Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review
Abstract
:1. Introduction
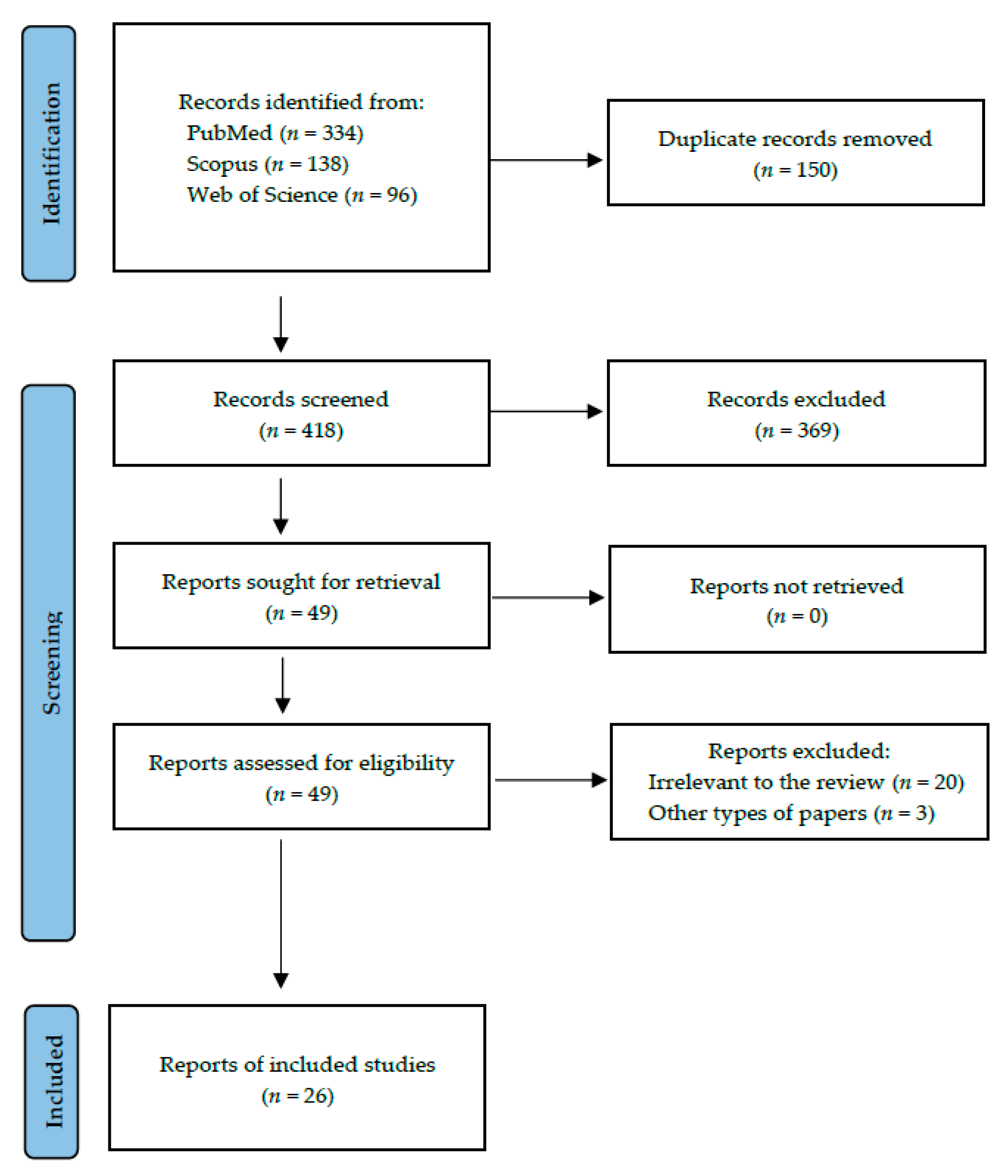
2. Materials and Methods
2.1. Search Strategy and Data Extraction
- -
- for PubMed: (myeloperoxidase AND saliva) AND (disease OR disorder OR syndrome OR therapy)
- -
- for Scopus: TITLE-ABS-KEY ((myeloperoxidase AND saliva) AND (disease OR disorder OR syndrome OR therapy))
- -
- for Web of Science: TS = ((myeloperoxidase AND saliva) AND (disease OR disorder OR syndrome OR therapy)).
2.2. Quality Assessment and Critical Appraisal for the Systematic Review of Included Studies
3. Results
4. Discussion
4.1. Cardiovascular Diseases
4.2. Respiratory Disorders
4.3. Gastrointestinal Diseases
4.4. Haematological Disorders
4.5. Infectious and Immunological Disorders
4.6. Autoimmunological Disorders
4.7. Other Disorders
4.8. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, M.J. Myeloperoxidase-Derived Oxidation: Mechanisms of Biological Damage and Its Prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, J.S.; Sumagin, R. Non-Canonical Functions of Myeloperoxidase in Immune Regulation, Tissue Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 12250. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- van der Veen, B.S.; de Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal 2009, 11, 2899–2937. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A Bridge Linking Inflammation and Oxidative Stress with Cardiovascular Disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Nijakowski, K.; Surdacka, A. Salivary Biomarkers for Diagnosis of Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 7477. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef]
- Nijakowski, K.; Zdrojewski, J.; Nowak, M.; Gruszczyński, D.; Knoll, F.; Surdacka, A. Salivary Metabolomics for Systemic Cancer Diagnosis: A Systematic Review. Metabolites 2023, 13, 28. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 August 2020).
- OCEBM Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 22 August 2020).
