Leaf Photosynthesis and Its Temperature Response Are Different between Growth Stages and N Supplies in Rice Plants
Abstract
1. Introduction
2. Results
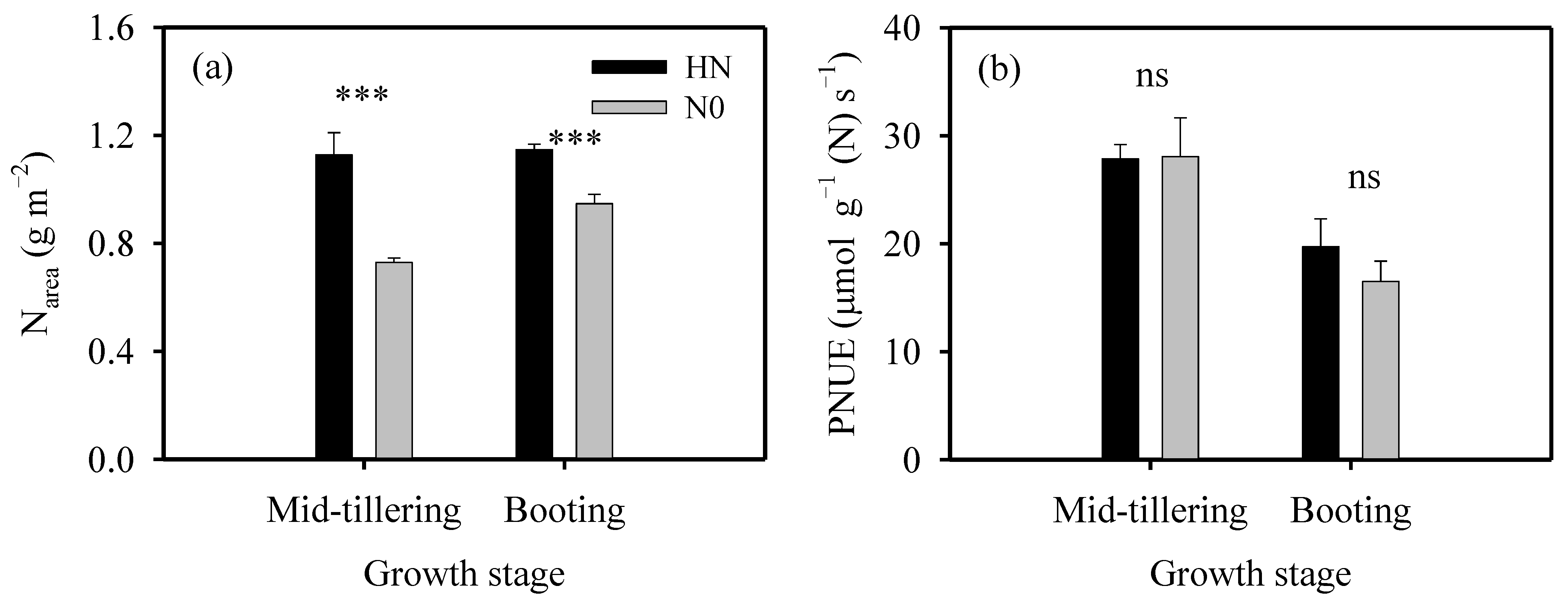
2.1. Response of Leaf N Content to N Supplies and Growth Stages
2.2. The Responses of Gas-Exchange Parameters to Temperature under Different N Supplies and at Different Growth Stages
2.3. Effects of N Supply and Growth Stage on Leaf Anatomical Traits
2.4. The Responses of Leaf Hydraulic Traits to Temperature under Different N Supplies and at Different Growth Stages
3. Discussion
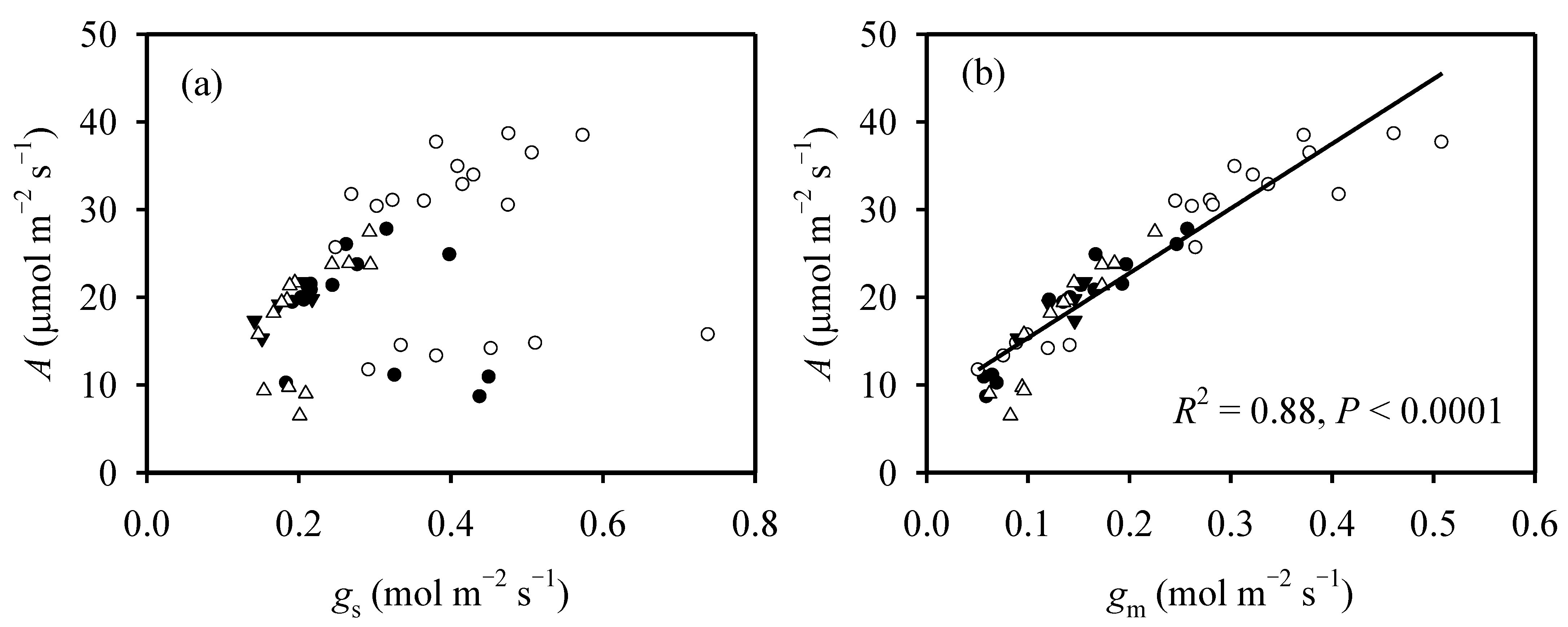
3.1. The Variation in Photosynthesis between Growth Stage and N Supply Is Related to CO2-Diffusion Capacity
3.2. Temperature Response of gm Varies with Growth Stage and N Supply
4. Materials and Methods
4.1. Plant Materials and N Treatments
4.2. Gas-Exchange Measurements
4.3. Measurements of Leaf Hydraulic Parameters
4.4. Leaf N Content Measurement
4.5. Measurements of Leaf Anatomical Traits
4.6. Quantification of the Temperature Response of gm
4.7. Modelling the Temperature Response of gm
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kathuria, H.; Giri, J.; Tyagi, H.; Tyagi, A.K. Advances in transgenic rice biotechnology. Crit. Rev. Plant Sci. 2007, 26, 65–103. [Google Scholar] [CrossRef]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Xu, X.; Shen, Q.; Guo, S. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J. Exp. Bot. 2009, 60, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Nagai, T.; Makino, A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011, 34, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Cago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef]
- Adachi, S.; Nakae, T.; Uchida, M.; Soda, K.; Takai, T.; Oi, T.; Hirasawa, T. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J. Exp. Bot. 2013, 64, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carriquí, M.; Nadal, M.; Clemente-Moreno, M.J.; Coopman, R.E.; Fernie, A.R.; Flexas, J. Photosynthesis optimized across land plant phylogeny. Trends Plant Sci. 2019, 24, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (Genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef] [PubMed]
