Emission Trade-Off between Isoprene and Other BVOC Components in Pinus massoniana Saplings May Be Regulated by Content of Chlorophylls, Starch and NSCs under Drought Stress
Abstract
:1. Introduction
2. Results
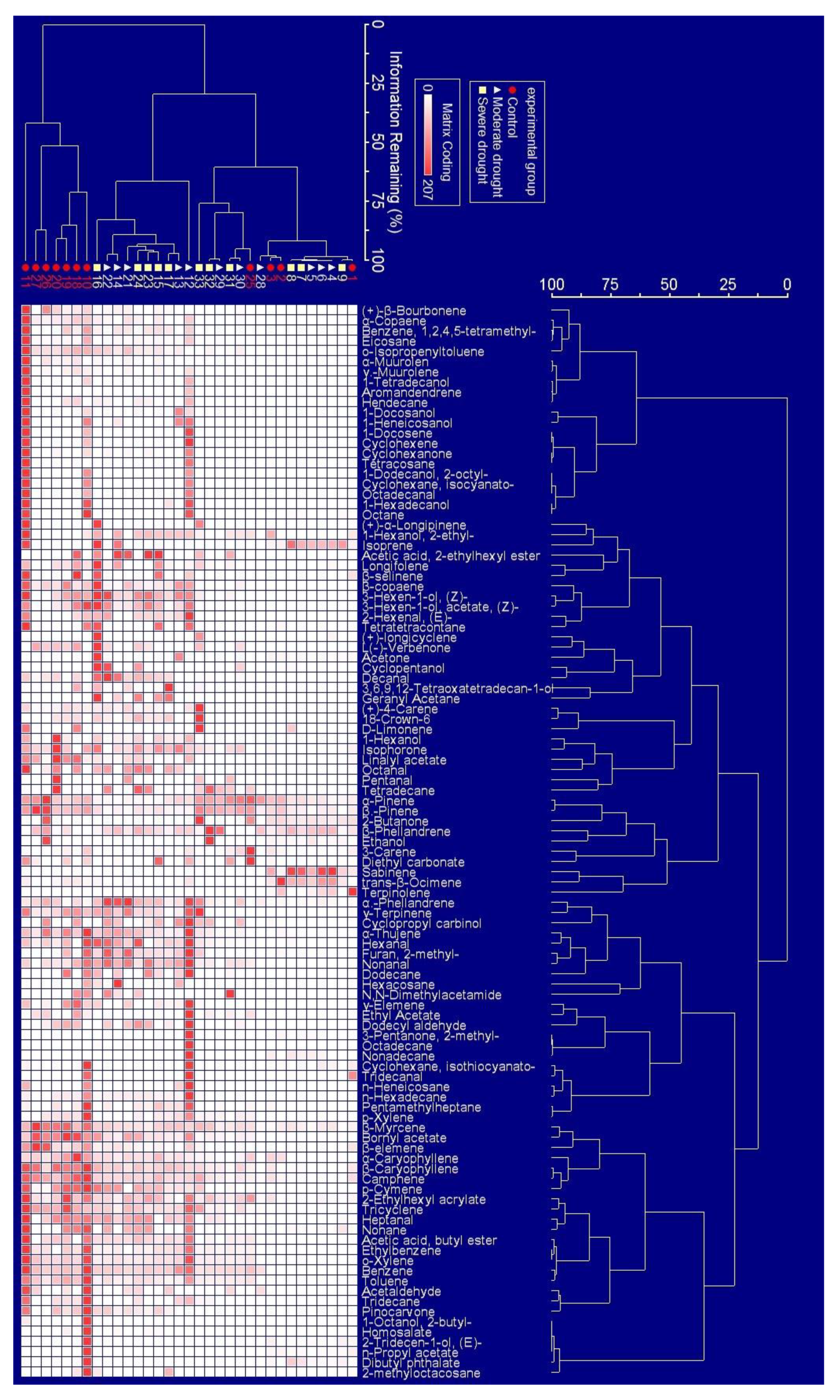
2.1. The Appearance Frequency of BVOCs Components in Response to Drought Stress
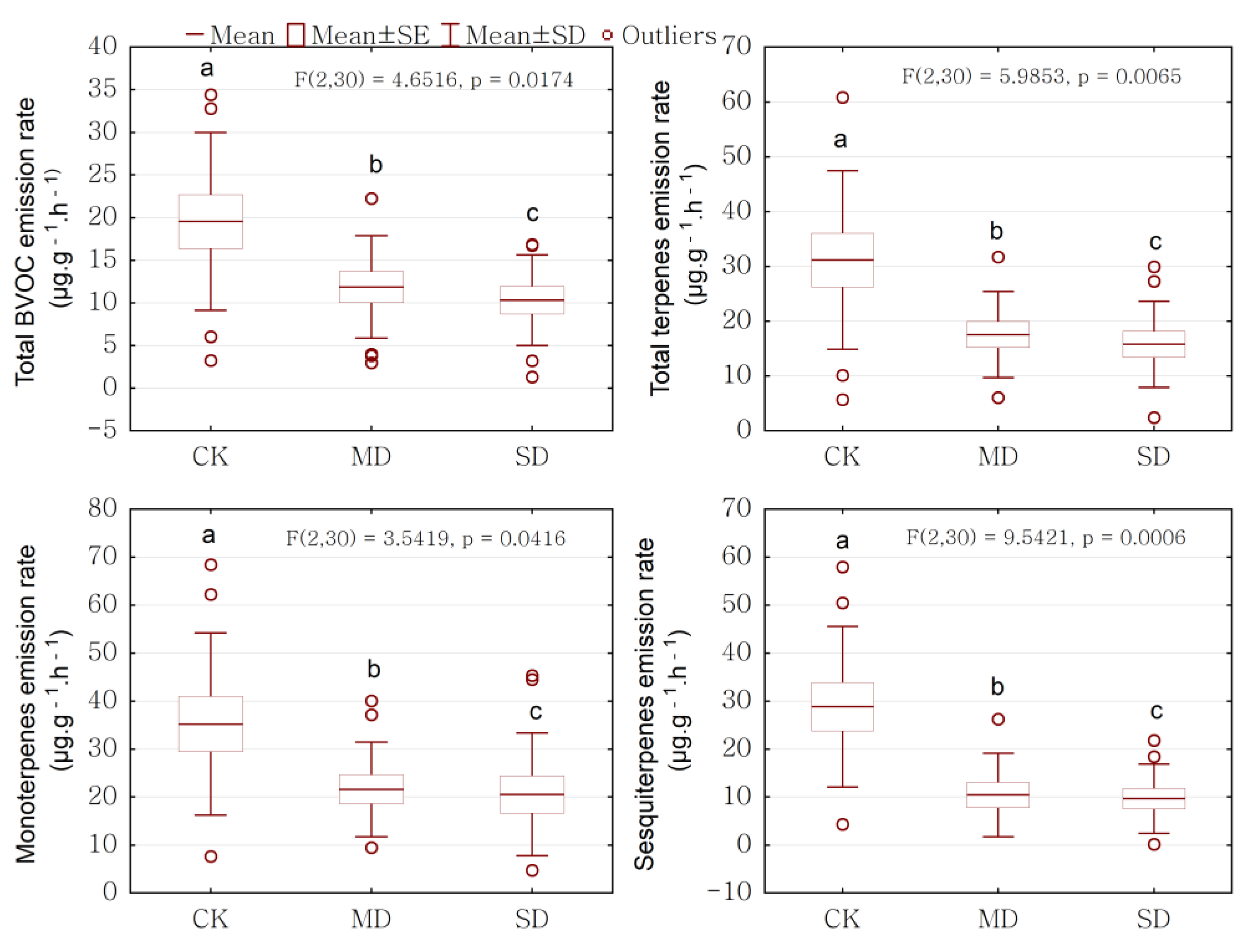
2.2. Drought Effect on BVOCs Emission Rate
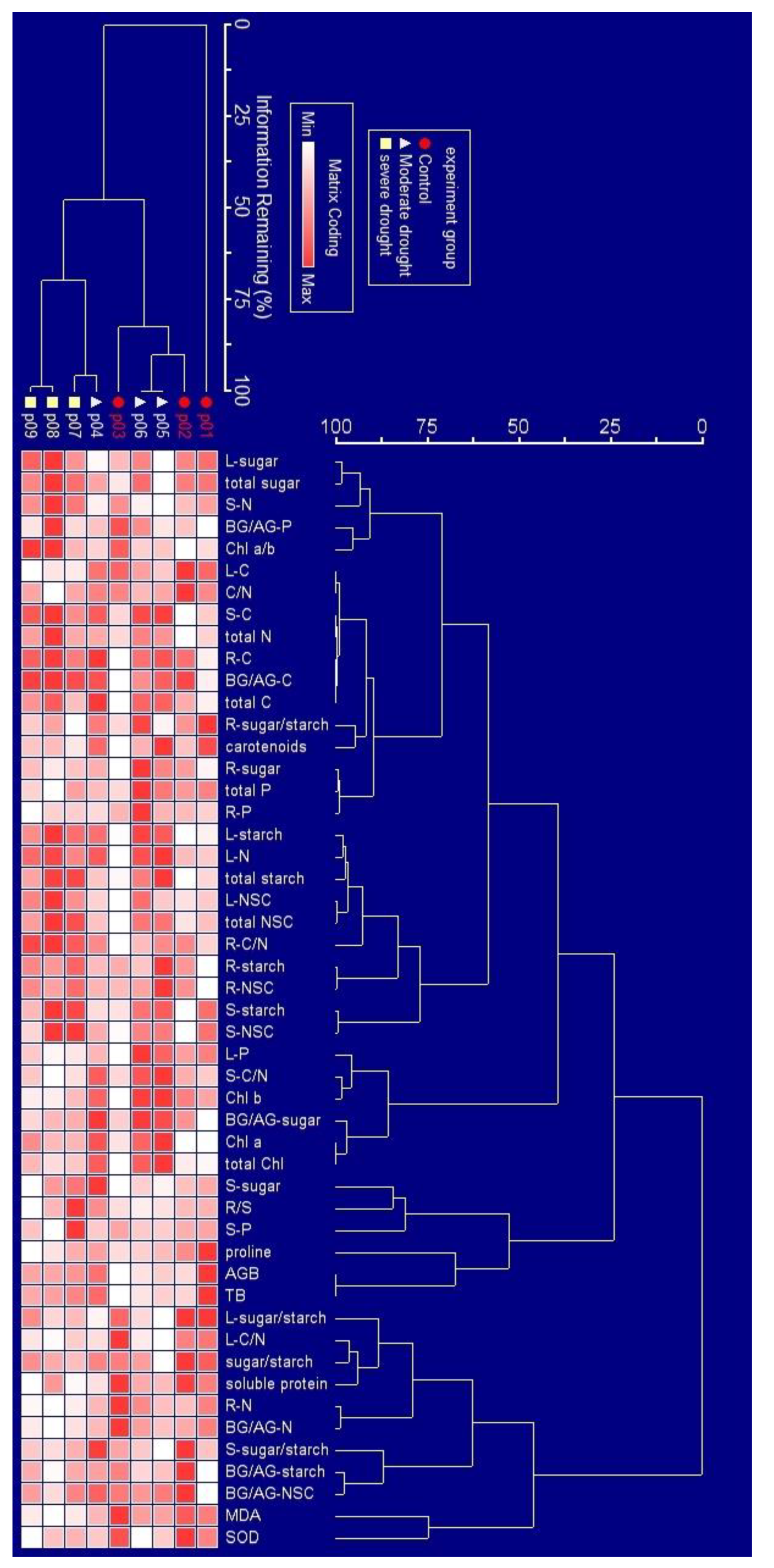
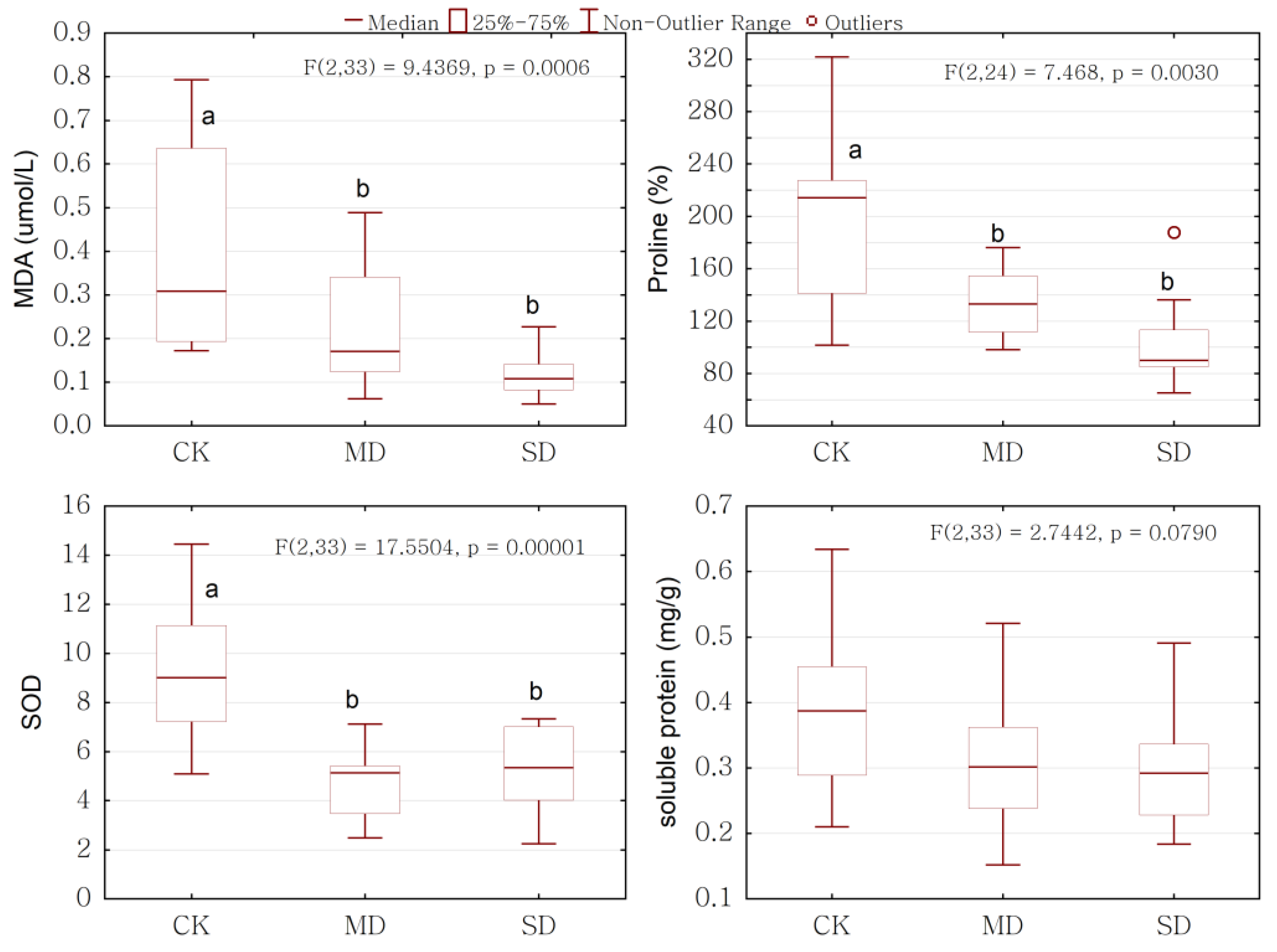
2.3. Overall Response of Physiology to Drought Stress
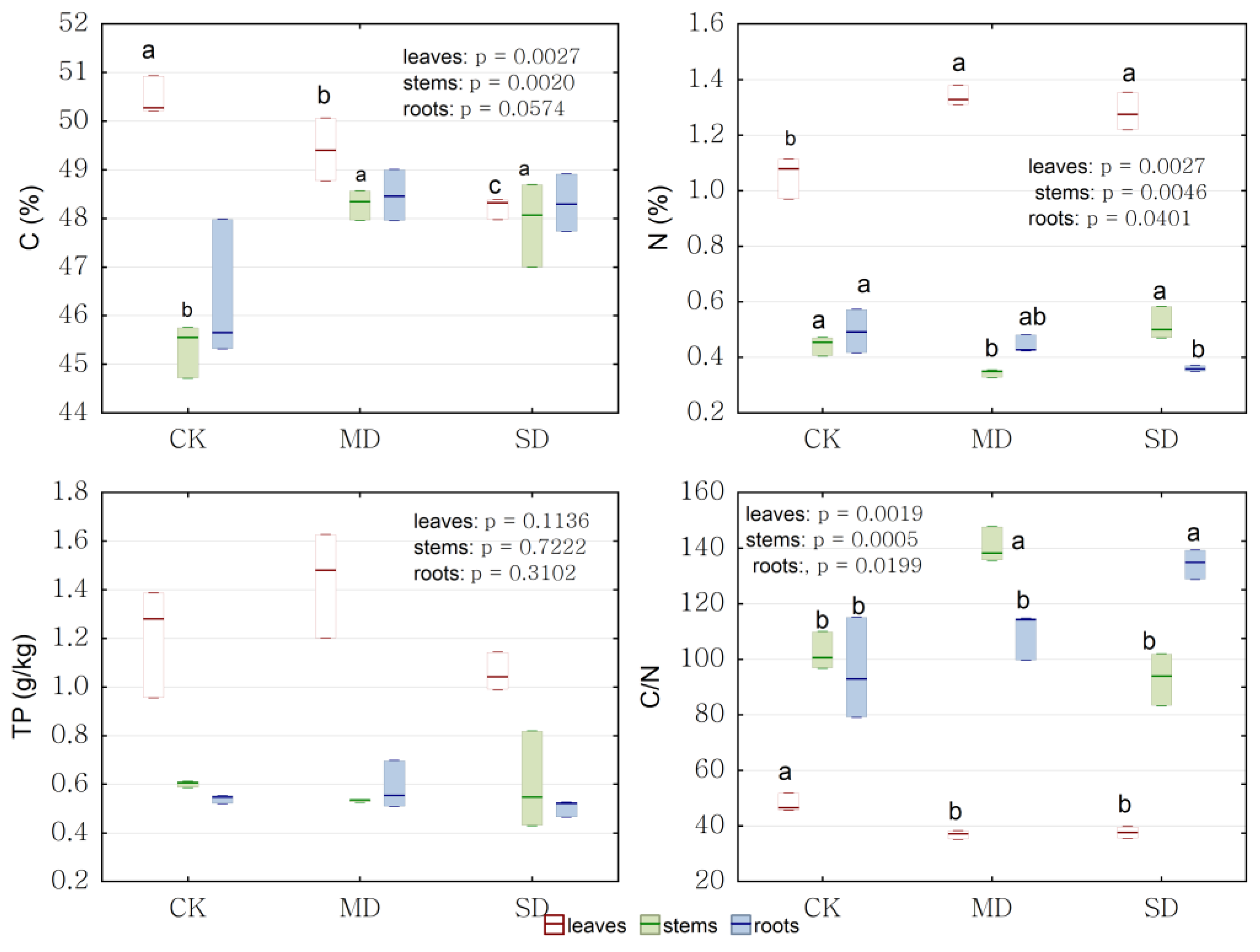
2.4. Physiological Response to Drought Stress
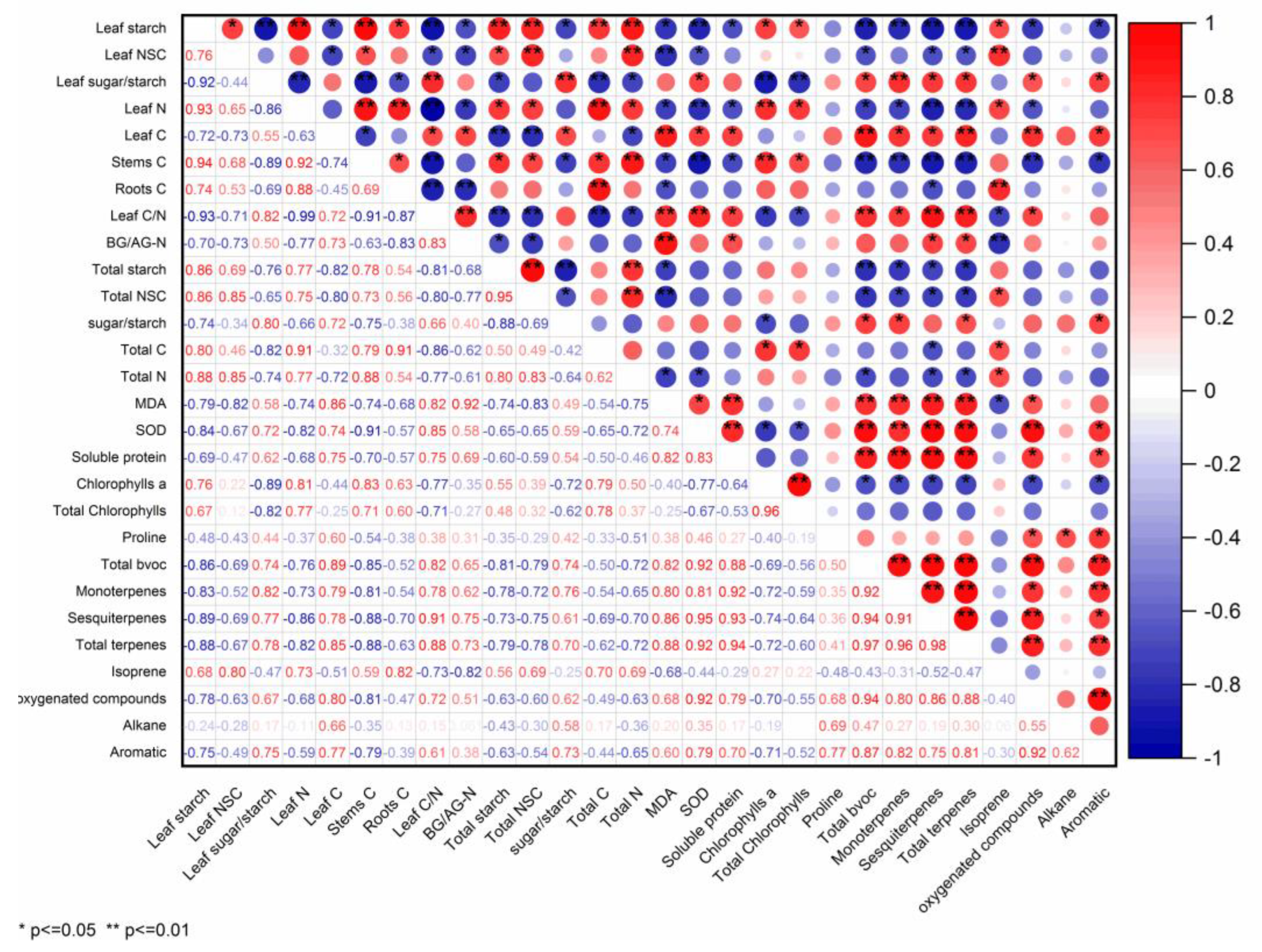
2.5. BVOCs Emission Associated with Physiological Traits
3. Discussions
3.1. BVOCs Emission in Responses to Drought Stress
3.2. Physiological Mechanism of BVOCs Emission under Drought Stress
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Physiological and Biochemical Characteristics Analysis
4.3. Biogenic Volatile Organic Compound (BVOCs) Sampling and Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peñuelas, J.; Llusia, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Geron, C.; Pierce, T.; Lamb, B.; Harley, P.; Fall, R. Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos. Environ. 2000, 34, 2205–2230. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res.-Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Guenther, A. The contribution of reactive carbon emissions from vegetation to the carbon balance of terrestrial ecosystems. Chemosphere 2002, 49, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Huang, J.B.; Hartmann, H.; Hellén, H.; Wisthaler, A.; Perreca, E.; Weinhold, A.; Rücker, A.; Van Dam, N.M.; Gershenzon, J.; Trumbore, S.; et al. New perspectives on CO2, temperature, and light effects on BVOC emissions using online measurements by PTR-MS and cavity ring-down spectroscopy. Environ. Sci. Technol. 2018, 52, 13811–13823. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2010, 131, 211–225. [Google Scholar] [CrossRef]
