Sexual Dimorphism of Skeletal Muscle in a Mouse Model of Breast Cancer: A Functional and Molecular Analysis
Abstract
:1. Introduction
2. Results
2.1. Bulk RNA Sequencing
2.2. Skeletal Muscle Properties
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ex Vivo Muscle Physiological Analysis
4.3. RNA Isolation, Sequencing, and Bioinformatics
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023.
- White, J.; Kearins, O.; Dodwell, D.; Hogan, K.; Hanby, A.M.; Speirs, V. Male breast carcinoma: Increased awareness needed. Breast Cancer Res. 2011, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Johnson, K.J.; Ma, C.X. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin. Breast Cancer 2018, 18, e997–e1002. [Google Scholar] [CrossRef]
- Sosa Rivera, A.M.; Espinoza, S.L.; Aguilar, R.M.; Palencia, R. Male Breast Cancer: Case Report. Rev. Colomb. Cardiol. 2017, 28, 4810–4815. [Google Scholar]
- Maglione, J.E.; Moghanaki, D.; Young, L.J.T.; Manner, C.K.; Ellies, L.G.; Joseph, S.O.; Nicholson, B.; Cardiff, R.D.; MacLeod, C.L. Transgenic Polyoma Middle-T Mice Model Premalignant Mammary Disease. Cancer Res. 2001, 61, 8298–8305. [Google Scholar] [PubMed]
- Shishido, S.; Delahaye, A.; Beck, A.; Nguyen, T.A. The MMTV-PyVT Trangenic Mouse as a Multistage Model for Mammary Carcinoma and the Efficacy of Antineoplastic Treatment. J. Cancer Ther. 2013, 4, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Attalla, S.; Taifour, T.; Bui, T.; Muller, W. Insights from transgenic mouse models of PyMT-induced breast cancer: Recapitulating human breast cancer progression in vivo. Oncogene 2021, 40, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, A.; Christofori, G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006, 8, 212. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, C.H.; Lee, H. Mouse models of breast cancer in preclinical research. Lab. Anim. Res. 2018, 34, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Regua, A.T.; Arrigo, A.; Doheny, D.; Wong, G.L.; Lo, H.-W. Transgenic mouse models of breast cancer. Cancer Lett. 2021, 516, 73–83. [Google Scholar] [CrossRef]