- Navazesh, M. Methods for Collecting Saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Mansson-Rahemtulla, B.; Baldone, D.C.; Pruitt, K.M.; Rahemtulla, F. Specific Assays for Peroxidases in Human Saliva. Arch. Oral Biol. 1986, 31, 661–668. [Google Scholar] [CrossRef]
- Akcalı, A.; Bostanci, N.; Özçaka, Ö.; Gümüş, P.; Öztürk-Ceyhan, B.; Tervahartiala, T.; Husu, H.; Buduneli, N.; Sorsa, T.; Belibasakis, G.N. Gingival Inflammation and Salivary or Serum Granulocyte-Secreted Enzymes in Patients with Polycystic Ovary Syndrome. J. Periodontol. 2017, 88, 1145–1152. [Google Scholar] [CrossRef]
- Akpinar, M.E.; Yigit, O.; Altundag, A.; Demirel, G.Y.; Kocak, I. Salivary and Serum Myeloperoxidase in Obstructive Sleep Apnea. J. Otolaryngol. Head Neck Surg. 2012, 41, 215–221. [Google Scholar]
- Dodds, M.W.; Yeh, C.K.; Johnson, D.A. Salivary Alterations in Type 2 (Non-Insulin-Dependent) Diabetes Mellitus and Hypertension. Community Dent. Oral Epidemiol. 2000, 28, 373–381. [Google Scholar] [CrossRef]
- Drążewski, D.; Grzymisławska, M.; Korybalska, K.; Czepulis, N.; Grzymisławski, M.; Witowski, J.; Surdacka, A. Oral Health Status of Patients with Lysosomal Storage Diseases in Poland. Int. J. Environ. Res. Public Health 2017, 14, 281. [Google Scholar] [CrossRef] [Green Version]
- Floriano, P.N.; Christodoulides, N.; Miller, C.S.; Ebersole, J.L.; Spertus, J.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; Steinhubl, S.; Acosta, S.; et al. Use of Saliva-Based Nano-Biochip Tests for Acute Myocardial Infarction at the Point of Care: A Feasibility Study. Clin. Chem. 2009, 55, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.D.; Sneed, J.D.; Steinhubl, S.R.; Kolasa, J.R.; Ebersole, J.L.; Lin, Y.; Kryscio, R.J.; McDevitt, J.T.; Campbell, C.L.; Miller, C.S. Salivary Biomarkers Associated with Myocardial Necrosis: Results from an Alcohol Septal Ablation Model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janšáková, K.; Escudier, M.; Tóthová, Ľ.; Proctor, G. Salivary Changes in Oxidative Stress Related to Inflammation in Oral and Gastrointestinal Diseases. Oral Dis. 2021, 27, 280–289. [Google Scholar] [CrossRef]
- Johansson, I.; Lenander-Lumikari, M.; Saellström, A.K. Saliva Composition in Indian Children with Chronic Protein-Energy Malnutrition. J. Dent. Res. 1994, 73, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Karolewska, E.; Konopka, T.; Pupek, M.; Chybicka, A.; Mendak, M. Antibacterial Potential of Saliva in Children with Leukemia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kirstilä, V.; Tenovuo, J.; Ruuskanen, O.; Nikoskelainen, J.; Irjala, K.; Vilja, P. Salivary Defense Factors and Oral Health in Patients with Common Variable Immunodeficiency. J. Clin. Immunol. 1994, 14, 229–236. [Google Scholar] [CrossRef]
- Lahdentausta, L.S.J.; Paju, S.; Mäntylä, P.; Buhlin, K.; Tervahartiala, T.; Pietiäinen, M.; Alfthan, H.; Nieminen, M.S.; Sinisalo, J.; Sorsa, T.; et al. Saliva and Serum Biomarkers in Periodontitis and Coronary Artery Disease. J. Clin. Periodontol. 2018, 45, 1045–1055. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Laurikainen, K.; Kuusisto, P.; Vilja, P. Stimulated Salivary Flow Rate and Composition in Asthmatic and Non-Asthmatic Adults. Arch. Oral Biol. 1998, 43, 151–156. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Ihalin, R.; Lähteenoja, H. Changes in Whole Saliva in Patients with Coeliac Disease. Arch. Oral Biol. 2000, 45, 347–354. [Google Scholar] [CrossRef]
- Mahmood, Z.; Enocsson, H.; Bäck, M.; Chung, R.W.S.; Lundberg, A.K.; Jonasson, L. Salivary and Plasma Levels of Matrix Metalloproteinase-9 and Myeloperoxidase at Rest and after Acute Physical Exercise in Patients with Coronary Artery Disease. PLoS ONE 2019, 14, e0207166. [Google Scholar] [CrossRef]
- Mellanen, L.; Ingman, T.; Lähdevirta, J.; Lauhio, A.; Ainamo, A.; Konttinen, Y.T.; Sukura, A.; Salo, T.; Sorsa, T. Matrix Metalloproteinases-1, -3 and -8 and Myeloperoxidase in Saliva of Patients with Human Immunodeficiency Virus Infection. Oral Dis. 1996, 2, 263–271. [Google Scholar] [CrossRef]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Simon, M.; Korybalska, K.; Witowski, J.; Surdacka, A. Potential Salivary Markers for Differential Diagnosis of Crohn’s Disease and Ulcerative Colitis. Life 2021, 11, 943. [Google Scholar] [CrossRef]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Korybalska, K.; Witowski, J.; Surdacka, A. Changes in Salivary Parameters of Oral Immunity after Biologic Therapy for Inflammatory Bowel Disease. Life 2021, 11, 1409. [Google Scholar] [CrossRef]
- Nizam, N.; Basoglu, O.K.; Tasbakan, M.S.; Holthöfer, A.; Tervahartiala, T.; Sorsa, T.; Buduneli, N. Do Salivary and Serum Collagenases Have a Role in an Association between Obstructive Sleep Apnea Syndrome and Periodontal Disease? A Preliminary Case-Control Study. Arch. Oral Biol. 2015, 60, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Lahdentausta, L.; Sorsa, T.; Tervahartiala, T.; Gokel, P.; Buggle, F.; Safer, A.; Becher, H.; Grau, A.J.; Pussinen, P. Biomarkers of Periodontitis and Inflammation in Ischemic Stroke: A Case-Control Study. Innate Immunol. 2014, 20, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polizzi, A.; Torrisi, S.; Santonocito, S.; Stefano, M.D.; Indelicato, F.; Giudice, A.L. Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study. Appl. Sci. 2020, 10, 1037. [Google Scholar] [CrossRef] [Green Version]