- Carriquí, M.; Cabrera, H.M.; Conesa, M.À.; Coopman, R.E.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Tomás, M.; et al. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 2015, 38, 448–460. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y. Varietal difference in the correlation between leaf nitrogen content and photosynthesis in rice (Oryza sativa L.) plants is related to specific leaf weight. J. Integr. Agr. 2016, 15, 2002–2011. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Peng, S.B.; Li, Y. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. 2019, 70, 5259–5269. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Yang, Y.H.; Peng, S.B.; Li, Y. Nighttime transpirational cooling enabled by circadian regulation of stomatal conductance is related to stomatal anatomy and leaf morphology in rice. Planta 2021, 254, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Shu, Y.; Peng, S.B.; Li, Y. Leaf photosynthesis is positively correlated with xylem and phloem areas in leaf veins in rice (Oryza sativa) plants. Ann. Bot. 2022, 2022, mcac020. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [PubMed]
- North, G.B.; Lynch, F.H.; Maharaj, F.D.; Phillips, C.A.; Woodside, W.T. Leaf hydraulic conductance for a tank bromeliad: Axial and radial pathways for moving and conserving water. Front. Plant Sci. 2013, 4, e78. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant Cell Environ. 2015, 38, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Mesophyll conductance: Walls, membranes and spatial complexity. New Phytol. 2020, 229, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, B.; Ding, L.; Shen, Q.; Peng, S.; Guo, S. Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS ONE 2013, 8, e62036. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Zhang, Q.Q.; Wei, X.H.; Peng, S.B.; Li, Y. Nitrogen can alleviate the inhibition of photosynthesis caused by high temperature stress under both steady-state and flecked irradiance. Front. Plant Sci. 2017, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Q.Q.; Huang, G.J.; Peng, S.B.; Li, Y. Temperature responses of photosynthesis and leaf hydraulic conductance in rice and wheat. Plant Cell Environ. 2020, 43, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant. Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Evans, J.R.; von Caemmerer, S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. 2013, 36, 745–756. [Google Scholar] [CrossRef] [PubMed]
- von Caemmerer, S.; Evans, J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, X.; Li, S.; Salter, W.T.; Barbour, M.M. The role of leaf water potential in the temperature response of mesophyll conductance. New Phytol. 2020, 225, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Shimada, T.; Takumi, S.; Kaneko, K.; Matsuoka, M.; Shimamoto, K.; Nakano, H.; Miyao-Tokutomi, M.; Mae, T.; Yamamoto, N. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense rbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol. 1997, 114, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–479. [Google Scholar] [CrossRef]