- Lin, E.Y.; Jones, J.G.; Li, P.; Zhu, L.; Whitney, K.D.; Muller, W.J.; Pollard, J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003, 163, 2113–2126. [Google Scholar] [CrossRef] [Green Version]
- Fluck, M.M.; Schaffhausen, B.S. Lessons in Signaling and Tumorigenesis from Polyomavirus Middle T Antigen. Microbiol. Mol. Biol. Rev. 2009, 73, 542–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Sundaram, S.; Rust, B.M.; Picklo, M.J.; Bukowski, M.R. Mammary Tumorigenesis and Metabolome in Male Adipose Specific Monocyte Chemotactic Protein−1 Deficient MMTV-PyMT Mice Fed a High-Fat Diet. Front. Oncol. 2021, 11, 667843. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J.; Lambert, C.P. Physiological Basis of Fatigue. Am. J. Phys. Med. Rehabil. 2007, 86, S29–S46. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Wilson, H.E.; Rhodes, K.K.; Rodriguez, D.; Chahal, I.; Stanton, D.A.; Bohlen, J.; Davis, M.; Infante, A.M.; Hazard-Jenkins, H.; Klinke, D.J.; et al. Human Breast Cancer Xenograft Model Implicates Peroxisome Proliferator–activated Receptor Signaling as Driver of Cancer-induced Muscle Fatigue. Clin. Cancer Res. 2019, 25, 2336–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, H.E.; Stanton, D.A.; Montgomery, C.; Infante, A.M.; Taylor, M.; Hazard-Jenkins, H.; Pugacheva, E.N.; Pistilli, E.E. Skeletal muscle reprogramming by breast cancer regardless of treatment history or tumor molecular subtype. NPJ Breast Cancer 2020, 6, 18. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.S.; Chang, C.H.; Peterman, A.; Slavin, M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002, 94, 528–538. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in Breast Cancer Survivors: Occurrence, Correlates, and Impact on Quality of Life. J. Clin. Oncol. 2000, 18, 743–753. