gombos, T.; Udvardy, M.L.; Hársfalvi, J.; Prohászka, Z. Increased Plasma von Willebrand Factor Antigen Levels but Normal von Willebrand Factor Cleaving Protease (ADAHTS13) Activity in Preeclampsia. Thromb. Haemost. 2009, 101, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Matsumoto, M.; Yagi, H.; Isonishi, A.; Sakai, K.; Hayakawa, M.; Hori, Y.; Sado, T.; Kobayashi, H.; Fujimura, Y. Severe reduction of free-form ADAMTS13, unbound to von Willebrand factor, in plasma of patients with HELLP syndrome. Blood Adv. 2017, 1, 1628–1631. [Google Scholar] [CrossRef] [Green Version]
- Krogh, A.-S.; von Kremer Hovinga, J.A.; Romundstad, P.R.; Roten, L.T.; Lämmle, B.; Waage, A.; Quist-Paulsen, P. ADAMTS13 Gene Variants and Function in Women with Preeclampsia: A Population-Based Nested Case-Control Study from the HUNT Study. Thromb. Res. 2015, 136, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molvarec, A.; Derzsy, Z.; Kocsis, J.; Bőze, T.; Nagy, B.; Balogh, K.; Makó, V.; Cervenak, L.; Mézes, M.; Karádi, I.; et al. Circulating anti-heat-shock-protein antibodies in normal pregnancy and preeclampsia. Cell Stress Chaperon 2009, 14, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury-Stewart, D.N.; Lannert, K.W.; Chung, D.; Teramura, G.T.; Zimring, J.C.; Konkle, B.; Gammill, H.S.; Johnsen, J.M. Complex Changes in von Willebrand Factor-Associated Parameters Are Acquired during Uncomplicated Pregnancy. PLoS ONE 2014, 9, e112935. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, P.; Han, C.; Li, J.; Liu, L.; Zhao, Z.; Gao, Y.; Qin, Y.; Xu, Q.; Yan, Y.; et al. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: A clinical observational study. BJOG: Int. J. Obstet. Gynaecol. 2020, 128, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Balduit, A.; Aghamajidi, A.; Ricci, G.; Kishore, U.; Bulla, R. COVID-19, Pre-Eclampsia, and Complement System. Front. Immunol. 2021, 12, 4744. [Google Scholar] [CrossRef] [PubMed]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Agostinis, C.; Stampalija, T.; Tannetta, D.; Loganes, C.; Brumatti, L.V.; De Seta, F.; Celeghini, C.; Radillo, O.; Sargent, I.; Tedesco, F.; et al. Complement component C1q as potential diagnostic but not predictive marker of preeclampsia. Am. J. Reprod. Immunol. 2016, 76, 475–481. [Google Scholar] [CrossRef]
- Rampersad, R.; Barton, A.; Sadovsky, Y.; Nelson, D. The C5b-9 Membrane Attack Complex of Complement Activation Localizes to Villous Trophoblast Injury in vivo and Modulates Human Trophoblast Function in vitro. Placenta 2008, 29, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Banadakoppa, M.; Vidaeff, A.C.; Yallampalli, U.; Ramin, S.M.; Belfort, M.A.; Yallampalli, C. Complement Split Products in Amniotic Fluid in Pregnancies Subsequently Developing Early-Onset Preeclampsia. Dis. Markers 2015, 2015, 263109. [Google Scholar] [CrossRef] [Green Version]
- Vaught, A.J.; Braunstein, E.M.; Jasem, J.; Yuan, X.; Makhlin, I.; Eloundou, S.; Baines, A.C.; Merrill, S.A.; Chaturvedi, S.; Blakemore, K.; et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight 2018, 3, e99128. [Google Scholar] [CrossRef] [Green Version]