- Nogués, I.; Muzzini, V.; Loreto, F.; Bustamante, M.A. Drought and soil amendment effects on monoterpene emission in rosemary plants. Sci. Total Environ. 2015, 538, 768–778. [Google Scholar] [CrossRef]
- Staudt, M.; Morin, X.; Chuine, I. Contrasting direct and indirect effects of warming and drought on isoprenoid emissions from Mediterranean oaks. Reg. Environ. Change 2017, 17, 2121–2133. [Google Scholar] [CrossRef]
- Wu, C.; Pullinen, I.; Andres, S.; Carriero, G.; Fares, S.; Goldbach, H.; Hacker, L.; Kasal, T.; Kiendler-Scharr, A.; Kleist, E.; et al. Impacts of soil moisture on de novo monoterpene emissions from European beech, Holm oak, Scots pine, and Norway spruce. Biogeosciences 2015, 12, 177–191. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Jamie, I.M.; Prentice, I.C.; Atwell, B.J. Increased ratio of electron transport to net assimilation rate supports elevated isoprenoid emission rate in Eucalypts under drought. Plant Physiol. 2014, 166, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef]
- Pegoraro, E.; Rey, A.; Greenberg, J.; Harley, P.; Grace, J.; Malhi, Y.; Guenther, A. Effect of drought on isoprene emission rates from leaves of Quercus virginiana Mill. Atmos. Environ. 2004, 38, 6149–6156. [Google Scholar] [CrossRef]
- Staudt, M.; Ennajah, A.; Mouillot, F.; Joffre, R. Do volatile organic compound emissions of Tunisian cork oak populations originating from contrasting climatic conditions differ in their responses to summer drought? Can. J. Forest Res. 2008, 38, 2965–2975. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Calatayud, V.; Gao, F.; Fares, S.; Paoletti, E.; Tian, Y.; Feng, Z.Z. