Effect of Chemotherapy Cytarabine and Acute Myeloid Leukemia on the Development of Spermatogenesis at the Adult Age of Immature Treated Mice
Abstract
:1. Introduction
2. Results
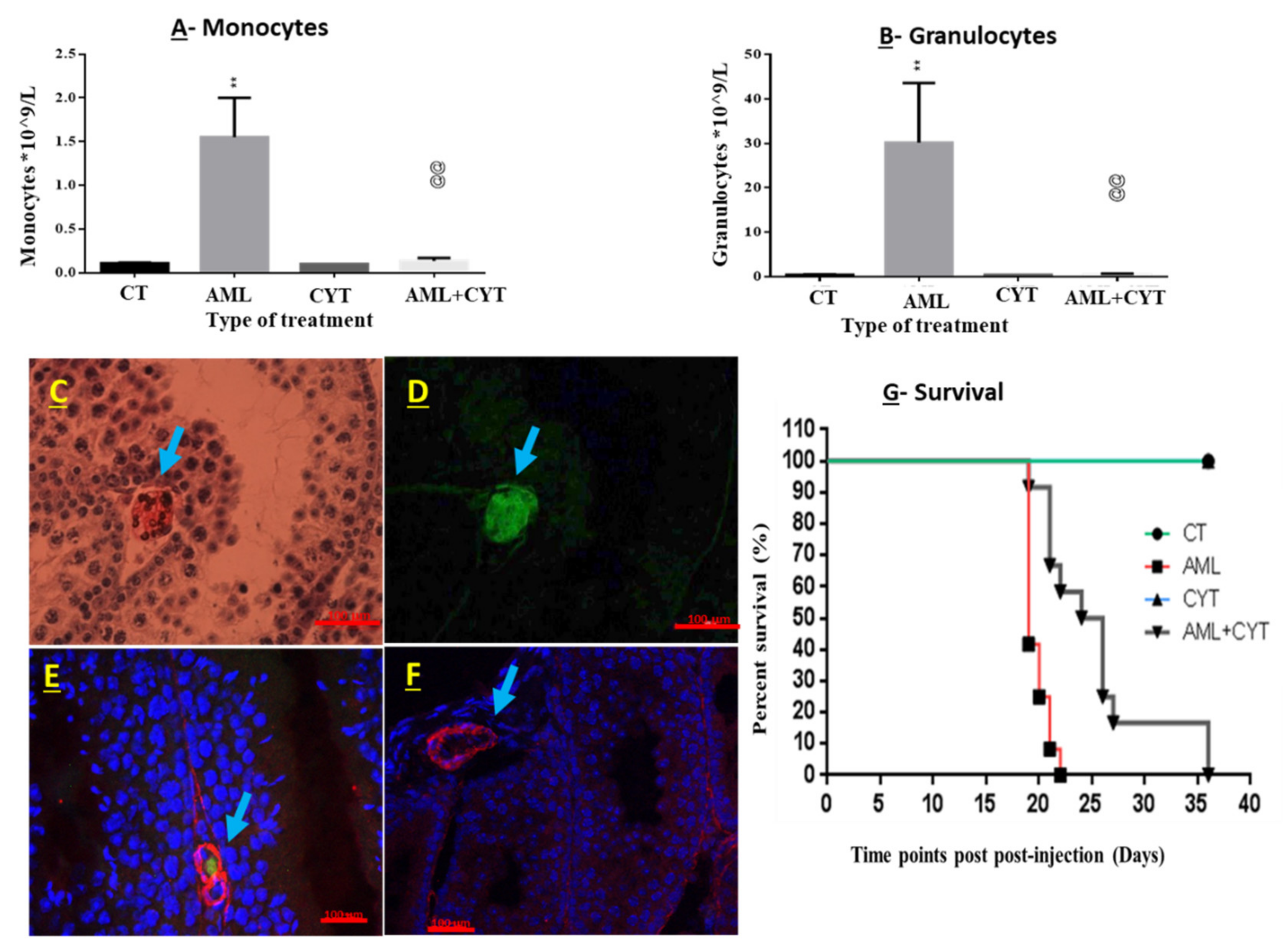
2.1. Development of AML in Immature Mice
2.2. Effect of AML and CYT on the Survival of Immature Treated Mice
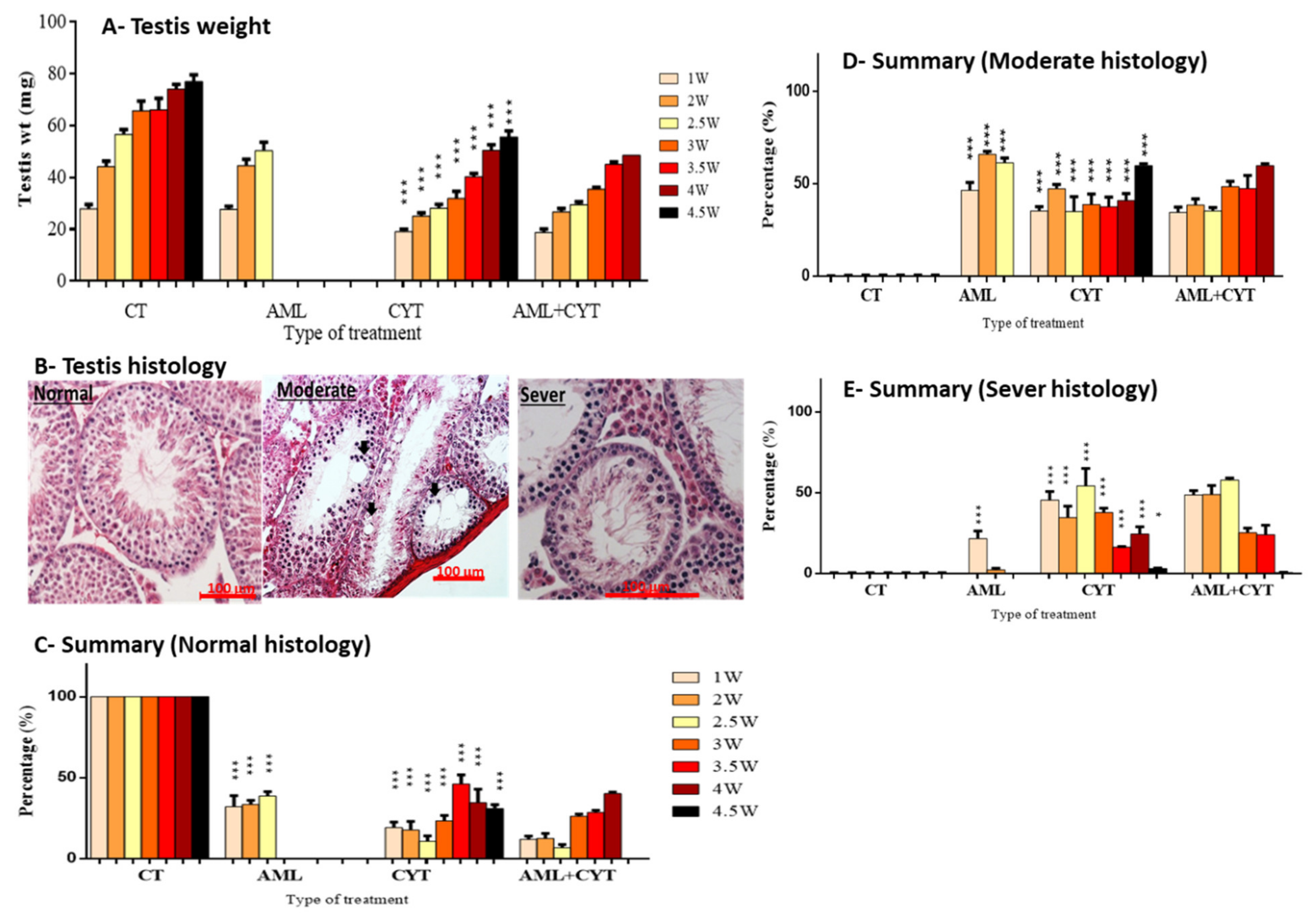
2.3. Effect of AML and CYT on Testes Weight and the Testicular Histology of Immature Treated Mice
2.4. Effect of AML and CYT on Apoptosis of Spermatogenic Cells in Testicular Tissue of Immature Treated Mice
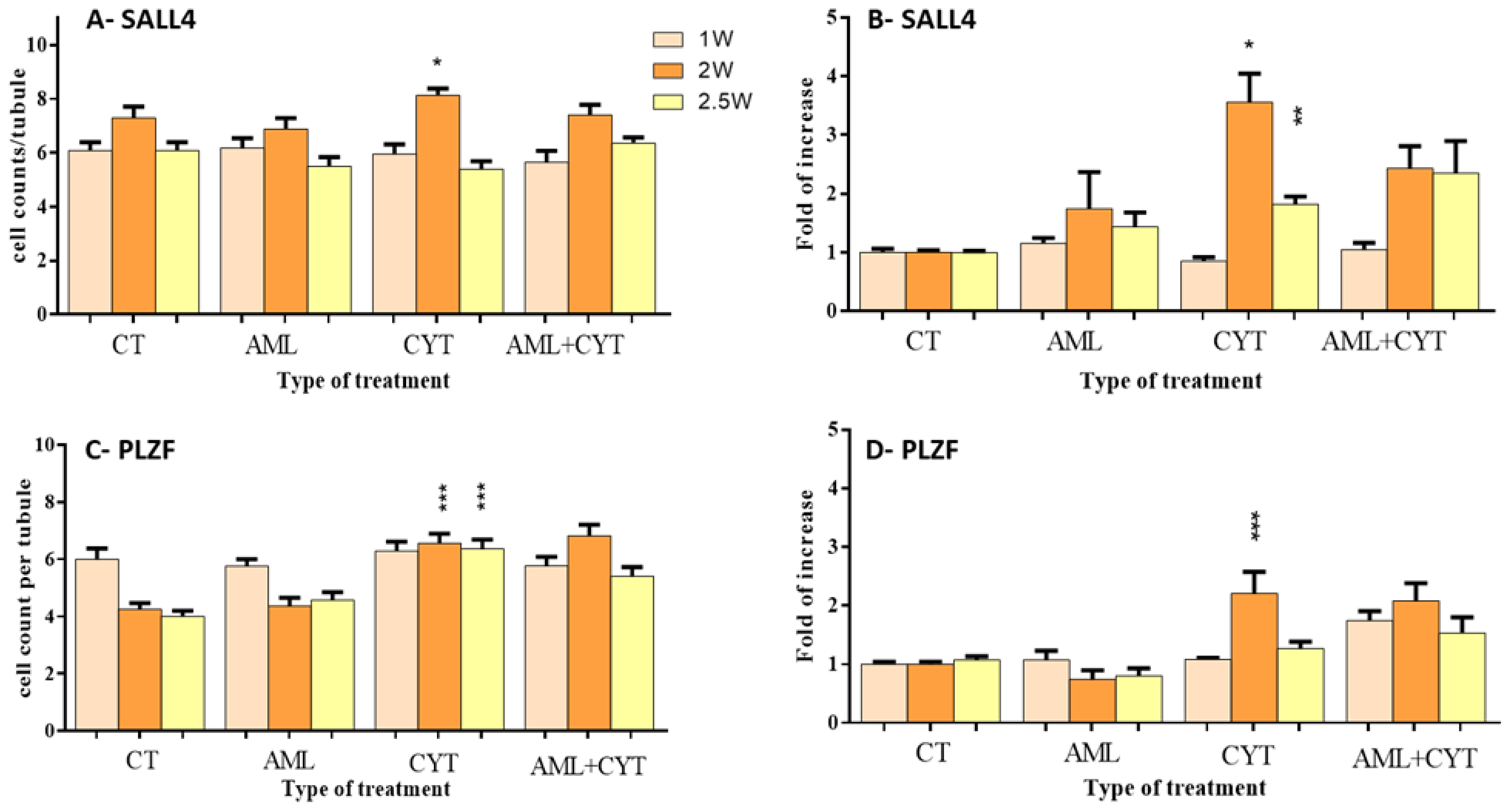
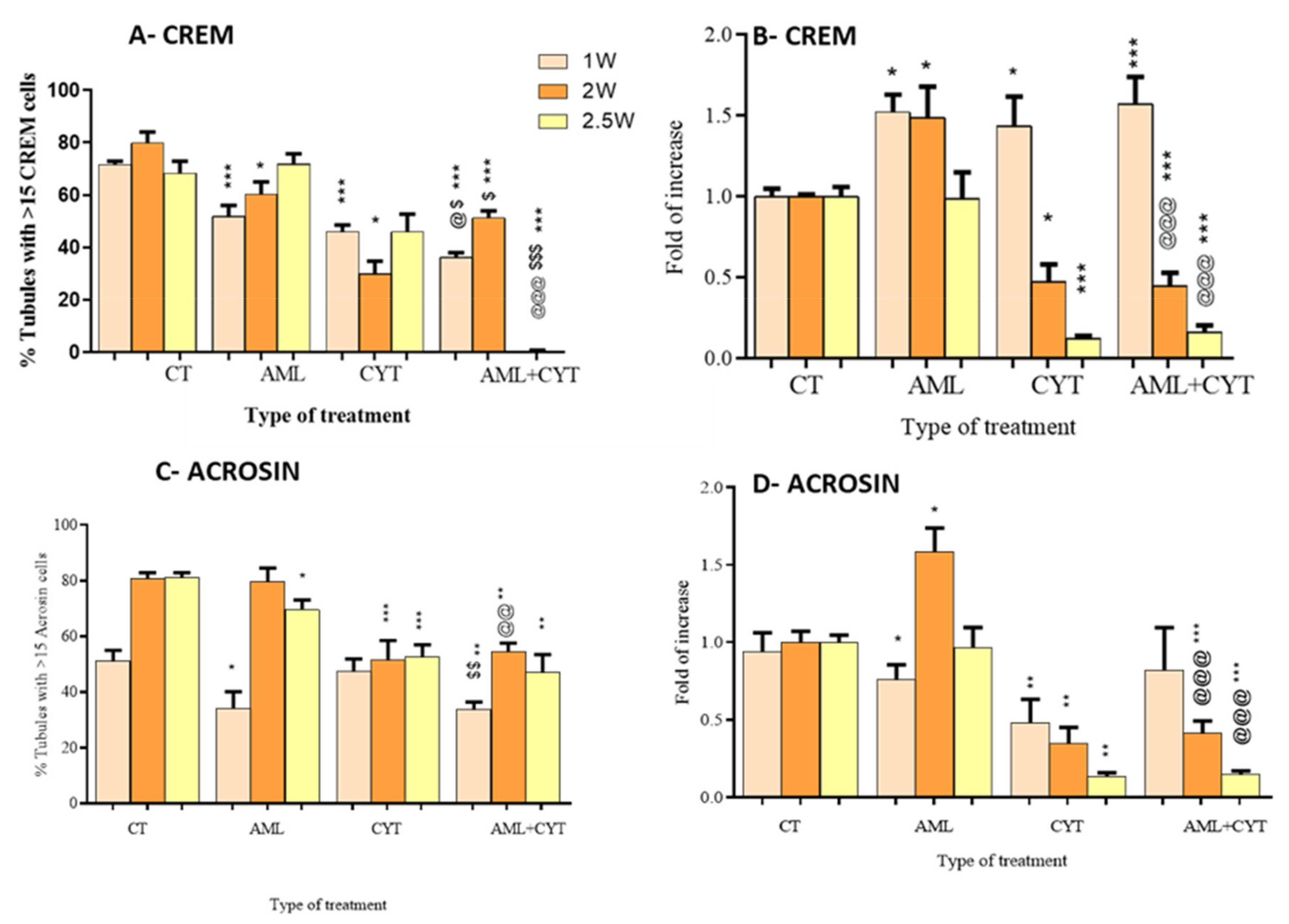
2.5. Effect of AML and CYT on the Expression Levels and Number of Spermatogenic Cells of Immature Treated Mice
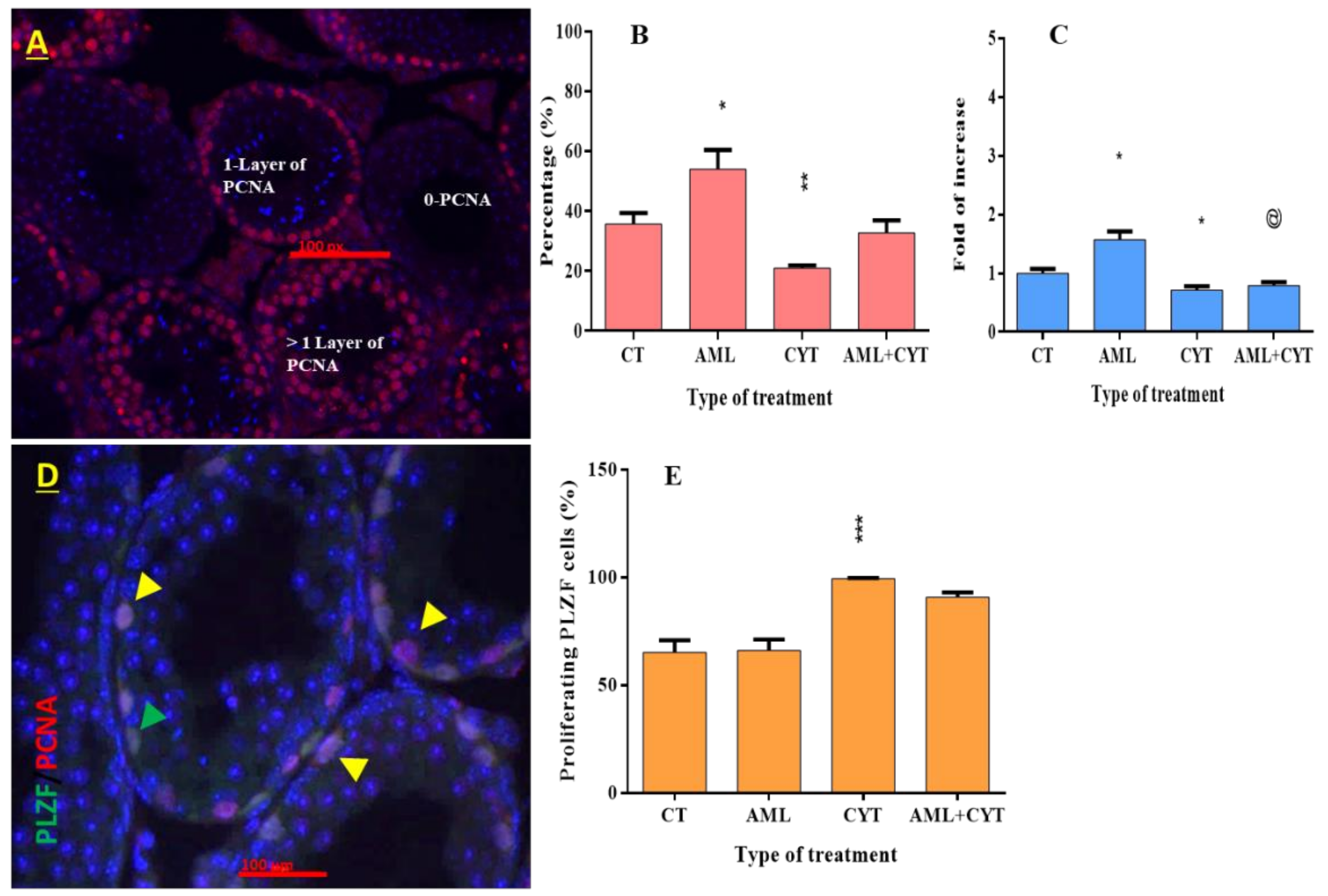
2.6. Effect of AML and CYT on Cell Proliferation of Spermatogenic Cells in Testicular Tissue of Immature Treated Mice
2.7. Effect of CYT and AML+CYT on Sperm Concentration
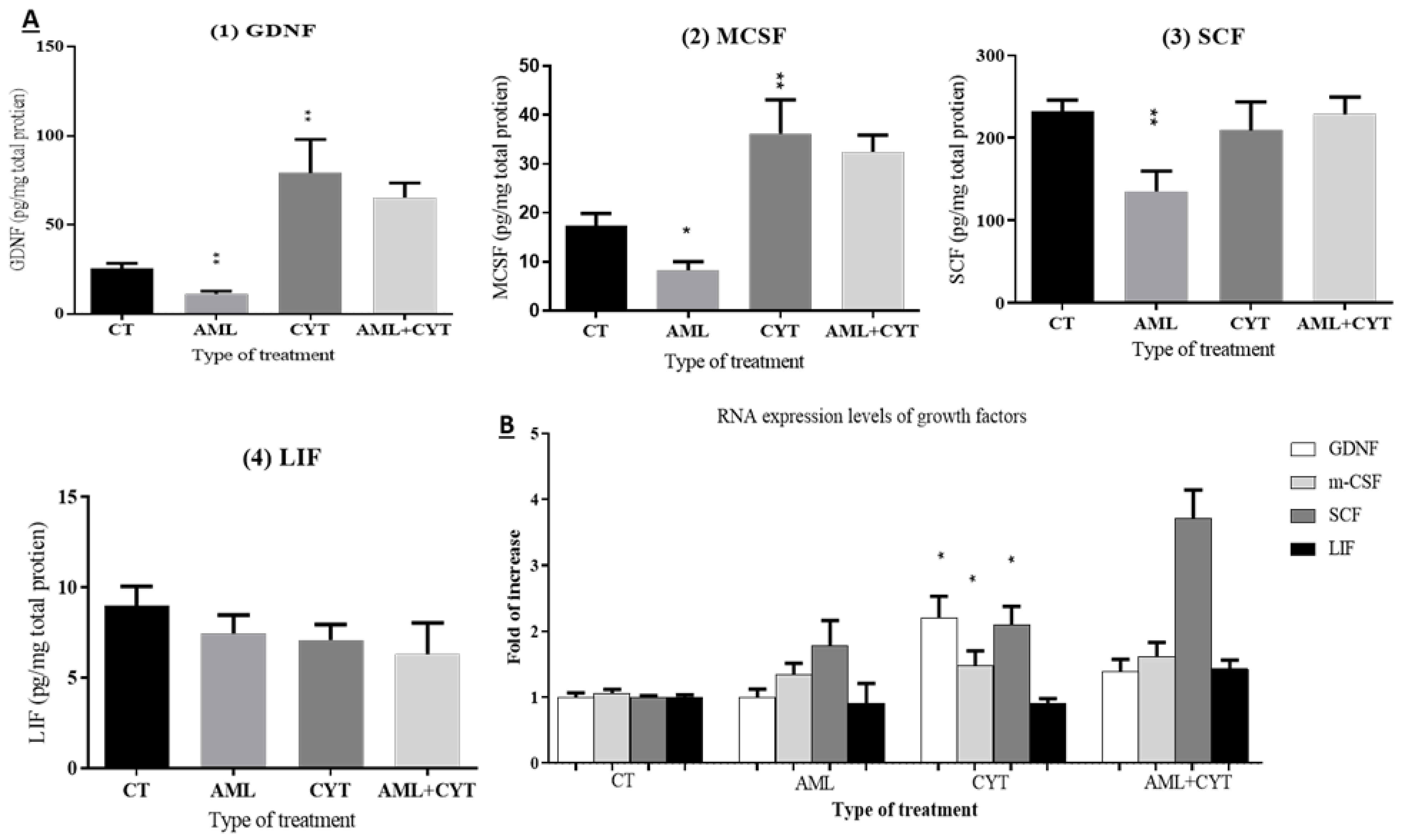
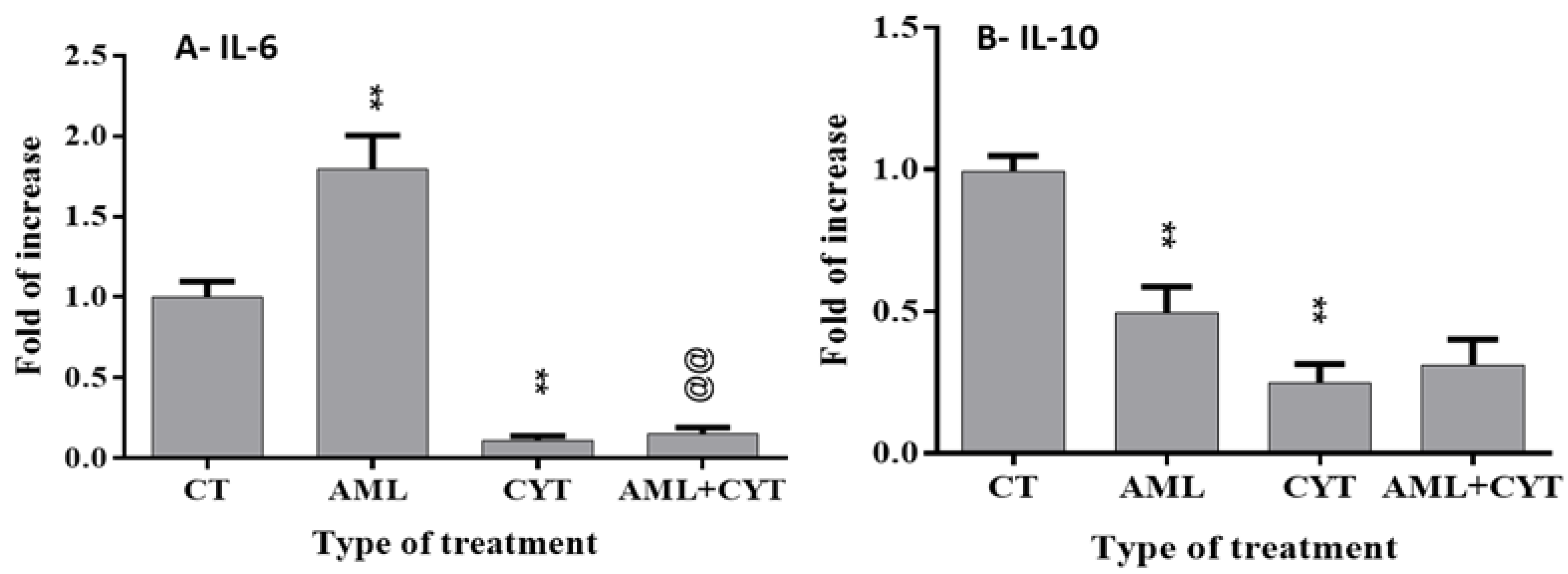
2.8. Effect of AML and CYT on the Levels of Testicular Growth Factors, Proinflammatory and Anti-Inflammatory Cytokines of Immature Treated Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Development of a Model of AML in Immature Mice
4.3. Hematological Analysis
4.4. Immunofluorescent Staining of Testicular Tissues
4.5. Immunohistochemistry Staining of Testicular Tissues
4.6. Hematoxylin Eosin Staining
4.7. Real-Time Quantitative PCR
4.8. TUNEL Immunohistochemistry