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr. Treat. Options Oncol. 2020, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Counts, B.R.; Carson, J.A. Understanding Sex Differences in the Regulation of Cancer-Induced Muscle Wasting. Curr. Opin. Support. Palliat. Care 2018, 12, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.E.; Stanton, D.A.; Rellick, S.; Geldenhuys, W.; Pistilli, E.E. Breast cancer-associated skeletal muscle mitochondrial dysfunction and lipid accumulation is reversed by PPARG. Am. J. Physiol. Cell Physiol. 2021, 320, C577–C590. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.; Ono-Moore, K.D.; Chintapalli, S.V.; Rutkowsky, J.M.; Tolentino, T.; Lloyd, K.C.K.; Olfert, I.M.; Adams, S.H. Sex differences in skeletal muscle revealed through fiber type, capillarity, and transcriptomics profiling in mice. Physiol. Rep. 2021, 9, e15031. [Google Scholar] [CrossRef]
- MacDougall, K.B.; Devrome, A.N.; Kristensen, A.M.; MacIntosh, B.R. Force-frequency relationship during fatiguing contractions of rat medial gastrocnemius muscle. Sci. Rep. 2020, 10, 11575. [Google Scholar] [CrossRef]
- Brooks, S.V.; Faulkner, J.A. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988, 404, 71–82. [Google Scholar] [CrossRef]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E., 3rd. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Biswas, A.K.; Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 2020, 20, 274–284. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, A.; Vicioso, L.; Santonja, A.; Álvarez, M.; Plata-Fernández, Y.; Miramón, J.; Zarcos, I.; Ramírez-Tortosa, C.L.; Montes-Torres, J.; Jerez, J.M.; et al. Male breast cancer: Correlation between immunohistochemical subtyping and PAM50 intrinsic subtypes, and the subsequent clinical outcomes. Mod. Pathol. 2018, 31, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P.; et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.F.; Jatoi, I.; Tse, J.; Rosenberg, P.S. Male breast cancer: A population-based comparison with female breast cancer. J. Clin. Oncol. 