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 2016, 39, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, E.; Rey, A.; Barron-Gafford, G.; Monson, R.; Malhi, Y.; Murthy, R. The interacting effects of elevated atmospheric CO2 concentration, drought and leaf-to-air vapour pressure deficit on ecosystem isoprene fluxes. Oecologia 2005, 146, 120–129. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Sun, K.; Wu, J.S.; Sheng, W.X.; Jiang, P.K.; Zhang, Y.Q.; Ge, J.F. Potential of phytolith-occluded organic carbon sequestration in Masson Pine stands at different ages in subtropical China. Sci. Silvae Sin. 2020, 56, 10–18. [Google Scholar]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, 93–102. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Paul, N.D.; Vickers, C.E.; Possell, M.; Taylor, J.E.; Mullineaux, P.M.; Hewitt, C.N. Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ. 2008, 31, 1410–1415. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Baker, B.; Bai, J.H.; Johnson, C.; Cai, Z.T.; Li, Q.J.; Wang, Y.F.; Guenther, A.; Greenberg, J.; Klinger, L.; Geron, C.; et al. Wet and dry season ecosystem level fluxes of isoprene and monoterpenes from a Southeast Asian secondary forest and rubber tree plantation. Atmos. Environ. 2005, 39, 381–390. [Google Scholar] [CrossRef]
- Plaza, J.; Nunez, L.; Pujadas, M.; Perrez-Pastor, R.; Bermejo, V.; Garcia- Alonso, S.; Elvira, S. Field monoterpene emission of Mediterranean oak (Quercus ilex) in the central Iberian Peninsula measured by enclosure and micrometeorological techniques: Observation of drought stress effect. J. Geophys Res. Atmos. 2005, 110, D03303. [Google Scholar] [CrossRef]