4.9. Sperm Extraction and Sperm Concentration Measurement
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Evaluation of Total Protein in Testicular Homogenates
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saultz, J.N.; Garzon, R. Acute Myeloid Leukemia: A Concise Review. J. Clin. Med. 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belson, M.; Kingsley, B.; Holmes, A. Risk Factors for Acute Leukemia in Children: A Review. Environ. Health Perspect. 2007, 115, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, E.A.; Meshinchi, S. Acute myeloid leukemia in children and adolescents: Identification of New Molecular Targets Brings Promise of New Therapies. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, J.Y.; Park, N.C.; Jeon, T.G.; Park, H.J. Semen Analysis in Cancer Patients Referred for Sperm Cryopreservation before Chemotherapy over a 15-Year Period in Korea. World J. Mens Health 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Hallak, J.; Kolettis, P.N.; Sekhon, V.S.; Thomas, A.J., Jr.; Agarwal, A. Cryopreservation of sperm from patients with leukemia: Is it Worth the Effort? Cancer 1999, 85, 1973–1978. [Google Scholar] [CrossRef]
- Johnson, M.D.; Cooper, A.R.; Jungheim, E.S.; Lanzendorf, S.E.; Odem, R.R.; Ratts, V.S. Sperm banking for fertility preservation: A 20-year Experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Michailov, Y.; Lunenfeld, E.; Kapilushnik, J.; Friedler, S.; Meese, E.; Huleihel, M. Acute Myeloid Leukemia Affects Mouse Sperm Parameters, Spontaneous Acrosome Reaction, and Fertility Capacity. Int. J. Mol. Sci. 2019, 20, 219. [Google Scholar] [CrossRef] [Green Version]