- Rathnayake, N.; Gustafsson, A.; Norhammar, A.; Kjellström, B.; Klinge, B.; Rydén, L.; Tervahartiala, T.; Sorsa, T. PAROKRANK Steering Group Salivary Matrix Metalloproteinase-8 and -9 and Myeloperoxidase in Relation to Coronary Heart and Periodontal Diseases: A Subgroup Report from the PAROKRANK Study (Periodontitis and Its Relation to Coronary Artery Disease). PLoS ONE 2015, 10, e0126370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Mdkhana, B.; Hussain Alsayed, H.A.; Alsafar, H.; Alrais, Z.F.; Hamid, Q.; Halwani, R. Upregulation of Oxidative Stress Gene Markers during SARS-CoV-2 Viral Infection. Free Radic. Biol. Med. 2021, 172, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.R.N.; Soave, D.F.; Sacono, N.T.; de Castro, E.F.; Silva, G.B.L.; E Silva, L.P.; Silva, T.A.; Valadares, M.C.; Mendonça, E.F.; Batista, A.C. Effect of Photobiomodulation Therapy on Reducing the Chemo-Induced Oral Mucositis Severity and on Salivary Levels of CXCL8/Interleukin 8, Nitrite, and Myeloperoxidase in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. Lasers Med. Sci. 2017, 32, 1801–1810. [Google Scholar] [CrossRef]
- van Leeuwen, S.; Proctor, G.B.; Potting, C.; Ten Hoopen, S.; van Groningen, L.; Bronkhorst, E.M.; Blijlevens, N.; Huysmans, M. Early Salivary Changes in Multiple Myeloma Patients Undergoing Autologous HSCT. Oral Dis. 2018, 24, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Yamane, K.; Nakamura, H.; Hamasaki, M.; Minei, Y.; Aibara, N.; Shimizu, T.; Kawakami, A.; Nakashima, M.; Kuroda, N.; Ohyama, K. Immune Complexome Analysis Reveals the Presence of Immune Complexes and Identifies Disease-Specific Immune Complex Antigens in Saliva Samples from Patients with Sjögren’s Syndrome. Clin. Exp. Immunol. 2021, 204, 212–220. [Google Scholar] [CrossRef]
- Yilmaz, D.; Niskanen, K.; Gonullu, E.; Tervahartiala, T.; Gürsoy, U.K.; Sorsa, T. Salivary and Serum Levels of Neutrophil Proteases in Periodontitis and Rheumatoid Arthritis. Oral Dis. 2023. [Google Scholar] [CrossRef]
- Garvin, P.; Jonasson, L.; Nilsson, L.; Falk, M.; Kristenson, M. Plasma Matrix Metalloproteinase-9 Levels Predict First-Time Coronary Heart Disease: An 8-Year Follow-Up of a Community-Based Middle Aged Population. PLoS ONE 2015, 10, e0138290. [Google Scholar] [CrossRef]
- Tang, W.W.; Wu, Y.; Nicholls, S.J.; Hazen, S.L. Plasma Myeloperoxidase Predicts Incident Cardiovascular Risks in Stable Patients Undergoing Medical Management for Coronary Artery Disease. Clin. Chem. 2011, 57, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jönsson, S.; Lundberg, A.; Kälvegren, H.; Bergström, I.; Szymanowski, A.; Jonasson, L. Increased Levels of Leukocyte-Derived MMP-9 in Patients with Stable Angina Pectoris. PLoS ONE 2011, 6, e19340. [Google Scholar] [CrossRef] [Green Version]
- Khan, D.A.; Sharif, M.S.; Khan, F.A. Diagnostic Performance of High-Sensitivity Troponin T, Myeloperoxidase, and Pregnancy-Associated Plasma Protein A Assays for Triage of Patients with Acute Myocardial Infarction. Korean J. Lab. Med. 2011, 31, 172–178. [Google Scholar] [CrossRef]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The Roles of Myeloperoxidase in Coronary Artery Disease and Its Potential Implication in Plaque Rupture. Redox Rep. 2017, 22, 51–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Rosen, H.; Madtes, D.K.; Shao, B.; Martin, T.R.; Heinecke, J.W.; Fu, X. Myeloperoxidase Inactivates TIMP-1 by Oxidizing Its N-Terminal Cysteine Residue: An Oxidative Mechanism for Regulating Proteolysis during Inflammation. J. Biol. Chem. 2007, 282, 31826–31834. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, S.; Kugiyama, K.; Aikawa, M.; Nakamura, S.; Ogawa, H.; Libby, P. Hypochlorous Acid, a Macrophage Product, Induces Endothelial Apoptosis and Tissue Factor Expression: Involvement of Myeloperoxidase-Mediated Oxidant in Plaque Erosion and Thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1309–1314. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.; Oda, M.N.; Oram, J.F.; Heinecke, J.W. Myeloperoxidase: An Oxidative Pathway for Generating Dysfunctional High-Density Lipoprotein. Chem. Res. Toxicol. 2010, 23, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Fisher, E.A.; Feig, J.E.; Hewing, B.; Hazen, S.L.; Smith, J.D. High-Density Lipoprotein Function, Dysfunction, and Reverse Cholesterol Transport. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2813–2820. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Tsimikas, S. Candidate Biomarkers for the Detection of Coronary Plaque Destabilization and Rupture. Curr. Atheroscler. Rep. 2008, 10, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Obaid Abdullah, S.; Ramadan, G.M.; Makki Al-Hindy, H.A.-A.; Mousa, M.J.; Al-Mumin, A.; Jihad, S.; Hafidh, S.; Kadhum, Y. Serum Myeloperoxidase as a Biomarker of Asthma Severity Among Adults: A Case Control Study. Rep. Biochem. Mol. Biol. 2022, 11, 182–189. [Google Scholar] [CrossRef]
- Ekmekci, O.B.; Donma, O.; Sardoğan, E.; Yildirim, N.; Uysal, O.; Demirel, H.; Demir, T. Iron, Nitric Oxide, and Myeloperoxidase in Asthmatic Patients. Biochemistry 2004, 69, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Petrof, B.J.; Hamid, Q.; Fraser, R.; Kimoff, R.J. Upper Airway Muscle Inflammation and Denervation Changes in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 541–546. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Loukides, S.; Papatheodorou, G.; Roussos, C.; Alchanatis, M. Airway Inflammation in Obstructive Sleep Apnea: Is Leptin the Missing Link? Respir. Med. 2008, 102, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Sabato, R.; Guido, P.; Salerno, F.G.; Resta, O.; Spanevello, A.; Barbaro, M.P.F. Airway Inflammation in Patients Affected by Obstructive Sleep Apnea. Monaldi Arch. Chest Dis. 2006, 65, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanikoglu, F.; Huseyinoglu, N.; Ozben, S.; Cort, A.; Ozdem, S.; Ozben, T. Increased Plasma Soluble Tumor Necrosis Factor Receptor-1 and Myeloperoxidase Activity in Patients with Obstructive Sleep Apnea Syndrome. Int. J. Neurosci. 2015, 125, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Leong, R.W.; Wasinger, V.C.; Ip, M.; Yang, M.; Phan, T.G. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients with Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 2017, 153, 723–731. [Google Scholar] [CrossRef]