- Leegood, R.C.; Sharkey, T.D.; von Caemmerer, S. Photosynthesis: Physiology and Metabolism; Kluwer Academic Publishers: New York, NY, USA, 2004. [Google Scholar]
- Flexas, J.; Ribas-Carbó, M.; Hansom, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; von Caemmerer, S.; Setchell, B.A.; Hudson, S. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Funct. Plant. Biol. 1994, 21, 475–495. [Google Scholar] [CrossRef]
- Xiong, D.; Liu, X.; Liu, L.; Douthe, C.; Li, Y.; Peng, S.; Huang, J. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplies in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef]
- Harrison, M.T.; Edwards, E.J.; Farquhar, G.D.; Nicotra, A.B.; Evans, J.R. Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ. 2009, 32, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Clemente-Moreno, M.J.; Bota, J.; Brodribb, T.J.; Gago, J.; Mizokami, Y.; Nadal, M.; Perera-Castro, A.V.; Roig-Oliver, M.; Sugiura, D.; et al. Cell wall thickness and composition are involved in photosynthetic limitation. J. Exp. Bot. 2021, 72, 3971–3986. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant. Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.C.; Ahmed, M.A.; Abdalla, M.; Carminati, A. Root hydraulic phenotypes impacting water uptake in drying soils. Plant Cell Environ. 2022, 45, 650–663. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernandez, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef] [PubMed]
- Valentini, R.; Epron, D.; De Angelis, P.; Matteucci, G.; Dreyer, E. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: Diurnal cycles under different levels of water supply. Plant Cell Environ. 1995, 18, 631–640. [Google Scholar] [CrossRef]
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T.D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R.; Long, S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the Evaporative Flux Method (EFM). J. Vis. Exp. 2012, 70, e4179. [Google Scholar] [CrossRef] [PubMed]
- Taylaran, R.D.; Adachi, S.; Ookawa, T.; Usuda, H.; Hirasawa, T. Hydraulic conductance as well as nitrogen accumulation plays a role in the higher rate of leaf photosynthesis of the most productive variety of rice in Japan. J. Exp. Bot. 2011, 62, 4067–4077. [Google Scholar] [CrossRef]