- Michailov, Y.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model. Int. J. Mol. Sci. 2021, 22, 11157. [Google Scholar] [CrossRef]
- Walker, B.R.; Cartwright, P.C. Granulocytic sarcoma presenting as testicular and paratesticular masses in infancy. J. Urol. 2001, 165, 224. [Google Scholar] [CrossRef]
- Tran, C.N.; Collie, A.M.; Flagg, A.; Rhee, A. Testicular myeloid sarcoma: A Rare Manifestation of Acute Myeloid Leukemia in an Infant. Urology 2014, 84, 925–927. [Google Scholar] [CrossRef]
- Fonseca, A.; Scheinemann, K.; Jansen, J.; Barr, R. Testicular Myeloid Sarcoma: An Unusual Presentation of Infant Acute Myeloid Leukemia. J. Pediatric Hematol. Oncol. 2014, 36, e155–e157. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, J.; Tripathy, J.; Vij, M.; Scott, J.X. Unusual cause for testicular swelling in a child. BMJ Case Rep. 2017, 2017, bcr2016217656. [Google Scholar] [CrossRef] [PubMed]
- Valli-Pulaski, H.; Peters, K.A.; Gassei, K.; Steimer, S.R.; Sukhwani, M.; Hermann, B.P.; Dwomor, L.; David, S.; Fayomi, A.P.; Munyoki, S.K.; et al. Testicular tissue cryopreservation: 8 Years of Experience from a Coordinated Network of Academic Centers. Hum. Reprod. 2019, 34, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of Postmeiotic Cells In Vitro from Spermatogonial Cells of Prepubertal Cancer Patients. Stem Cells Dev. 2018, 27, 1007–1020. [Google Scholar] [CrossRef]