- Lavoir, A.V.; Staudt, M.; Schnitzler, J.P.; Landais, D.; Massol, F.; Rocheteau, A.; Rodriguez, R.; Zimmer, I.; Rambal, S. Drought reduced monoterpene emissions from the evergreen Mediterranean oak Quercus ilex: Results from a throughfall displacement experiment. Biogeosciences 2009, 6, 1167–1180. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Sardans, J.; Llusià, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, C.; Loreto, F. Volatile organic compounds from Italian vegetation and their interaction with ozone. Environ. Pollut. 2009, 157, 1478–1486. [Google Scholar] [CrossRef]
- Lundborg, L.; Nordlander, G.; Björklund, N.; Nordenhem, H.; Borg- Karlson, A.K. Methyl jasmonate-induced monoterpenes in Scots pine and Norway spruce tissues affect pine weevil orientation. J. Chem. Ecol. 2016, 42, 1237–1246. [Google Scholar] [CrossRef]
- Blanch, J.S.; Sampedro, L.; Llusia, J.; Moreira, X.; Zas, R.; Penuelas, J. Effects of phosphorus availability and genetic variation of leaf terpene content and emission rate in Pinus pinaster seedlings susceptible and resistant to the pine weevil, Hylobius abietis. Plant Biol. 2012, 14, 66–72. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.S.; Chen, F.; Stewart, C.N., Jr. Neal Stewart Ecological Functions of Terpenoids in Changing Climates. In Natural Product; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. [Google Scholar]
- Loreto, F.; Pinelli, P.; Brancaleoni, E.; Ciccioli, P. 13C labeling reveals chloroplastic and extrachloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol. 2004, 135, 1903–1907. [Google Scholar] [CrossRef]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why only someplants emit isoprene. Plant Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S. The role of a-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Fall, R. Light-dependent isoprene emission (characterization of a thylakoid-bound isoprene synthase in Salix discolor chloroplasts). Plant Physiol. 1996, 112, 171–182. [Google Scholar] [CrossRef]