- Burwick, R.M.; Fichorova, R.N.; Dawood, H.Y.; Yamamoto, H.S.; Feinberg, B.B. Urinary Excretion of C5b-9 in Severe Preeclampsia: Tipping the balance of complement activation in pregnancy. Hypertension 2013, 62, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Burwick, R.M.; Velasquez, J.; Valencia, C.M.; Gutiérrez-Marín, J.; Edna-Estrada, F.; Silva, J.L.; Trujillo-Otálvaro, J.; Vargas-Rodríguez, J.; Bernal, Y.; Quintero, A.; et al. Terminal Complement Activation in Preeclampsia. Obstet. Gynecol. 2018, 132, 1477–1485. [Google Scholar] [CrossRef] [Green Version]
- Buurma, A.; Cohen, D.; Veraar, K.; Schonkeren, D.; Claas, F.H.; Bruijn, J.A.; Bloemenkamp, K.W.; Baelde, H.J. Preeclampsia Is Characterized by Placental Complement Dysregulation. Hypertension 2012, 60, 1332–1337. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-D.; Xu, B.-N.; Wang, M.-L.; Wang, Y.-Q.; Yu, F.; Chen, Q.; Zhao, M.-H. Dysregulation of complement system during pregnancy in patients with preeclampsia: A prospective study. Mol. Immunol. 2020, 122, 69–79. [Google Scholar] [CrossRef]
- Fang, C.J.; Fremeaux-Bacchi, V.; Liszewski, M.K.; Pianetti, G.; Noris, M.; Goodship, T.H.J.; Atkinson, J.P. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood 2008, 111, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Vaught, A.J.; Kovell, L.; Szymanski, L.M.; Mayer, S.A.; Seifert, S.M.; Vaidya, D.; Murphy, J.D.; Argani, C.; O’Kelly, A.; York, S.; et al. Acute Cardiac Effects of Severe Pre-Eclampsia. J. Am. Coll. Cardiol. 2018, 72, 1–11. [Google Scholar] [CrossRef]
- Burwick, R.; Feinberg, B. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013, 34, 201–203. [Google Scholar] [CrossRef]
- Lokki, A.I.; Haapio, M.; Heikkinen-Eloranta, J. Eculizumab Treatment for Postpartum HELLP Syndrome and aHUS—Case Report. Front. Immunol. 2020, 11, 548. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Anagnostopoulos, A.; Mastellos, D.C. Complement in Thrombotic Microangiopathies: Unraveling Ariadne’s Thread into the Labyrinth of Complement Therapeutics. Front. Immunol. 2019, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.R.; Robinson, S.; Scully, M.A. How we manage thrombotic microangiopathies in pregnancy. Br. J. Haematol. 2016, 173, 821–830. [Google Scholar] [CrossRef]
- Pourrat, O.; Coudroy, R.; Pierre, F. Differentiation between Severe HELLP Syndrome and Thrombotic Microangiopathy, Thrombotic Thrombocytopenic Purpura and Other Imitators. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 68–72. [Google Scholar] [CrossRef]
- Rahman, T.M.; Wendon, J. Severe Hepatic Dysfunction in Pregnancy. Qjm 2002, 95, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.K.; Badr, D.; Hubeish, M.; Itani, S.; Hijazi, H.; Mogharbil, A. HELLP Syndrome, Thrombotic Thrombocytopenic Purpura or Both: Appraising the Complex Association and Proposing a Stepwise Practical Plan for Differential Diagnosis. J. Hematol. 2018, 7, 32–37. [Google Scholar] [CrossRef]