2010, 28, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, E.E.; Bogdanovich, S.; Garton, F.; Yang, N.; Gulbin, J.P.; Conner, J.D.; Anderson, B.G.; Quinn, L.S.; North, K.; Ahima, R.S.; et al. Loss of IL−15 receptor α alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J. Clin. Investig. 2011, 121, 3120–3132. [Google Scholar] [CrossRef] [Green Version]
- Pistilli, E.E.; Alway, S.E.; Hollander, J.M.; Wimsatt, J.H. Aging alters contractile properties and fiber morphology in pigeon skeletal muscle. J. Comp. Physiol. B 2014, 184, 1031–1039. [Google Scholar] [CrossRef]
- Kiriaev, L.; Kueh, S.; Morley, J.W.; Houweling, P.J.; Chan, S.; North, K.N.; Head, S.I. Dystrophin-negative slow-twitch soleus muscles are not susceptible to eccentric contraction induced injury over the lifespan of the mdx mouse. Am. J. Physiol. Cell Physiol. 2021, 321, C704–C720. [Google Scholar] [CrossRef]
- Lynch, G.S.; Hinkle, R.T.; Chamberlain, J.S.; Brooks, S.V.; Faulkner, J.A. Force and power output of fast and slow skeletal muscles from mdx mice 6−28 months old. J. Physiol. 2001, 535, 591–600. [Google Scholar] [CrossRef]
- Pistilli, E.E.; Jackson, J.R.; Alway, S.E. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis 2006, 11, 2115–2126. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 2009, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Hart, S.; Therneau, T.; Zhang, Y.; Poland, G.; Kocher, J. Calculating Sample Size Estimates for RNA Sequencing Data. J. Comput. Biol. 2013, 20, 970–978. [Google Scholar] [CrossRef] [Green Version]
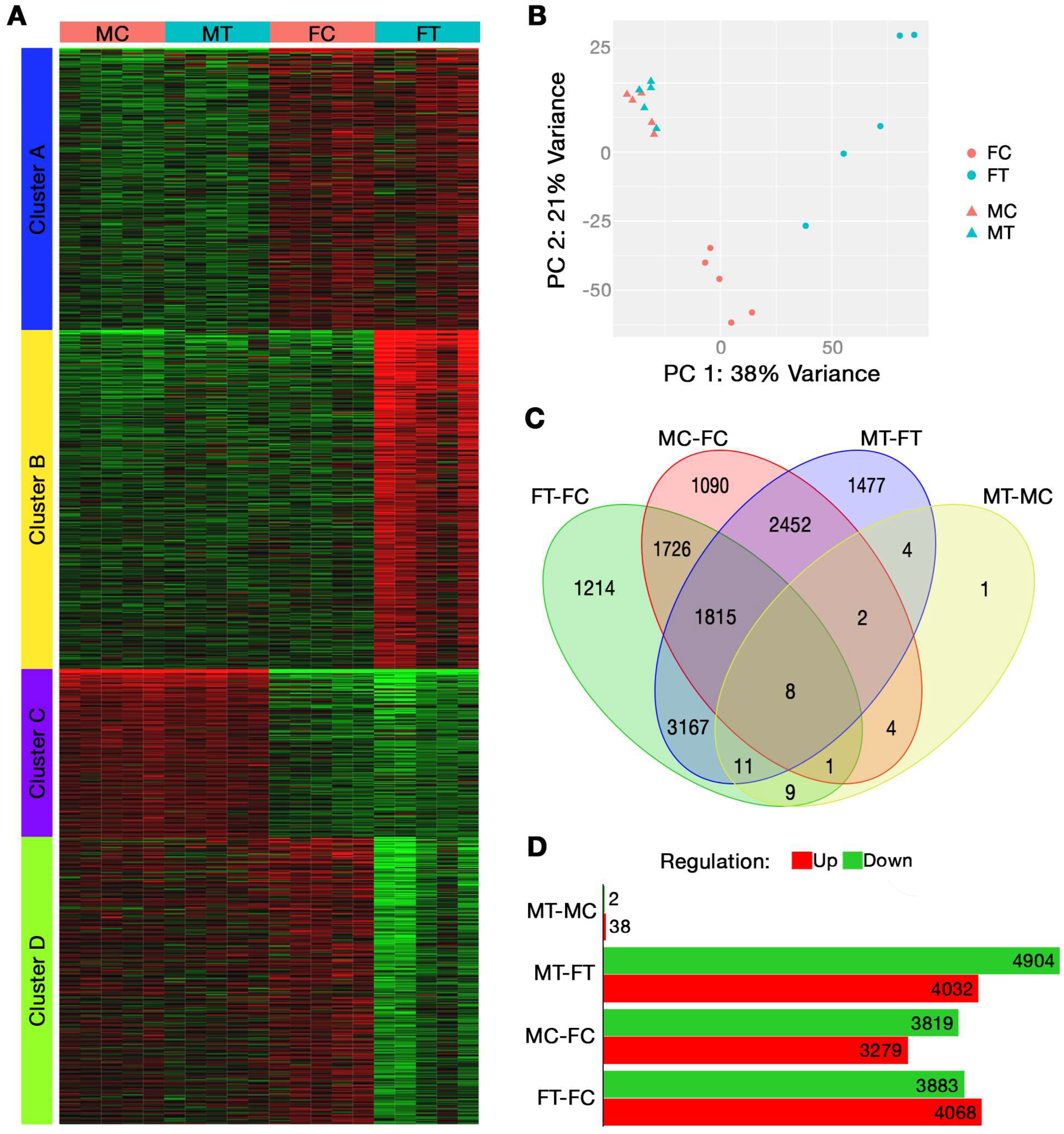
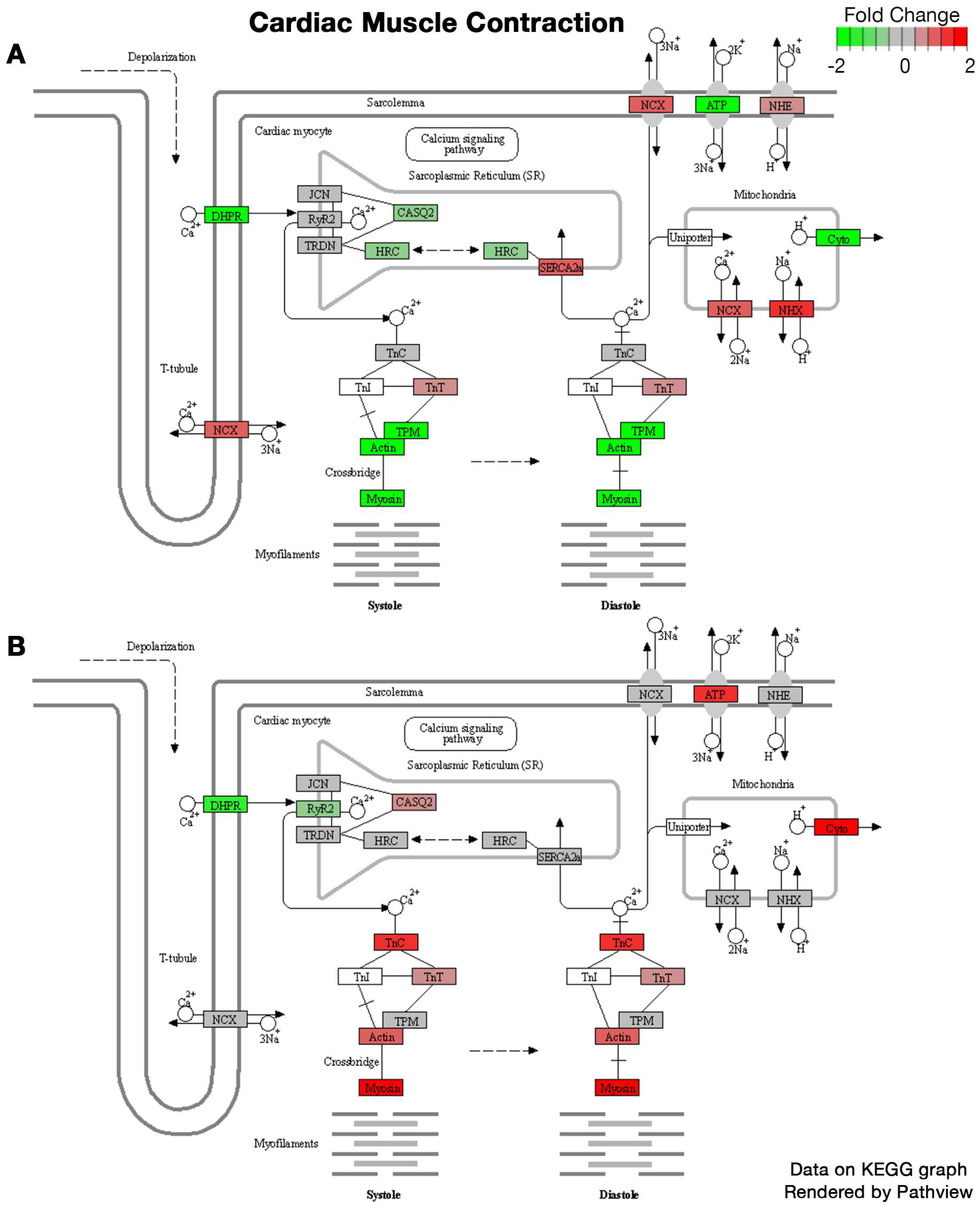
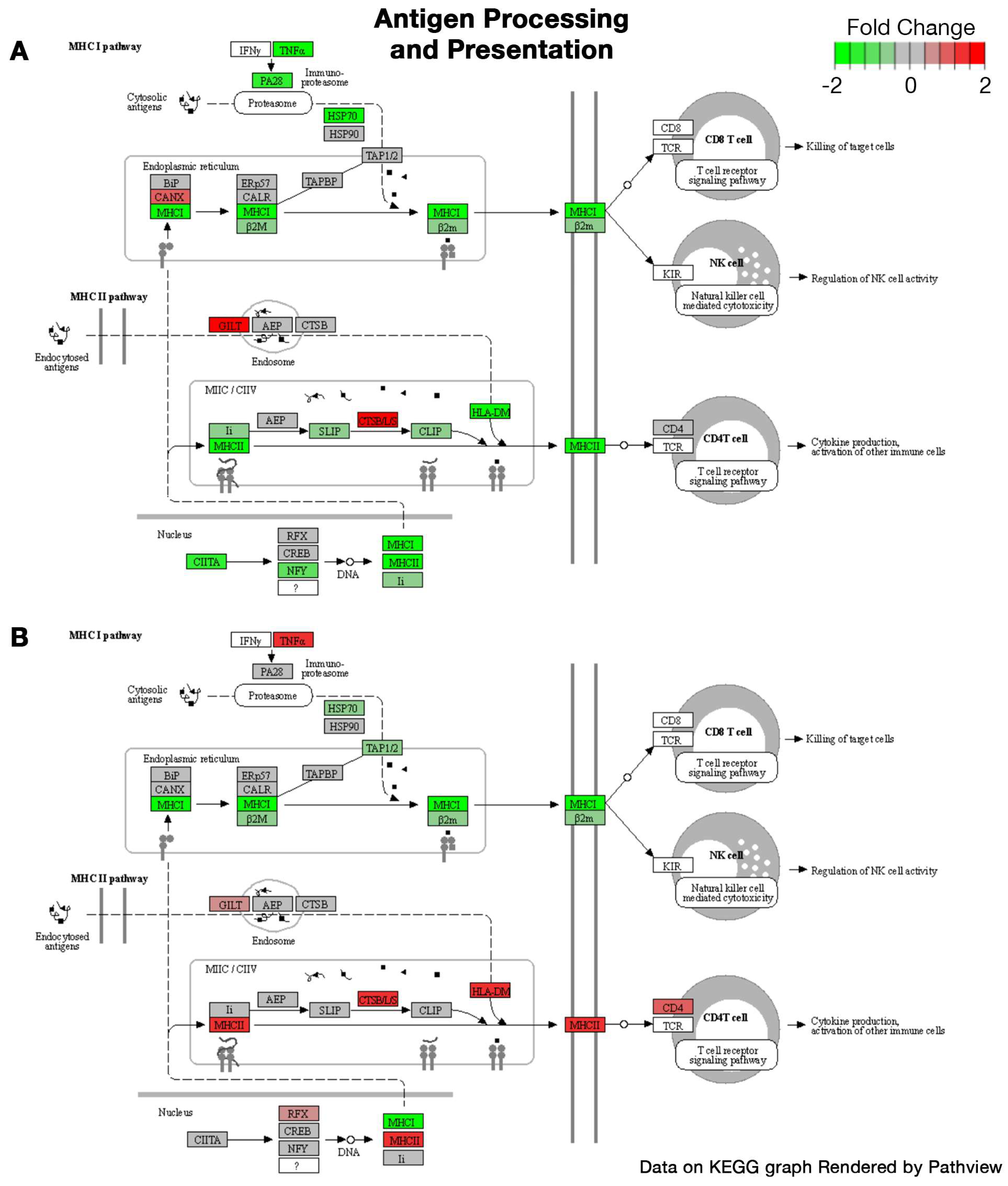

| Cluster | Adj. p-Value | # Genes | Pathways | Gene Ontology |
|---|---|---|---|---|
| A | 1.19 × 10−3 | 44 | Extracellular region | Cellular Component |
| 1.19 × 10−3 | 3 | Hemoglobin complex | Cellular Component | |