- Sack, L.; Streeter, C.M.; Holbrook, N.M. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiol. 2004, 134, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Sellin, A.; Kupper, P. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiol. 2007, 27, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Z.C.; Huang, G.J.; Xiong, Z.; Peng, S.B.; Li, Y. High leaf mass per area Oryza genotypes invest more leaf mass to cell wall and show a low mesophyll conductance. AoB Plants 2020, 12, plaa028. [Google Scholar] [CrossRef]
- Tosens, T.; Niinemets, Ü.; Westoby, M.; Wright, I.J. Anatomical basis of variation in mesophyll resistance in eastern australian sclerophylls: News of a long and winding path. J. Exp. Bot. 2012, 63, 5105–5119. [Google Scholar] [CrossRef] [PubMed]
- Tosens, T.; Nishida, K.; Gago, J.; Coopman, R.E.; Cabrera, H.M.; Carriquí, M.; Laanisto, L.; Morales, L.; Nadal, M.; Rojas, R.; et al. The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 2015, 209, 1576–1590. [Google Scholar] [CrossRef] [PubMed]

| Growth Stage | N Supply | Leaf Temperature (°C) | A (µmol m−2 s−1) | gs (mol m−2 s−1) | gm (mol m−2 s−1) |
|---|---|---|---|---|---|
| Mid-tillering | N0 | 15 | 10.2 ± 1.1 c | 0.350 ± 0.124 a | 0.063 ± 0.006 c |
| 25 | 20.3 ± 1.0 b | 0.213 ± 0.020 b | 0.149 ± 0.027 b | ||
| 35 | 24.6 ± 2.6 a | 0.294 ± 0.068 ab | 0.207 ± 0.043 a | ||
| HN | 15 | 14.0 ± 1.4 b | 0.452 ± 0.161 a | 0.096 ± 0.032 c | |
| 25 | 31.7 ± 4.1 a | 0.372 ± 0.087 a | 0.300 ± 0.073 b | ||
| 35 | 35.1 ± 2.7 a | 0.430 ± 0.105 a | 0.388 ± 0.066 a | ||
| Booting | N0 | 15 | - | - | - |
| 25 | 18.7 ± 2.5 | 0.178 ± 0.174 | 0.132 ± 0.121 | ||
| 35 | - | - | - | ||
| HN | 15 | 8.6 ± 1.5 c | 0.188 ± 0.024 b | 0.084 ± 0.016 c | |
| 25 | 18.9 ± 2.2 b | 0.174 ± 0.018 b | 0.127 ± 0.019 b | ||
| 35 | 24.0 ± 2.2 a | 0.257 ± 0.044 a | 0.188 ± 0.021 a | ||
| ANOVA | |||||
| T | *** | ns | *** | ||
| N | ** | ns | * | ||
| S | *** | ** | * | ||
| T × N | ** | ns | *** | ||
| T × S | ** | ns | *** | ||
| N × S | *** | * | *** |
| Growth Stage | N Supply | c | Ea,gm (kJ mol−1) | ΔS (kJ K−1 mol−1) | Ed,gm (kJ mol−1) |
|---|---|---|---|---|---|
| Mid-tillering | N0 | 11.2 | 28.2 | 1.25 | 437.4 |
| HN | 15.3 | 38.4 | 1.25 | 437.4 | |
| Booting | N0 | - | - | - | - |
| HN | 12.1 | 29.9 | 1.25 | 437.4 |
| Growth Stage | N Supply | l (µm) | Pmem,25 (mm s−1) | Ea,mem (kJ mol−1) | gliq,25’ (mol CO2 m−2 s−1) | gmem,25’ (mol CO2 m−2 s−1) | gm,25’ (mol CO2 m−2 s−1) | gliq,25’/gmem,25’ |
|---|---|---|---|---|---|---|---|---|
| Mid-tillering | N0 | 0.89 | 0.722 | 60.4 | 0.0732 | 0.0239 | 0.0180 | 3.07 |