- Poganitsch-Korhonen, M.; Masliukaite, I.; Nurmio, M.; Lähteenmäki, P.; van Wely, M.; van Pelt, A.M.M.; Jahnukainen, K.; Stukenborg, J.B. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017, 31, 1460–1463. [Google Scholar] [CrossRef]
- Fernandez, H.F. New trends in the standard of care for initial therapy of acute myeloid leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2010, 2010, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.T.; Karp, J.E. New agents in acute myeloid leukemia: Beyond Cytarabine and Anthracyclines. Curr. Oncol. Rep. 2009, 11, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Howland, R.D. Lippincott’s Illustrated Reviews: Pharmacology, 4th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2009. [Google Scholar]
- Giwercman, A.; Petersen, P.M. Cancer and male infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2000, 14, 453–471. [Google Scholar] [CrossRef]
- Namoju, R.C.; Khan, S.; Patel, R.S.; Shera, F.Y.; Trivedi, P.P.; Kushwaha, S.; Jena, G.B. Pre-pubertal exposure of cytarabine-induced testicular atrophy, impaired spermatogenesis and germ cell DNA damage in SD rats. Toxicol. Mech. Methods 2014, 24, 703–712. [Google Scholar] [CrossRef]
- Creasy, D.M.; Chapin, R.E. Chapter 17—Male reproductive system. In Fundamentals of Toxicologic Pathology, 3rd ed.; Wallig, M.A., Haschek, W.M., Rousseaux, C.G., Bolon, B., Eds.; Academic Press of Elsevier: London, UK, 2018; pp. 459–516. [Google Scholar] [CrossRef]
- de Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar] [CrossRef]
- Gassei, K.; Orwig, K.E. SALL4 Expression in Gonocytes and Spermatogonial Clones of Postnatal Mouse Testes. PLoS ONE 2013, 8, e53976. [Google Scholar] [CrossRef] [Green Version]