- Ehsanipoor, R.M.; Rajan, P.; Holcombe, R.F.; Wing, D.A. Limitations of ADAMTS-13 Activity Level in Diagnosing Thrombotic Thrombocytopenic Purpura in Pregnancy. Clin. Appl. Thromb. 2008, 15, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Patrick, T.; Najera, J.E.; Eastwood, J.; Carlan, S.J. Management of Thrombotic Thrombocytopenic Purpura with Autoantibodies to ADAMTS-13 and Concurrent Preeclampsia in Pregnancy: Multidisciplinary Team Approach. Am. J. Perinatol. Rep. 2012, 2, 37–38. [Google Scholar] [CrossRef] [Green Version]
- Mousseaux, C.; Joly, B.S.; Mohamadou, I.; Arrestier, R.; Hertig, A.; Rafat, C. Severe HELLP syndrome masquerading as thrombocytopenic thrombotic purpura: A case report. BMC Nephrol. 2020, 21, 204. [Google Scholar] [CrossRef]
- Bhute, A.; Acharya, S.; Acharya, N.; Mohammad, S. Moschcowitz Syndrome or Thrombotic Thrombocytopenic Purpura and Antiphospholipid Antibody Syndrome as a Rare Cause of Thrombocytopenia in Pregnancy Mimicking Hemolysis, Elevated Liver Enzymes, and Low Platelets Syndrome in a Patient with Bad Obstetric History: A Diagnostic Dilemma. J. South Asian Fed. Obstet. Gynaecol. 2020, 12, 250–253. [Google Scholar] [CrossRef]
- González-Mesa, E.; Narbona, I.; Blasco, M.; Cohen, I. Unfavorable Course in Pregnancy-Associated Thrombotic Thrombocytopenic Purpura Necessitating a Perimortem Cesarean Section: A Case Report. J. Med. Case Rep. 2013, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fakhouri, F.; Scully, M.; Provôt, F.; Blasco, M.; Coppo, P.; Noris, M.; Paizis, K.; Kavanagh, D.; Pène, F.; Quezada, S.; et al. Management of thrombotic microangiopathy in pregnancy and postpartum: Report from an international working group. Blood 2020, 136, 2103–2117. [Google Scholar] [CrossRef]
| Authors (Publication Date) | Sample | n | Control Group | n | vWF | ADAMTS13 |
|---|---|---|---|---|---|---|
| Kalem et al. (2017) [13] | PE | 12 | HP | 24 | Decrease | |
| Chen et al. (2021) [24] | PE | 54 | HP NP | 33 | Hyperadhesive | Decrease Decrease |
| Stepanian et al. (2011) [14] | PE | 140 | HP | 140 | No difference | Decrease |
| Alpoim et al. (2011) [15] | PE | 55 | HP NP | 35 50 | Increase Increase | Decrease Decrease |
| Sabau et al. (2016) [16] | HELLP | 16 | - | <30% in 13% | ||
| Molvarec et al. (2009) [19] | PE | 67 | HP NP | 70 59 | Normal multimeric pattern of vWF Increased vWF:Ag | No difference |
| Yoshida et al. (2017) [20] | PE HELLP | 5 8 | HP | 128 | Increased vWF:Ag | No difference |
| Aref et al. (2013) [2] | PE | 110 | HP NP | 50 10 | Increase | Decrease |
| Xiao et al. (2017) [17] | PE | - | Decrease | |||
| Hulstein et al. (2006) [18] | HELLP | 14 | HP PE | 9 6 | Increase Increase | Decrease No difference |
| von Krogh et al. (2015) [21] | PE | 500 | HP | 500 | No difference (levels, genotype, allele, haplotype, levels) | |
| Drury Stewart et al. (2014) [23] | NP | 46 | - | vWF: Ag increase vWFpp increase (later stage) No high molecular weight vWF multimers | ||
| Molvarec et al. (2009) [22] | PE | 93 | HP | 127 | Increase |
| Mississippi System | Tennessee System | |
|---|---|---|
| Class 1 | PLTs < 50.000/mm3 | Complete syndrome |
| Class 2 | PLTs: 50.000–100.000/mm3 | Incomplete syndrome |
| Class 3 | -PLTs> 100.000–150.000/mm3 -Hemolysis and elevated liver enzymes—LDH > 600 IU/I | |