| HN | 0.73 | 1.272 | 81.0 | 0.0897 | 0.0421 | 0.0286 | 2.13 | |
| Booting | N0 | - | - | - | - | - | - | - |
| HN | 1.01 | 0.910 | 66.4 | 0.0643 | 0.0301 | 0.0205 | 2.14 |
| Growth Stage | N Supply | Sm (µm2 µm−2) | Sc (µm2 µm−2) | Tcw (µm) |
|---|---|---|---|---|
| Mid-tillering | N0 | 10.95 ± 1.70 a | 6.92 ± 1.82 b | 0.226 ± 0.030 a |
| HN | 10.95 ± 1.51 a | 8.64 ± 1.90 a | 0.193 ± 0.024 b | |
| Booting | N0 | 11.37 ± 1.01 a | 5.25 ± 0.97 b | 0.245 ± 0.022 a |
| HN | 11.69 ± 0.98 a | 6.40 ± 1.76 a | 0.249 ± 0.042 a | |
| ANOVA | ||||
| N | ns | ** | * | |
| S | ** | *** | *** | |
| N × S | ns | ns | * |
| Growth Stage | N Supply | Leaf Temperature (°C) | Ψleaf (MPa) | Kleaf (mmol m−2 s−1 MPa−1) |
|---|---|---|---|---|
| Mid-tillering | N0 | 15 | −0.293 ± 0.087 a | 8.57 ± 1.11 a |
| 25 | −0.285 ± 0.090 a | 7.86 ± 1.80 a | ||
| 35 | −0.423 ± 0.097 b | 7.05 ± 1.56 a | ||
| HN | 15 | −0.360 ± 0.099 a | 11.39 ± 2.53 a | |
| 25 | −0.430 ± 0.114 a | 10.71 ± 1.45 a | ||
| 35 | −0.370 ± 0.106 a | 10.13 ± 2.58 a | ||
| Booting | N0 | 15 | −0.155 ± 0.064 a | 7.48 ± 2.25 a |
| 25 | −0.278 ± 0.068 b | 5.98 ± 1.78 a | ||
| 35 | −0.560 ± 0.140 c | 6.03 ± 1.55 a | ||
| HN | 15 | −0.395 ± 0.137 a | 8.83 ± 1.16 a | |
| 25 | −0.310 ± 0.081 a | 9.20 ± 2.17 a | ||
| 35 | −0.425 ± 0.044 a | 9.59 ± 1.38 a | ||
| ANOVA | ||||
| T | *** | ns | ||
| N | ns | *** | ||
| S | ns | ** | ||
| T × N | *** | ns | ||
| T × S | * | ns | ||
| N × S | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Zhang, Z.; Huang, G.; Li, Y. Leaf Photosynthesis and Its Temperature Response Are Different between Growth Stages and N Supplies in Rice Plants. Int. J. Mol. Sci. 2022, 23, 3885. https://doi.org/10.3390/ijms23073885
Ye M, Zhang Z, Huang G, Li Y. Leaf Photosynthesis and Its Temperature Response Are Different between Growth Stages and N Supplies in Rice Plants. International Journal of Molecular Sciences. 2022; 23(7):3885. https://doi.org/10.3390/ijms23073885
Chicago/Turabian StyleYe, Miao, Zhengcan Zhang, Guanjun Huang, and Yong Li. 2022. "Leaf Photosynthesis and Its Temperature Response Are Different between Growth Stages and N Supplies in Rice Plants" International Journal of Molecular Sciences 23, no. 7: 3885. https://doi.org/10.3390/ijms23073885
APA StyleYe, M., Zhang, Z., Huang, G., & Li, Y. (2022). Leaf Photosynthesis and Its Temperature Response Are Different between Growth Stages and N Supplies in Rice Plants. International Journal of Molecular Sciences, 23(7), 3885. https://doi.org/10.3390/ijms23073885