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D. Vitro Spermatogenesis: Past, Present, and Future. In Spermatozoa: Facts and Perspectives; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Prihatno, S.A.; Adi, Y.K.; Budipitojo, T. Immunolocalization of IL-6 and IL-10 in the testicular tissue of testicular dysfunction rat treated with secretome. J. Adv. Vet. Anim. Res. 2020, 7, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Abuelhija, M.; Lunenfeld, E. In vitro culture of testicular germ cells: Regulatory Factors and Limitations. Growth Factors 2007, 25, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M.; Marshall, G.R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001, 22, 764–786. [Google Scholar] [CrossRef] [PubMed]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, G.F.; Luetjens, C.M.; Simoni, M.; Nieschlag, E. Physiology of testicular function. In Andrology: Male Reproductive Health and Dysfunction; Nieschlag, E., Behre, H.M., Nieschlag, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 11–59. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S. Disruption of spermatogenesis by the cancer disease process. J. Natl. Cancer Inst. Monogr. 2005, 2005, 9–12. [Google Scholar] [CrossRef]
- The American Cancer Society Medical and Editorial Content Team. Childhood Leukemia Early Detection, Diagnosis, and Types. In American Cancer Society Medical Information; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Michailov, Y.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Leukemia and male infertility: Past, Present, and Future. Leuk. Lymphoma 2019, 60, 1126–1135. [Google Scholar] [CrossRef]