- Schnitzler, J.P.; Zimmer, I.; Bachl, A.; Arend, M.; Fromm, J.; Fischbach, R.J. Biochemical properties of isoprene synthase in poplar (Populus × canescens). Planta 2005, 222, 777–786. [Google Scholar] [CrossRef]
- Loreto, F.; Fischbach, R.J.; Schnitzler, J.P.; Ciccioli, P.; Brancaleoni, E.; Calfapietra, C.; Seufert, G. Monoterpene emission and monoterpene synthase activities in the Mediterranean evergreen oak Quercus ilex L. grown at elevated CO2 concentrations. Global Change Biol. 2001, 7, 709–717. [Google Scholar] [CrossRef]
- Brüggemann, N.; Schnitzler, J.P. Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens Willd. and Quercus robur L.) saplings. Plant Biol. 2002, 4, 456–463. [Google Scholar] [CrossRef]
- Ghirado, A.; Koch, K.; Taipale, R.; Zimmer, I.; JÖRG-PETER Schnitzler, J.P.; Rinne, J. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ. 2010, 33, 781–792. [Google Scholar]
- Sharkey, T.D.; Loreto, F.; Delwiche, C.F. The biochemistry of isoprene emission from leaves during photosynthesis. In Trace Gas Emissions by Plants; Sharkey, T.D., Holland, B., Mooney, H.A., Eds.; Academic Press: New York, NY, USA, 1991; pp. 153–184. [Google Scholar]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 155–166. [Google Scholar] [CrossRef]
- Huang, J.B.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; Van Dam, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.; et al. Eyes on the future-evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef]
- Funk, J.L.; Jones, C.G.; Lerdau, M.T. Defoliation effects on isoprene emission from Populus deltoides. Oecologia 1999, 118, 333–339. [Google Scholar] [CrossRef]