| 1.50 × 10−3 | 3 | Haptoglobin binding | Molecular Function | |
| 1.62 × 10−3 | 3 | Haptoglobin–hemoglobin complex | Cellular Component | |
| 7.09 × 10−3 | 30 | Extracellular space | Cellular Component | |
| 7.09 × 10−3 | 3 | Troponin complex | Cellular Component | |
| 7.09 × 10−3 | 15 | Collagen-containing extracellular matrix | Cellular Component | |
| 7.23 × 10−3 | 4 | Transition between fast and slow fiber | Biological Process | |
| 7.23 × 10−3 | 28 | Positive regulation of immune system process | Biological Process | |
| 7.30 × 10−3 | 3 | Oxygen carrier activity | Molecular Function | |
| B | 1.53 × 10−14 | 132 | Immune system process | Biological Process |
| 3.38 × 10−14 | 136 | Response to external stimulus | Biological Process | |
| 7.07 × 10−13 | 88 | Defense response | Biological Process | |
| 5.08 × 10−12 | 40 | Leukocyte migration | Biological Process | |
| 2.28 × 10−11 | 30 | Leukocyte chemotaxis | Biological Process | |
| 3.04 × 10−11 | 35 | Cell chemotaxis | Biological Process | |
| 3.50 × 10−11 | 22 | Neutrophil migration | Biological Process | |
| 3.50 × 10−11 | 54 | Inflammatory response | Biological Process | |
| 3.65 × 10−11 | 150 | Response to organic substance | Biological Process | |
| 4.94 × 10−11 | 29 | Myeloid leukocyte migration | Biological Process | |
| C | 1.22 × 10−5 | 27 | Monocarboxylic acid metabolic process | Biological Process |
| 1.76 × 10−4 | 49 | Small molecule metabolic process | Biological Process | |
| 2.02 × 10−4 | 31 | Carboxylic acid metabolic process | Biological Process | |
| 2.02 × 10−4 | 31 | Oxoacid metabolic process | Biological Process | |
| 3.42 × 10−4 | 29 | Metal ion transport | Biological Process | |
| 3.70 × 10−4 | 20 | Purine-containing compound metabolic process | Biological Process | |
| 7.77 × 10−4 | 40 | Ion transport | Biological Process | |
| 7.77 × 10−4 | 34 | Cation transport | Biological Process | |
| 1.03 × 10−3 | 27 | Oxidoreductase activity | Molecular Function | |