| Parameters | HELLP Syndrome | TTP |
|---|---|---|
| Hemolysis | + to +++ | +++ |
| Schistocytosis | + to +++ | +++ |
| LDH | ++ to +++ | +++ |
| Liver enzymes | ++ to +++ | No or + |
| Platelets | ++ to +++ | +++ |
| Total Bilirubin | + | + to ++ |
| Proteinuria | +++ | + to ++ |
| ADAMTS13 | Detectable (decreased) | Undetectable |
| Fever | No | ++ |
| Authors Age (Years)/Gestational Week | Signs/Symptoms of Admission | Initial Diagnosis | Clinical Course | Final Diagnosis |
|---|---|---|---|---|
| Ramadan et al. (2018) 26 y/33 w [43] | Seizures high blood pressure | HELLP | PLTs decrease and LDH increase on postpartum day 6 after initial amelioration | HELLP & TTP |
| Ramadan et al. (2018) 36 y/27 w [43] | Thrombocytopenia | Acquired TTP | headache, epigastric pain, and tachypnea manifestation, high blood pressure | TTP& PE/HELLP |
| Ramadan et al. (2018) 24 y/37 w [43] | High blood pressure, edema, mild anemia | PE | hypertension, headache, thrombocytopenia, hyperuricemia, SGOT, serum creatinine, and LDH elevation, schistocytes | PE & TTP |
| González-Mesa et al. (2013) 30 y/28 w [48] | Dizziness, headache, Other neurological manifestation, anemia, thrombocytopenia | TTP | Dyspnea, hypoxia, cardiopulmonary arrest | TTP |
| Ehsanipoor et al. (2005) 26 y/22 w [44] | epigastric pain, proteinuria, and elevated blood pressure | HELLP | persistent thrombocytopenia and hemolytic anemia | HELLP & TTP |
| Patrick et al. (2012) 28 y/32.2 w [45] | Headache Elevated blood pressure | TTP | Persistent thrombocytopenia, liver enzymes elevation | TTP& PE |
| Mousseaux et al. (2020) 28 y/37 w [46] | Elevated Blood pressure, proteinuria | PE | Epigastric pain, fetal bradycardia, anuria (after delivery) | PE &TTP |
| Bhute et al. (2020) 25 y/34 w [47] | Headache, seizures, jaundice, high blood pressure, edema | HELLP | Worsening proteinuria, liver enzymes elevation | HELLP & TTP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardikioti, A.; Venou, T.-M.; Gavriilaki, E.; Vetsiou, E.; Mavrikou, I.; Dinas, K.; Daniilidis, A.; Vlachaki, E. Molecular Advances in Preeclampsia and HELLP Syndrome. Int. J. Mol. Sci. 2022, 23, 3851. https://doi.org/10.3390/ijms23073851
Gardikioti A, Venou T-M, Gavriilaki E, Vetsiou E, Mavrikou I, Dinas K, Daniilidis A, Vlachaki E. Molecular Advances in Preeclampsia and HELLP Syndrome. International Journal of Molecular Sciences. 2022; 23(7):3851. https://doi.org/10.3390/ijms23073851
Chicago/Turabian StyleGardikioti, Angeliki, Theodora-Maria Venou, Eleni Gavriilaki, Evangelia Vetsiou, Ioulia Mavrikou, Konstantinos Dinas, Angelos Daniilidis, and Efthymia Vlachaki. 2022. "Molecular Advances in Preeclampsia and HELLP Syndrome" International Journal of Molecular Sciences 23, no. 7: 3851. https://doi.org/10.3390/ijms23073851
APA StyleGardikioti, A., Venou, T.-M., Gavriilaki, E., Vetsiou, E., Mavrikou, I., Dinas, K., Daniilidis, A., & Vlachaki, E. (2022). Molecular Advances in Preeclampsia and HELLP Syndrome. International Journal of Molecular Sciences, 23(7), 3851. https://doi.org/10.3390/ijms23073851