- Schnitzler, J.P.; Graus, M.; Kreuzwieser, J.; Heizmann, U.; Rennenberg, H.; Wisthaler, A.; Hansel, A. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol. 2004, 135, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.L.; Sujatha, M.; Alivelu, K.; Sujatha, K. Sources of resistance to Alternariaster leaf blight in sunflower pre-breeding lines derived from interspecific crosses and wild helianthus species. Crop Prot. 2017, 92, 70–78. [Google Scholar] [CrossRef]
- Liu, C.C.; Cui, B.J.; Hu, C.; Wu, H.Q.; Ma, H.H.; Ma, T.; Gao, F. Response of crop physiological characteristics to mixed irrigation of brackish water and reclaimed water. Water Sav. Irrig. 2021, 12, 35–40. [Google Scholar]
- Sun, M.S.; Hu, Y.; Chen, X.; Luo, Q.F.; Yang, Z.Q. Effects of exogenous regulating substances on physiological characteristics of erythrophleum fordii seedlings under drought stress. Sci. Silvae Sin. 2020, 56, 165–172. [Google Scholar]
- Wen, J.K.; Xing, C.J.; Wang, Y.P.; Yang, L.; Zheng, W.; Li, W.Y. Effects of different drought stress and spermidine rehydration on physiological characteristics of Taxus chinensis var. mairei. Water Sav. Irrig. 2023, 109–115. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Possell, M.; Cojocariu, C.I.; Velikova, V.B.; Laothawornkitkul, J.; Ryan, A.; Mullineaux, P.M.; Hewitt, C.N. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 2009, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Li, M.H.; Xiao, W.F.; Shi, P.; Wang, S.G.; Zhong, Y.D.; Liu, X.L.; Wang, X.D.; Cai, X.H.; Shi, Z.M. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res.-Atmos. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Okumura, M.; Kosugi, Y.; Tani, A. Biogenic volatile organic compound emissions from bamboo species in Japan. J. Agric. Meteorol. 2018, 74, 40–44. [Google Scholar] [CrossRef]
- Geron, C.; Harley, P.; Guenther, A. Isoprene emission capacity for US tree species. Atmos. Environ. 2001, 35, 3341–3352. [Google Scholar] [CrossRef]