| 1.31 × 10−3 | 18 | Ribonucleotide metabolic process | Biological Process | |
| D | 1.30 × 10−37 | 138 | Extracellular region | Cellular Component |
| 3.71 × 10−32 | 104 | Extracellular space | Cellular Component | |
| 5.10 × 10−27 | 64 | External encapsulating structure | Cellular Component | |
| 5.10 × 10−27 | 64 | Extracellular matrix | Cellular Component | |
| 2.66 × 10−22 | 51 | Collagen-containing extracellular matrix | Cellular Component | |
| 2.44 × 10−20 | 33 | Extracellular matrix structural constituent | Molecular Function | |
| 1.17 × 10−11 | 196 | System development | Biological Process | |
| 1.26 × 10−11 | 19 | Collagen trimer | Cellular Component | |
| 1.26 × 10−11 | 9 | Fibrillar collagen trimer | Cellular Component | |
| 1.26 × 10−11 | 9 | Banded collagen fibril | Cellular Component |
| Sex | Regulation | Pathways | Gene Ontology | Statistic | # Genes | Adj. p-Value |
|---|---|---|---|---|---|---|
| Female | Down | External encapsulating structure | Cellular Component | −7.348 | 363 | 1.4 × 10−10 |
| Extracellular matrix | Cellular Component | −7.2978 | 362 | 1.4 × 10−10 | ||
| Extracellular matrix structural constituent | Molecular Function | −7.236 | 111 | 4.1 × 10−9 | ||
| Collagen-containing extracellular matrix | Cellular Component | −6.8648 | 277 | 2.1 × 10−9 | ||
| Fibrillar collagen trimer | Cellular Component | −5.5438 | 10 | 7.5 × 10−4 | ||
| Banded collagen fibril | Cellular Component | −5.5438 | 10 | 7.5 × 10−4 | ||
| Mitochondrial inner membrane | Cellular Component | −5.4532 | 408 | 5.8 × 10−6 | ||
| Extracellular matrix organization | Biological Process | −5.2292 | 241 | 2.0 × 10−4 | ||
| Extracellular structure organization | Biological Process | −5.2292 | 241 | 2.0 × 10−4 | ||
| External encapsulating structure organization | Biological Process | −5.2292 | 241 | 2.0 × 10−4 | ||
| Up | Ribonucleoprotein complex biogenesis | Biological Process | 6.2942 | 397 | 1.7 × 10−6 | |