- Wéra, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Dos Santos Ramos, A.; Viana, G.C.S.; de Macedo Brigido, M.; Almeida, J.F. Neutrophil Extracellular Traps in Inflammatory Bowel Diseases: Implications in Pathogenesis and Therapeutic Targets. Pharmacol. Res. 2021, 171, 105779. [Google Scholar] [CrossRef]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef] [Green Version]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase Is Required for Neutrophil Extracellular Trap Formation: Implications for Innate Immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Chami, B.; Martin, N.J.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the Inflamed Colon: A Novel Target for Treating Inflammatory Bowel Disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Motylewska, B.; Banasik, E.; Rutkowski, R.; Tsaryk, V.; Łuczak, J.; Korybalska, K.; Witowski, J.; Surdacka, A.; Eder, P. The Treatment Regimens and Disease Activity Could Alter the Salivary Myeloperoxidase Levels in Patients with Inflammatory Bowel Diseases. Pol. Arch. Intern. Med. 2023; accepted. [Google Scholar]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac Disease, Inflammation and Oxidative Damage: A Nutrigenetic Approach. Nutrients 2012, 4, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Maluf, S.W.; Wilhelm, D.; Parisotto, E.B.; da Silva de Medeiros, G.; Pereira, C.H.J.; Maraslis, F.T.; Dornelles Schoeller, C.C.; da Rosa, J.S.; Fröde, T.S. DNA Damage, Oxidative Stress, and Inflammation in Children with Celiac Disease. Genet. Mol. Biol. 2023, 43, e20180390. [Google Scholar] [CrossRef]
- Tedjosasongko, U.; Pramudita, R.A.; Puteri, M.M. Biomarker of Malnutrition in Terms of Total Salivary Protein in Stunting Children (Literature Review). Int. J. Sci. Adv. 2022, 3, 398–402. [Google Scholar] [CrossRef]
- Mishra, R.; Kapur, A.; Goyal, A.; Gauba, K.; Trehan, A. Salivary Parameters and Their Correlation with Neutrophil Counts in Children with Acute Lymphoblastic Leukemia. Eur. Arch. Paediatr. Dent. 2022, 23, 281–287. [Google Scholar] [CrossRef]
- Madu, A.J.; Ibegbulam, O.G.; Ocheni, S.; Madu, K.A.; Aguwa, E.N. Absolute Neutrophil Values in Malignant Patients on Cytotoxic Chemotherapy. Niger. J. Med. 2011, 20, 120–123. [Google Scholar] [PubMed]
- Vistoso Monreal, A.; Polonsky, G.; Shiboski, C.; Sankar, V.; Villa, A. Salivary Gland Dysfunction Secondary to Cancer Treatment. Front. Oral Health 2022, 3, 907778. [Google Scholar] [CrossRef] [PubMed]
- Imanguli, M.M.; Atkinson, J.C.; Harvey, K.E.; Hoehn, G.T.; Ryu, O.H.; Wu, T.; Kingman, A.; Barrett, A.J.; Bishop, M.R.; Childs, R.W.; et al. Changes in Salivary Proteome Following Allogeneic Hematopoietic Stem Cell Transplantation. Exp. Hematol. 2007, 35, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Lieschke, G.J.; Ramenghi, U.; O’Connor, M.P.; Sheridan, W.; Szer, J.; Morstyn, G. Studies of Oral Neutrophil Levels in Patients Receiving G-CSF after Autologous Marrow Transplantation. Br. J. Haematol. 1992, 82, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front. Immunol. 2021, 12, 687627. [Google Scholar] [CrossRef] [PubMed]
- Avivi, I.; Avraham, S.; Koren-Michowitz, M.; Zuckerman, T.; Aviv, A.; Ofran, Y.; Benyamini, N.; Nagler, A.; Rowe, J.M.; Nagler, R.M. Oral Integrity and Salivary Profile in Myeloma Patients Undergoing High-Dose Therapy Followed by Autologous SCT. Bone Marrow Transplant. 2009, 43, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, S.S.; de Oliveira, E.M.; de Araújo, T.H.; Rodrigues, M.R.; Albuquerque, R.C.; Mortara, R.A.; Taniwaki, N.N.; Nakaya, H.I.; Campa, A.; Moreno, A.C.R. Myeloperoxidase in Human Peripheral Blood Lymphocytes: Production and Subcellular Localization. Cell Immunol. 2016, 300, 18–25. [Google Scholar] [CrossRef]
- Gangcuangco, L.M.A.; Mitchell, B.I.; Siriwardhana, C.; Kohorn, L.B.; Chew, G.M.; Bowler, S.; Kallianpur, K.J.; Chow, D.C.; Ndhlovu, L.C.; Gerschenson, M.; et al. Mitochondrial Oxidative Phosphorylation in Peripheral Blood Mononuclear Cells Is Decreased in Chronic HIV and Correlates with Immune Dysregulation. PLoS ONE 2020, 15, e0231761. [Google Scholar] [CrossRef]
- Mohammed, R.N.; Tamjidifar, R.; Rahman, H.S.; Adili, A.; Ghoreishizadeh, S.; Saeedi, H.; Thangavelu, L.; Shomali, N.; Aslaminabad, R.; Marofi, F.; et al. A Comprehensive Review about Immune Responses and Exhaustion during Coronavirus Disease (COVID-19). Cell Commun. Signal 2022, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 Infection Pathogenesis Is Related to Oxidative Stress as a Response to Aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.; Chan, L.L.Y.; Cheung, C.; Chia, P.Y.; Ong, S.W.X.; Fong, S.W.; Ng, L.F.P.; Renia, L.; Lye, D.C.; Young, B.E.; et al. Myeloperoxidase Inhibition May Protect against Endothelial Glycocalyx Shedding Induced by COVID-19 Plasma. Commun. Med. 2023, 3, 62. [Google Scholar] [CrossRef]
- Guellec, D.; Cornec-Le Gall, E.; Groh, M.; Hachulla, E.; Karras, A.; Charles, P.; Dunogué, B.; Abad, S.; Alvarez, F.; Gérard, F.; et al. ANCA-Associated Vasculitis in Patients with Primary Sjögren’s Syndrome: Detailed Analysis of 7 New Cases and Systematic Literature Review. Autoimmun. Rev. 2015, 14, 742–750. [Google Scholar] [CrossRef]
- Iwafuchi, Y.; Morioka, T.; Oyama, Y.; Narita, I. A Case of Smoldering Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Development during the Course of Primary Sjögren’s Syndrome. CEN Case Rep. 2022, 11, 247–253. [Google Scholar] [CrossRef]