| Variables | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Total BVOC | 538.08 | 2 | 269.04 | 4.65 | 0.017 |
| Total terpenes | 1552.38 | 2 | 776.19 | 5.99 | 0.006 |
| Monoterpenes | 1469.33 | 2 | 734.67 | 3.54 | 0.042 |
| Sesquiterpenes | 2590.79 | 2 | 1295.39 | 9.54 | 0.001 |
| Isoprene | 0.112834 | 2 | 0.056417 | 1.622 | 0.245 |
| Variable | Control | Moderate Drought | Severe Drought |
|---|---|---|---|
| Total BVOC | 19.56 ± 10.44 a | 11.88 ± 6.02 b | 10.32 ± 5.33 c |
| Total terpenes | 31.15 ± 16.29 a | 17.57 ± 7.87 b | 15.79 ± 7.87 c |
| Monoterpenes (MT) | 35.22 ± 19.01 a | 21.61 ± 9.85 b | 20.57 ± 12.82 c |
| Sesquiterpenes (ST) | 28.83 ± 16.73 a | 10.44 ± 8.69 b | 9.66 ± 7.19 c |
| Isoprene | 0.18 ± 0.27 | 0.28 ± 0.09 | 0.42 ± 0.17 |
| Alcohol | 17.63 ± 24.21 | 10.17 ± 12.63 | 7.85 ± 10.74 |
| Aldehyde | 9.81 ± 13.33 | 16.74 ± 25.67 | 11.42 ± 18.90 |
| Ketone | 9.05 ± 11.69 | 4.77 ± 6.61 | 8.59 ± 9.84 |
| Ester | 20.09 ± 25.26 a | 9.23 ± 12.67 b | 7.93 ± 11.14 c |
| Alkane | 12.20 ± 18.87 a | 10.14 ± 17.74 ab | 4.82 ± 9.24 b |
| oxygenated compounds (OCs) | 14.62 ± 20.68 a | 10.31 ± 16.44 b | 8.56 ± 13.63 c |
| Aromatic | 13.51 ± 20.74 a | 6.39 ± 10.11 b | 5.74 ± 10.82 c |
| MT: total BVOCs (%) | 48.13 ± 21.41 | 60.42 ± 31.59 | 55.12 ± 30.59 |
| ST: total BVOCs (%) | 20.90 ± 7.46 a | 9.24 ± 7.49 b | 9.95 ± 8.23 b |
| OCs: total BVOCs (%) | 21.99 ± 9.57 | 21.59 ± 17.66 | 26.88 ± 18.57 |
| Variable | Compounds | Control | Moderate Drought | Severe Drought |
|---|---|---|---|---|
| Monoterpenes | α-Pinene | 94.85 ± 53.01 a | 52.08 ± 42.19 b | 49.77 ± 40.41 c |
| β-Pinene | 92.04 ± 56.83 a | 29.07 ± 29.09 c | 43.14 ± 37.39 b | |
| β-Myrcene | 68.32 ± 51.24 a | 23.27 ± 12.88 c | 27.29 ± 15.82 b | |
| β-Phellandrene | 27.73 ± 21.28 | 43.70 ± 22.92 | 36.39 ± 40.32 | |
| α-Phellandrene | 9.89 ± 10.42 | 33.32 ± 35.19 | 15.87 ± 16.02 | |