| Ribosome biogenesis | Biological Process | 5.7665 | 278 | 2.3 × 10−5 | ||
| RRNA metabolic process | Biological Process | 5.2363 | 210 | 2.9 × 10−4 | ||
| RRNA processing | Biological Process | 5.1772 | 203 | 3.0 × 10−4 | ||
| Cytosolic ribosome | Cellular Component | 4.8633 | 100 | 9.8 × 10−4 | ||
| NcRNA metabolic process | Biological Process | 4.6176 | 424 | 0.0027 | ||
| NcRNA processing | Biological Process | 4.5756 | 352 | 0.0028 | ||
| Posttranscriptional regulation of gene expression | Biological Process | 4.045 | 437 | 0.023 | ||
| Cytoplasmic translation | Biological Process | 4.0078 | 93 | 0.035 | ||
| Autophagy | Biological Process | 3.8234 | 390 | 0.041 | ||
| Male | Up | Inflammatory response | Biological Process | 4.9953 | 475 | 0.002 |
| Leukocyte migration | Biological Process | 4.7888 | 264 | 0.0033 | ||
| Innate immune response | Biological Process | 4.606 | 469 | 0.0045 | ||
| Cytokine-mediated signaling pathway | Biological Process | 4.3149 | 279 | 0.014 | ||
| Myeloid leukocyte migration | Biological Process | 4.1515 | 158 | 0.024 | ||
| Response to bacterium | Biological Process | 4.0783 | 450 | 0.024 | ||
| Regulation of cell activation | Biological Process | 3.8591 | 425 | 0.043 | ||
| Regulation of leukocyte activation | Biological Process | 3.8372 | 391 | 0.043 | ||
| Tumor necrosis factor superfamily cytokine production | Biological Process | 3.8201 | 136 | 0.043 | ||
| Regulation of tumor necrosis factor superfamily cytokine production | Biological Process | 3.8201 | 136 | 0.043 |
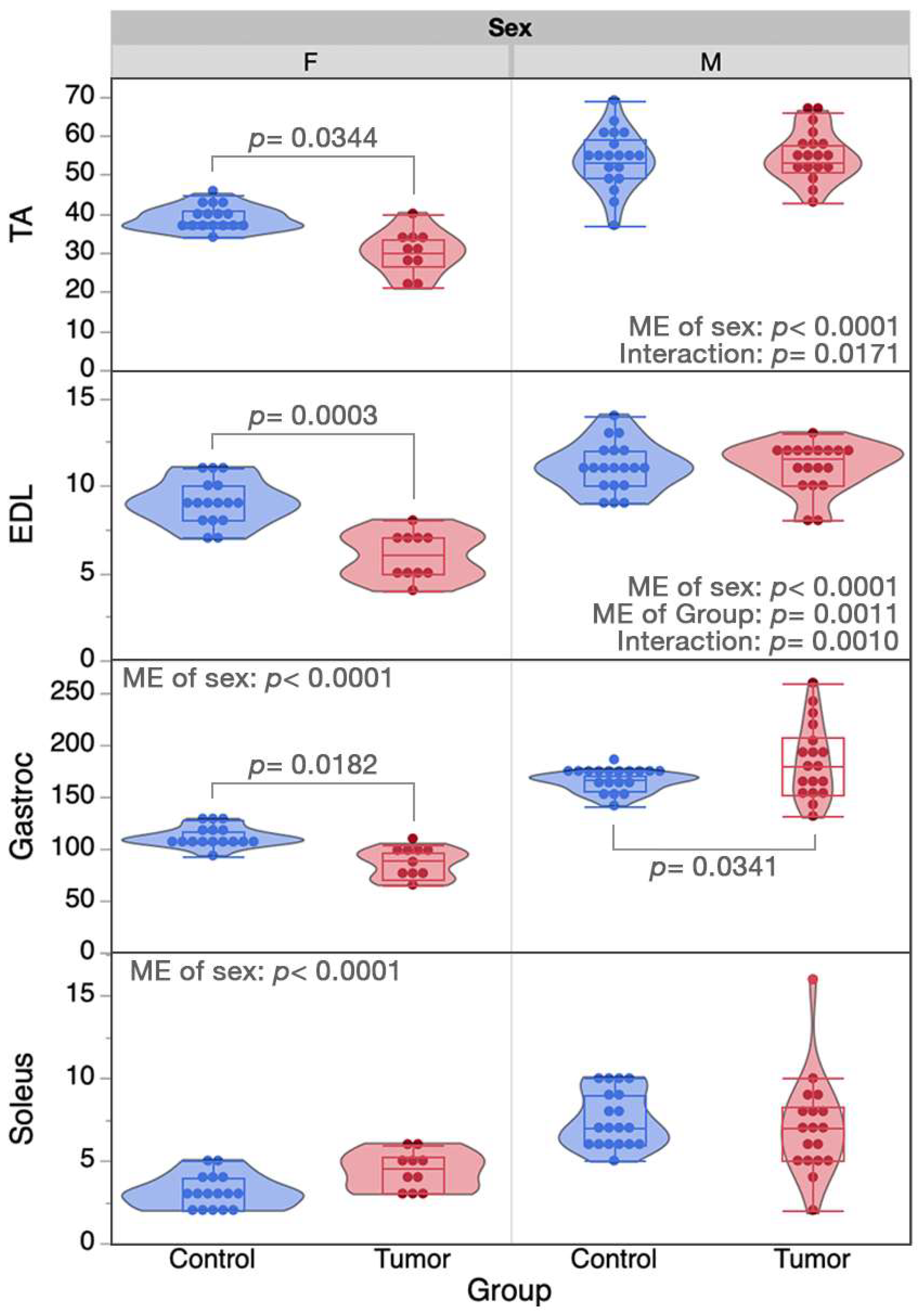
| Group LSM ± SE | Fixed Effect p-Values | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Female Control | Female Tumor | Male Control | Male Tumor | ME of Sex | ME of Group | Sex*Group Interaction |
| Lo a,b | 11.63 ± 0.17 | 10.76 ± 0.21 | 11.01 ± 0.42 | 12.05 ± 0.20 | 0.2243 | 0.7577 | 0.0018 |
| CT | 22.5 ± 2.07 | 24.0 ± 2.61 | 26.0 ± 2.61 | 25.0 ± 1.69 | 0.3332 | 0.9136 | 0.5884 |