- O’Neil, L.J.; Kaplan, M.J. Neutrophils in Rheumatoid Arthritis: Breaking Immune Tolerance and Fueling Disease. Trends Mol. Med. 2019, 25, 215–227. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and Redox Stress in the Pathogenesis of Autoimmune Disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Arvikar, S.L.; Hasturk, H.; Strle, K.; Stephens, D.; Bolster, M.B.; Collier, D.S.; Kantarci, A.; Steere, A.C. Periodontal Inflammation and Distinct Inflammatory Profiles in Saliva and Gingival Crevicular Fluid Compared with Serum and Joints in Rheumatoid Arthritis Patients. J. Periodontol. 2021, 92, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, W.; Ning, X. Increased Levels of Neutrophil Extracellular Trap Remnants in the Serum of Patients with Rheumatoid Arthritis. Int. J. Rheum. Dis. 2018, 21, 415–421. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating Inflammatory Markers in Polycystic Ovary Syndrome: A Systematic Review and Metaanalysis. Fertil. Steril. 2011, 95, 1048–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Li, S.; Liu, H.; Bai, H.; Liu, Q.; Hu, K.; Guan, L.; Fan, P. Myeloperoxidase and CYBA Genetic Variants in Polycystic Ovary Syndrome. Eur. J. Clin. Investig. 2021, 51, e13438. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Martinez de Marañon, A.; Rios-Navarro, C.; Alvarez, A.; Gomez, M.; Rocha, M.; Hernández-Mijares, A. Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS ONE 2016, 11, e0151960. [Google Scholar] [CrossRef]
- Yogalingam, G.; Lee, A.R.; Mackenzie, D.S.; Maures, T.J.; Rafalko, A.; Prill, H.; Berguig, G.Y.; Hague, C.; Christianson, T.; Bell, S.M.; et al. Cellular Uptake and Delivery of Myeloperoxidase to Lysosomes Promote Lipofuscin Degradation and Lysosomal Stress in Retinal Cells. J. Biol. Chem. 2017, 292, 4255–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelić-Knezović, N.; Galijašević, S.; Lovrić, M.; Vasilj, M.; Selak, S.; Mikulić, I. Levels of Nitric Oxide Metabolites and Myeloperoxidase in Subjects with Type 2 Diabetes Mellitus on Metformin Therapy. Exp. Clin. Endocrinol. Diabetes 2019, 127, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, J.J.; Meuwese, M.C.; van Miert, J.N.I.; Kastelein, A.; Tijssen, J.G.P.; Piek, J.J.; Trip, M.D. Diabetes Mellitus Type 2 Is Associated with Higher Levels of Myeloperoxidase. Med. Sci. Monit. 2008, 14, 406–410. [Google Scholar]
- Liu, D.; Liu, L.; Hu, Z.; Song, Z.; Wang, Y.; Chen, Z. Evaluation of the Oxidative Stress-Related Genes ALOX5, ALOX5AP, GPX1, GPX3 and MPO for Contribution to the Risk of Type 2 Diabetes Mellitus in the Han Chinese Population. Diab Vasc. Dis. Res. 2018, 15, 336–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez García, A.; Rivera Rodríguez, M.; Gómez Alonso, C.; Rodríguez Ochoa, D.Y.; Alvarez Aguilar, C. Myeloperoxidase Is Associated with Insulin Resistance and Inflammation in Overweight Subjects with First-Degree Relatives with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2015, 39, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients aged from 0 to 99 years, both genders | |
| Intervention/Exposure | systemic diseases (e.g., cardiovascular diseases, gastrointestinal diseases) | other diseases (e.g., oral diseases) |
| Comparison | not applicable | |
| Outcomes | salivary levels of myeloperoxidase | serum levels of myeloperoxidase, salivary levels of other antioxidants |
| Study design | case-control, cohort, and cross-sectional studies | literature reviews, case reports, expert opinion, letters to the editor, conference reports |
| published until 5 May 2023 | not published in English |
| Author, Year | Setting | Study Group (F/M), Age | Control Group (F/M), Age | Diagnosis | Inclusion Criteria | Exclusion Criteria | Smoking Status |
|---|---|---|---|---|---|---|---|
| Akcalı et al., 2017 [16] | Turkey | 80 (80/0): 45 periodontally healthy, 35 with gingivitis; NR | 45 (45/0): 25 periodontally healthy, 20 with gingivitis; NR | Polycystic ovary syndrome (PCOS) | PCOS diagnosed based on Rotterdam criteria | Hyperandrogenism, diabetes mellitus, hyperprolactemia, congenital adrenal hyperplasia, thyroid disorders, Cushing’s syndrome, hypertension, hepatic or renal dysfunction, BMI > 30 kg/m2, and cardiovascular diseases, medication such as oral contraceptive agents, steroid hormones, insulin-sensitizing drugs and antibiotics or anti-inflammatory drugs that could affect their periodontal status during the last 6 months prior to the study, smoking | No smokers |
| Akpinar et al., 2012 [17] | Turkey | 32 (8/24); 43.79 ± 12.72 | 24 (8/16); 44.7 ± 13.75 | Obstructive sleep apnea (OSA) | Patients with complaints of snoring, excessive daytime sleepiness, and apnea, whereas control subjects had no distinct complaints | Oropharyngeal and dental infections, ongoing dental treatments, periodontal and gingival inflammation, laryngeal pathologies, a history of asthma, chronic bronchitis, allergic rhinitis, chronic obstructive pulmonary disease, and acute airway infection, smoking, a history of continuous positive airway pressure use and upper airway surgery for OSA, including nasal surgery | No smokers |
| Dodds et al., 2000 [18] | USA | 233 (113/120) DM, mean 61.2/63.9; 227 (136/91) hypertension, 64.7/62.2 | 240 (106/134), mean 55.3/55.9 | Diabetes mellitus (DM) type 2, hypertension | DM identified by modified WHO criteria or currently taking diabetic medications; hypertension classified by diastolic blood pressure ±95 mm Hg or currently taking anti-hypertensive medications | NR | NR |
| Drążewski et al., 2017 [19] | Poland | 36 (13/23), range: 7–30 | NA | Lysosomal storage diseases (LSDs) | Patients participated in rehabilitation training courses in the SMRD center | NR | NR |
| Floriano et al., 2009 [20] | USA | 56 (36/20), mean 54.8 | 59 (34/25), mean 49.3 | Acute myocardial infarction (AMI) | Patients within 48 h of onset of symptoms of AMI, at least 18 years old | Fever, stroke, immune disorders, use of steroidal medications, organ complications/failure, and inability to provide saliva | NR |
| Foley et al., 2012 [21] | USA | 19 (12/7), 58.58 ± 13.41 | 97 (59/38), 48.6 ± 8.6 | Hypertrophic cardiomyopathy (HCM) | Underwent alcohol septal ablation as treatment for HCM | Age < 18 years, unable or unwilling to provide informed consent or provide samples, recently treated with chemotherapeutic drugs, anti-organ rejection drugs, or significant immune modulators within the past 3 months or during the course of the study, febrile illness, or active infection at the time of enrollment, pregnancy | Current smokers (n = 9) |