- Hedger, M.P.; Meinhardt, A. Cytokines and the immune-testicular axis. J. Reprod. Immunol. 2003, 58, 1–26. [Google Scholar] [CrossRef]
- Potashnik, H.; Elhija, M.A.; Lunenfeld, E.; Potashnik, G.; Schlatt, S.; Nieschlag, E.; Huleihel, M. Interleukin-6 expression during normal maturation of the mouse testis. Eur. Cytokine Netw. 2005, 16, 161–165. [Google Scholar] [PubMed]
- Elhija, M.A.; Potashnik, H.; Lunenfeld, E.; Potashnik, G.; Schlatt, S.; Nieschlag, E.; Huleihel, M. Testicular interleukin-6 response to systemic inflammation. Eur. Cytokine Netw. 2005, 16, 167–172. [Google Scholar] [PubMed]
- Białas, M.; Fiszer, D.; Rozwadowska, N.; Kosicki, W.; Jedrzejczak, P.; Kurpisz, M. The role of IL-6, IL-10, TNF-alpha and its receptors TNFR1 and TNFR2 in the local regulatory system of normal and impaired human spermatogenesis. Am. J. Reprod. Immunol. 2009, 62, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A.; Jacobo, P.; Theas, M.S.; Lustig, L. Cytokines and chemokines in testicular inflammation: A Brief Review. Microsc. Res. Tech. 2009, 72, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Loveland, K.; Klein, B.; Püschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.; Hedger, M.; Schuppe, H.-C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, H.; Katayama, K.; Ueno, M.; Uetsuka, K.; Nakayama, H.; Doi, K. Involvement of p53 in 1-beta-D-arabinofuranosylcytosine-induced trophoblastic cell apoptosis and impaired proliferation in rat placenta. Biol. Reprod. 2004, 70, 1762–1767. [Google Scholar] [CrossRef]