| ½ RT | 35.0 ± 2.50 | 36.0 ± 3.16 | 26.0 ± 3.16 | 35.5 ± 2.49 | 0.1090 | 0.0792 | 0.1507 |
| Twitch/CSA | 56.5 ± 6.40 | 56.8 ± 8.10 | 42.0 ± 8.10 | 62.4 ± 6.25 | 0.5478 | 0.1664 | 0.1816 |
| Tetanus/CSA | 247.2 ± 17.0 | 247.6 ± 21.5 | 156.9 ± 21.5 | 199.6 ± 18.2 | 0.0020 | 0.2846 | 0.2924 |
| RFD a,b | 2069.5 ± 168.0 | 1286.0 ± 212.5 | 1700.4 ± 212.5 | 2341.1 ± 154.2 | 0.0814 | 0.7083 | 0.0009 |
| RR a,b | −1048.2 ± 81.0 | −683.1 ± 102.5 | −1346.6 ± 115.7 | −1306.1 ± 72.2 | <0.0001 | 0.0412 | 0.0975 |
| Pmin b | 54.4 ± 5.47 | 39.4 ± 6.92 | 58.1 ± 6.92 | 77.5 ± 5.85 | 0.0033 | 0.7289 | 0.0130 |
| Pmax a,c | 256.8 ± 12.2 | 185.2 ± 15.4 | 201.1 ± 15.4 | 234.8 ± 13.01 | 0.8330 | 0.1924 | 0.0012 |
| Kf | 32.1 ± 1.96 | 35.3 ± 2.48 | 32.0 ± 2.48 | 28.8 ± 2.10 | 0.1623 | 0.9959 | 0.1759 |
| h | 2.45 ± 1.67 | 2.34 ± 2.11 | 4.19 ± 1.20 | 5.28 ± 0.18 | 0.1295 | 0.7430 | 0.6888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentz, L.E.; Whetsell, M.A.; Clayton, S.A.; Mizener, A.D.; Holásková, I.; Chapa, M.G.; Hoblitzell, E.H.; Eubank, T.D.; Pistilli, E.E. Sexual Dimorphism of Skeletal Muscle in a Mouse Model of Breast Cancer: A Functional and Molecular Analysis. Int. J. Mol. Sci. 2023, 24, 11669. https://doi.org/10.3390/ijms241411669
Rentz LE, Whetsell MA, Clayton SA, Mizener AD, Holásková I, Chapa MG, Hoblitzell EH, Eubank TD, Pistilli EE. Sexual Dimorphism of Skeletal Muscle in a Mouse Model of Breast Cancer: A Functional and Molecular Analysis. International Journal of Molecular Sciences. 2023; 24(14):11669. https://doi.org/10.3390/ijms241411669
Chicago/Turabian StyleRentz, Lauren E., Marcella A. Whetsell, Stuart A. Clayton, Alan D. Mizener, Ida Holásková, Matthew G. Chapa, Emily H. Hoblitzell, Timothy D. Eubank, and Emidio E. Pistilli. 2023. "Sexual Dimorphism of Skeletal Muscle in a Mouse Model of Breast Cancer: A Functional and Molecular Analysis" International Journal of Molecular Sciences 24, no. 14: 11669. https://doi.org/10.3390/ijms241411669