| Janšáková et al., 2021 [22] | United Kingdom | 27 (17/10) OFG, 38.76 ± 14.53; 29 (10/19) CD, 37.13 ± 11.78; 14 (3/11) CD + OFG, 44.5 ± 15.1 | 22 (10/12), 35.09 ± 10.12 | Crohn’s disease (CD), orofacial granulomatosis (OFG) | In a remission phase of the disease; no acute complications or presenting of co-morbidities at the date of the collection | Presence of another systemic disease, oral diseases, e.g., periodontitis, oral lichen planus, acute illnesses, e.g., cold, gastroenteritis, infection of urogenital tract and taking anticholinergic drugs or treatment affecting salivary flow, pregnancy, age under 18 years | Smokers included (NR) |
| Johansson et al., 1994 [23] | India | 34 (NR), range: 8–12 | 34 (sex- and age-matched) | Chronic protein-energy malnutrition (PEM) | Selected from 8- to 12-year-old pupils attending St. Mary’s School, Madras, India; with a defined date of birth and without other conditions or any known disease at the time of examination | NR | NA |
| Karolewska et al., 2008 [24] | Poland | 44 (19/25), mean 10.26, range: 3–17 | 23 (NR), mean 8.7, range: 5–14 | Leukemia | Children with newly diagnosed acute leukemia, able to participate in saliva collection and whose parents signed the informed consent | Children younger than 3 years | NA |
| Kirstilä et al., 1994 [25] | Finland | 15 (6/9), range: 7–67 | 15 (6/9), range: 7–63 | Common Variable Immunodeficiency (CVI) | Diagnosed for CVI in Turku University Central Hospital | NR | NR |
| Lahdentausta et al., 2018 [26] | Finland | 163 (45/118), mean 63.0 ± 9.5 | 290 (102/188), mean 64.1 ± 8.7 | Acute coronary syndrome (ACS) | ≥50% stenosis in at least one coronary artery, episode of typical chest pain for ischemia and elevated cardiac enzymes. | “ACS-like, no significant CAD” (including takotsubo patients) | Smokers (n = 94) |
| Lenander-Lumikari et al., 1998 [27] | Finland | 26 (21/5), range: 25–50 | 33 (23/10), range: 25–50 | Asthma | Age ranging from 25 to 50 years and diagnosed asthma | Medication for psychiatric diseases, diabetes or any other disease that may directly or indirectly affect the oral cavity | Smokers (n = 5) |
| Lenander-Lumikari et al., 2000 [28] | Finland | 128 (104/24), mean 42.7 ± 14.7 | 55 (41/14), mean 39.1 ± 12.7 | Coeliac disease | Invited members of the Coeliac Association of Turku, Finland, without any discrimination based on age or sex, followed a strict gluten-free diet | Diagnosis based only on positive serological tests | NR |
| Mahmood et al., 2019 [29] | Sweden | 23 (5/18), range: 60–69 | NA | Coronary artery disease (CAD) | Recent coronary event, i.e., acute coronary syndrome and/or revascularization with either percutaneous coronary intervention or coronary artery bypass grafting | Age > 75 years, severe heart failure, neoplastic disease, major clinical depression, chronic liver and renal failure, chronic immunologic disorders or treatment with immunosuppressive/anti-inflammatory agents, serious physical or psychological diseases interfering with performing an exercise test, and inability to understand the Swedish language | Smokers (n = 5) |
| Mellanen et al., 1996 [30] | Finland | 56 (10/46), mean 37.7, range: 23–68 | 10 (5/5), mean 36, range: 27–58 | Human immunodeficiency virus (HIV) | Voluntary HIV-seropositive patients visiting the Aurora Hospital Dental Clinic, Helsinki, Finland | NR | NR |
| Nijakowski et al., 2021 [31] | Poland | 27 (10/17) CD, range: 28–48; 24 (7/17) UC, range: 24–40.5 | 51 (17/34), range: 26–40 | Crohn’s disease (CD), ulcerative colitis (UC) | Adult patients of both sexes, with inflammatory bowel diseases, qualified for biologic treatment | Concomitant autoimmune diseases (including diabetes), periodontal disease or other overt inflammatory lesions in the oral cavity, taking medications known to affect salivation | Smokers (CD n = 7, UC n = 1) |
| Nijakowski et al., 2021 [32] | Poland | 27 (10/17) CD, range: 28–48; 24 (7/17) UC, range: 24–40.5 | NA | Crohn’s disease (CD), ulcerative colitis (UC) | Adult patients of both sexes, with inflammatory bowel diseases, qualified for biologic treatment - patients with active, moderate to severe disease, not responding to previous conventional full-dose therapy or showing intolerance to such therapy (e.g., allergic reactions) | Concomitant autoimmune diseases (including diabetes), periodontal disease or other overt inflammatory lesions in the oral cavity, taking medications known to affect salivation | Smokers (CD n = 7, UC n = 1) |
| Nizam et al., 2015 [33] | Turkey | 37 (12/25): 17 mild-to-moderate (8/9), range: 29–64; 20 severe (4/16), range: 26–61 | 13 (8/5), range: 21–59 | Obstructive sleep apnea (OSA) | Complaints of sleep apnea-related symptoms | Immunological disorders, diabetes mellitus, received antibiotic treatment within the last 3 months, and periodontal treatment within the last 6 months, or had less than 20 teeth and wearing removable dentures | Smokers (n = 8), ex-smokers (n = 6) |
| Palm et al., 2014 [34] | Germany | 98 (45/53), mean 68.2 ± 9.7 | 100 (47/53), mean 69.1 ± 5.2 | Acute ischemic stroke | Patients with a first-ever ischemic stroke between the age of 18 and 80 years | Clinical or laboratory signs of acute infection at time of stroke onset | Smokers (n = 28), ex-smokers (n = 58) |
| Polizzi et al., 2020 [35] | Italy | 62 (32/30): 31 periodontally healthy (17/14), range: 46–58; 31 with periodontitis (15/16), range: 47–56 | 62 (31/31): 31 periodontally healthy (16/15), range: 48–56; 31 with periodontitis (15/16), range: 47–57 | Cardiovascular disease (CVD) | At least ≥18 years; a diagnosis of CVD with ≥50% of stenosis of at least one coronary artery verified by coronary angiography, or past or current percutaneous coronary intervention | Contraceptive drugs, antibiotics, anti-inflammatory or immunosuppressive drugs during the three months previous the trial; gestation or suction; intake of alcohol; anesthetic allergy; intake of nifedipine, hydantoin or cyclosporin; any type of periodontal treatment in the three months before baseline | Smokers (n = 4), ex-smokers (n = 4) |