- Furukawa, S.; Hayashi, S.; Usuda, K.; Abe, M.; Hagio, S.; Ogawa, I. Toxicological pathology in the rat placenta. J. Toxicol. Pathol. 2011, 24, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Lindahl, M.; Hyvönen, M.E.; Parvinen, M.; de Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Naughton, C.K.; Jain, S.; Strickland, A.M.; Gupta, A.; Milbrandt, J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 2006, 74, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Shinohara, T. Spermatogonial stem cell self-renewal and development. Annu. Rev. Cell Dev. Biol. 2013, 29, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Braun, R.E. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Development 2018, 145, dev151555. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liang, M.; Wang, D. Progress on the study of the mechanism of busulfan cytotoxicity. Cytotechnology 2018, 70, 497–502. [Google Scholar] [CrossRef]
- Mopin, A.; Driss, V.; Brinster, C. A Detailed Protocol for Characterizing the Murine C1498 Cell Line and its Associated Leukemia Mouse Model. J. Vis. Exp. 2016, 116, e54270. [Google Scholar] [CrossRef] [Green Version]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2015, 96, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cano, F.; Pannel, R.; Follows, G.A.; Rabbitts, T.H. Preclinical modeling of cytosine arabinoside response in Mll-Enl translocator mouse leukemias. Mol. Cancer Ther. 