| Camphene | 54.65 ± 40.08 a | 14.36 ± 10.92 c | 19.75 ± 16.51 b | |
| Sesquiterpenes | α-caryophyllene | 40.07 ± 33.60 | 14.87 ± 8.20 | 19.29 ± 12.21 |
| β-Caryophyllene | 80.01 ± 62.01 a | 22.38 ± 18.24 c | 27.99 ± 22.23 b | |
| β-Bourbonene | 44.72 ± 42.39 | 6.72 ± 2.98 | 3.63 ± 2.41 | |
| β-elemene | 54.16 ± 39.91 a | 12.56 ± 10.31 b | 5.63 ± 2.21 c | |
| Longifolene | 6.03 ± 5.39 | 4.05 ± 6.04 | 7.13 ± 11.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhang, T.; Ge, X.; Cao, Y.; Li, Z.; Zhou, B. Emission Trade-Off between Isoprene and Other BVOC Components in Pinus massoniana Saplings May Be Regulated by Content of Chlorophylls, Starch and NSCs under Drought Stress. Int. J. Mol. Sci. 2023, 24, 8946. https://doi.org/10.3390/ijms24108946
Huang R, Zhang T, Ge X, Cao Y, Li Z, Zhou B. Emission Trade-Off between Isoprene and Other BVOC Components in Pinus massoniana Saplings May Be Regulated by Content of Chlorophylls, Starch and NSCs under Drought Stress. International Journal of Molecular Sciences. 2023; 24(10):8946. https://doi.org/10.3390/ijms24108946
Chicago/Turabian StyleHuang, Runxia, Tianning Zhang, Xiaogai Ge, Yonghui Cao, Zhengcai Li, and Benzhi Zhou. 2023. "Emission Trade-Off between Isoprene and Other BVOC Components in Pinus massoniana Saplings May Be Regulated by Content of Chlorophylls, Starch and NSCs under Drought Stress" International Journal of Molecular Sciences 24, no. 10: 8946. https://doi.org/10.3390/ijms24108946
APA StyleHuang, R., Zhang, T., Ge, X., Cao, Y., Li, Z., & Zhou, B. (2023). Emission Trade-Off between Isoprene and Other BVOC Components in Pinus massoniana Saplings May Be Regulated by Content of Chlorophylls, Starch and NSCs under Drought Stress. International Journal of Molecular Sciences, 24(10), 8946. https://doi.org/10.3390/ijms24108946