| Rathnayake et al., 2015 [36] | Sweden | 200 (32/168), mean 61 ± 8 | 200 (32/168), mean 61 ± 8 | Coronary Artery Disease (CAD) | <75 years old and admitted to a coronary care unit with a first myocardial infarction | Undergone cardiac valvular surgery or had language barriers preventing them to complete study procedures | Smokers (n = 19), ex-smokers (n = 103) |
| Saheb Sharif-Askari et al., 2021 [37] | United Arab Emirates | 7 asymptomatic (NR), mean 44 ± 6; 10 severe (NR), mean 53 ± 11 | 5 (NR), mean 34 ± 8 | SARS-CoV-2 infection | COVID-19 patients with different disease severity | NR | NR |
| Salvador et al., 2017 [38] | Brasil | 27 (12/15), mean 42 ± 17.8 | 24 (13/11), mean 41 ± 14.9 | Undergoing hematopoietic stem cell transplantation (HSCT) | At least 14 years of age, scheduled for autologous or allogeneic HSCT and planned treatment consisting of a myeloablative conditioning regimen with high-dose chemotherapy, without radiotherapy; intact oral mucosal lining, no infectious foci or other associated pathologies. | Patients who needed tracheal intubation, or presented mental confusion, or died | NR |
| van Leeuwen et al., 2018 [39] | The Netherlands | 20: part A: 12 (5/7), range: 43–68; part B: 8 (3/5), range: 53–67 | NA | Multiple myeloma (MM) | Adult MM patients undergoing HDM (200 mg/m2, infused during 1 h) and autologous HSCT | Patients who did not understand the Dutch language | NR |
| Yamane et al., 2021 [40] | Japan | 9 (8/1), range: 38–77 | 7 (7/0), range: 26–93 | Sjögren’s syndrome (SS) | Patients who met the internationally accepted 2002 American–European Consensus Group Sjögren’s syndrome classification criteria | NR | NR |
| Yilmaz et al., 2023 [41] | Finland | 49 (36/13): 26 with periodontitis (18/8), range: 33–68; 23 periodontally healthy (18/5), range: 40–68 | 48 (26/22): 24 periodontally healthy (14/10), range: 34–66; 24 with periodontitis (12/12), range: 35–63 | Rheumatoid arthritis (RA) | RA patients undergoing treatments and regular follow-ups | Having <16 teeth, periodontal treatment history and antibiotic use within at least 3 months or more before the initiation of the study, inflammatory and/or mucocutaneous disease and disorders of the oral cavity, additional general disorders or diseases such as diabetes mellitus, renal, hepatic disorders, or HIV as well as pregnancy and lactating period, a history of transplantation, diagnosed with other forms of arthritis, recent quitters (<2 years), and occasional smokers | Smokers included (NR) |
| Study | Type of Saliva and Method of Collection | Centrifugation and Storing | Method of MPO Determination | Salivary Biomarkers |
|---|---|---|---|---|
| Akcalı et al., 2017 [16] | Unstimulated whole saliva into sterile 50 mL tubes for 5 min | Placed on ice, supplemented with EDTA-free Protease Inhibitor Cocktail, centrifuged at 10,000× g for 15 min at 4 °C, stored at −80 °C until analysis | ELISA | MPO, MMP-9, NE, TIMP-1 |
| Akpinar et al., 2012 [17] | Unstimulated whole saliva 2–3 mL for 5 min | NR | Flow cytometry using a fluorescent bead immunoassay method | MPO |
| Dodds et al., 2000 [18] | Unstimulated whole saliva for 5 min, stimulated parotid for 5 min using a modified Carlson–Crittenden cup, and submandibular/sublingual saliva stimulated for 3 min and unstimulated for 5 min using a micropipette connected to a mini-vacuum pump; stimulation by swabbing the lateral surfaces of the tongue with 0.1 mol/L citric acid every 30 s | The volume determined gravimetrically, stored at −80 °C | ELISA | MPO, salivary peroxidase, cystatin, albumin, lactoferrin, lysozyme, secretory IgA |
| Drążewski et al., 2017 [19] | Unstimulated whole saliva into a sterile container | Centrifuged and stored at −80 °C until analysis | ELISA | MPO, TAS, MCP-1, TNF-R1 and TNF-R2, VEGF, sICAM-1, MMP-2 |
| Floriano et al., 2009 [20] | Unstimulated whole saliva | Transported on ice, centrifuged, stored at –80 °C until analysis | Luminex® IS100-based multiplex kits | MPO, CRP, MMP-9, IL-1β, slCAM-1, adiponectin, MCP-1, Gro-α, E-selectin, IL-18, ENA-78, sVCAM-1, MYO, CK-MB, cTnl, BNP, Fractalkine, RANTES, IL-6, sCD40-L, TNF-α |
| Foley et al., 2012 [21] | Unstimulated whole saliva 5 mL at baseline and at 8, 16, 24, and 48 h post-procedure; according to a modification in the method described by Navazesh | Immediately placed on ice, transported within 10 min, centrifuged, stored at –80 °C until analysis | Luminex® IS100-based multiplex kits | MPO, MMP-9, CK-MB, MYO, cTnI, BNP, CRP |
| Janšáková et al., 2021 [22] | Unstimulated whole saliva by passive drooling for 10 min into sterile tubes | Kept on ice, centrifuged at 7000× g for 5 min at 4 °C, stored at –20 °C until analysis | ELISA | MPO, IgA, lactoferrin |
| Johansson et al., 1994 [23] | Whole saliva: unstimulated by drooling for 10 min and paraffin-stimulated expectorated for 5 min into ice-chilled, graded test tubes | Centrifuged at 14,500× g for 15 min at 4 °C, stored at –20 °C | Non-isotopic immunometric assays with biotin-labeled antibodies and avidin alkaline phosphatase label | Unstimulated: MPO, thiocyanate, lactoferrin, lysozyme, BAGP, total IgG and IgA, specific anti-S. mutans IgA; stimulated: hexosamines, fucose, sialic acid, amylase, sodium, potassium, calcium, chloride |
| Karolewska et al., 2008 [24] | Unstimulated whole saliva into plastic test tubes placed in a flask of ice | Centrifuged at 3000× g for 15 min, stored at –30 °C until analysis | The modified method by Mansson-Rahemtulla et al., based on the time of oxidation of Cl– to OCl– present in the substrate Nbs-Cl | MPO, salivary peroxidase, lysozyme, lactoferrin, secretory IgA |
| Kirstilä et al., 1994 [25] | Paraffin-stimulated whole saliva | Centrifuged at 18,000× g for 10 min at 4 °C, stored at −20 °C until analysis | Non-isotopic immunometric assays with biotin-labeled antibodies and avidin alkaline phosphatase label | MPO, lysozyme, lactoferrin, salivary peroxidase, hypothiocyanite, thiocyanate, IgA, IgG, and IgM (total and specific anti-S. mutans) |