2008, 7, 730–735. [Google Scholar] [CrossRef] [Green Version]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of Spermatogenesis In Vitro in Three-Dimensional Culture from Spermatogonial Cells of Busulfan-Treated Immature Mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef] [Green Version]
- Bagdadi, N.; Sawaied, A.; AbuMadighem, A.; Lunenfeld, E.; Huleihel, M. The Expression Levels and Cellular Localization of Pigment Epithelium Derived Factor (PEDF) in Mouse Testis: Its Possible Involvement in the Differentiation of Spermatogonial Cells. Int. J. Mol. Sci. 2021, 22, 1147. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaleel, B.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Effect of Chemotherapy Cytarabine and Acute Myeloid Leukemia on the Development of Spermatogenesis at the Adult Age of Immature Treated Mice. Int. J. Mol. Sci. 2022, 23, 4013. https://doi.org/10.3390/ijms23074013
Khaleel B, Lunenfeld E, Kapelushnik J, Huleihel M. Effect of Chemotherapy Cytarabine and Acute Myeloid Leukemia on the Development of Spermatogenesis at the Adult Age of Immature Treated Mice. International Journal of Molecular Sciences. 2022; 23(7):4013. https://doi.org/10.3390/ijms23074013
Chicago/Turabian StyleKhaleel, Bara’ah, Eitan Lunenfeld, Joseph Kapelushnik, and Mahmoud Huleihel. 2022. "Effect of Chemotherapy Cytarabine and Acute Myeloid Leukemia on the Development of Spermatogenesis at the Adult Age of Immature Treated Mice" International Journal of Molecular Sciences 23, no. 7: 4013. https://doi.org/10.3390/ijms23074013