| Lahdentausta et al., 2018 [26] | Paraffin-stimulated whole saliva for 5 min and at least 2 mL by expectoration | Centrifuged at 9300× g for 3 min and used for the analysis | ELISA | MPO, MMP-8, MMP-9, TIMP-1 |
| Lenander-Lumikari et al., 1998 [27] | Paraffin-stimulated whole saliva into chilled, graduated glass tubes for 5 min | Centrifuged at 12,000× g for 10 min at 4 °C, stored at −20 °C until analysis | Non-isotopic immunometric assays with biotin-labeled antibodies and avidin alkaline phosphatase label | MPO, lactoferrin, lysozyme, salivary peroxidase, calcium, potassium, sodium, thiocyanate |
| Lenander-Lumikari et al., 2000 [28] | Paraffin-stimulated whole saliva into chilled, graduated glass tubes for 5 min | Immediately centrifuged at 16,000× g for 10 min at 4 °C, stored at −20 °C until analysis, analyzed within 2 months | The modified method by Mansson-Rahemtulla et al., based on the time of oxidation of Cl– to OCl– present in the substrate Nbs-Cl | MPO, salivary peroxidase, IgA, IgG and IgM, amylase, total protein |
| Mahmood et al., 2019 [29] | Unstimulated whole saliva with oral cotton swabs placed under the tongue for 2 min; prior to the bicycle test, directly after, and 30 min after the test completion | Immediately placed on ice, centrifuged 10,000× g for 5 min, stored at −70 °C until analysis | Magnetic bead-based luminex assay | MPO, MMP-9 |
| Mellanen et al., 1996 [30] | Paraffin-stimulated whole saliva about 5 mL of whole paraffin stimulated saliva | Immediately frozen and stored at −20 °C until analysis | Quantitative dot blotting | MPO, MMP-1, MMP-3, MMP-8 |
| Nijakowski et al., 2021 [31] | Unstimulated whole saliva by passive drooling up to 20 min, as described by Navazesh | Centrifuged and stored at −80 °C until analysis | ELISA | MPO, catalase, TNF-R1, serpin E1/PAI-1, S100A8/calprotectin, IgA |
| Nijakowski et al., 2021 [32] | Unstimulated whole saliva by passive drooling up to 20 min, as described by Navazesh | Centrifuged and stored at −80 °C until analysis | ELISA | MPO, IgA |
| Nizam et al., 2015 [33] | Unstimulated whole saliva expectorated into polypropylene tubes | Centrifuged at 800× g for 10 min at room temperature | ELISA | MPO, MMP-2, MMP-8, MMP-9, TIMP-1, NE, NGAL |
| Palm et al., 2014 [34] | Paraffin-stimulated whole saliva for 5 min and at least 2 mL by expectoration | Stored at −70 °C until analysis | ELISA | MMP-8, MPO, TIMP-1, IL-1β |
| Polizzi et al., 2020 [35] | Stimulated whole saliva by chewing a cotton roll for 2 min using the salivette method | Centrifuged at 1000× g for 2 min at 4 °C, stored at −20 °C | Magnetic bead-based luminex assay | MPO |
| Rathnayake et al., 2015 [36] | Paraffin-stimulated whole saliva up to 10 min into a graded test-tube until 2 mL | Centrifuged at 500× g for 5 min at 5 °C, stored at −80 °C until analysis | ELISA | MMP-8, MMP-9, MPO, TIMP-1 |
| Saheb Sharif-Askari et al., 2021 [37] | Unstimulated whole saliva | NR | qRT-PCR | 125 oxidative stress genes: 37 pro-oxidant genes, 32 oxidative-responsive genes, and 56 antioxidant genes (including MPO) |
| Salvador et al., 2017 [38] | Unstimulated whole saliva into sterile tubes for 5 min at three different moments of treatment | Centrifuged at 1500 rpm for 10 min, stored at −80 °C until analysis | ELISA | MPO, CXCL8/IL-8, nitrite |
| van Leeuwen et al., 2018 [39] | Whole saliva: unstimulated for 5 min and paraffin-stimulated for 1 min into previously weighed plastic cups | Centrifuged at 10,000 rpm for 5 min, stored at −80 °C until analysis | ELISA | MPO, mucin 5B, albumin, total IgA, lactoferrin |
| Yamane et al., 2021 [40] | Stimulated whole saliva using the Saxon test | NR | Immune complexome analysis | MPO, NE, neutrophil defensin 1, small proline-rich protein 2D cathepsin G, nuclear mitotic apparatus 1, phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit gamma |
| Yilmaz et al., 2023 [41] | Unstimulated whole saliva samples into calibrated 2 mL plastic tubes for 5 min | Centrifuged at 6000× g for 5 min, stored at −80 °C until transferred | ELISA | active-MMP-8, TIMP-1, NE |
| Study | Diagnosis | Concentration/Activity | Study Group | Control Group | p-Value | AUC |
|---|---|---|---|---|---|---|
| Akpinar et al., 2012 [17] | Obstructive sleep apnoea | ng/mL | 6.40 ± 2.82 | 3.92 ± 0.34 | <0.0001 | - |
| Dodds et al., 2000 [18] | Diabetes mellitus | µg/mL | 0.81 ± 0.10 | 0.21 ± 0.06 | 0.0117 | - |
| Floriano et al., 2009 [20] | Acute myocardial infarction | - | Mestudy/Mecontrol 1.91 | - | 0.71 | |
| Karolewska et al., 2008 [24] | Leukemia | IU/mg TP | 0.21 ± 0.16 | 0.27 ± 0.16 | 0.013 | - |
| Lenander-Lumikari et al., 1998 [27] | Asthma | ng/mL | 164.4 ± 136.5 | 95.2 ± 110.0 | <0.05 | - |
| Lenander-Lumikari et al., 2000 [28] | Coeliac disease | mU | 0.61 ± 0.29 | 0.36 ± 0.12 | ≤0.001 | - |
| Nijakowski et al., 2021 [31] | Crohn’s disease | ng/µg TP | 0.167 (0.046–0.577) | 0.239 (0.070–0.685) | <0.001 | CD vs. UC, ng/mL |
| Ulcerative colitis | 0.055 (0.011–0.124) | 0.69 | ||||
| Palm et al., 2014 [34] | Ischemic stroke | ng/mL | Me (IQR) 999.55 (1248.56) | Me (IQR) 2092.98 (3894.23) | <0.0001 | - |
| Polizzi et al., 2020 [35] | Cardiovascular disease | ng/mL | 83.2 (77.4–101.5) | - | <0.01 | - |
| Rathnayake et al., 2015 [36] | Coronary artery disease | ng/mL | 1637 ± 1386 | 1899 ± 1447 | 0.02 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12078. https://doi.org/10.3390/ijms241512078
Nijakowski K, Jankowski J, Gruszczyński D, Surdacka A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(15):12078. https://doi.org/10.3390/ijms241512078
Chicago/Turabian StyleNijakowski, Kacper, Jakub Jankowski, Dawid Gruszczyński, and Anna Surdacka. 2023. "Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review" International Journal of Molecular Sciences 24, no. 15: 12078. https://doi.org/10.3390/ijms241512078






