Restoring Angiotensin Type 2 Receptor Function Reverses PFOS-Induced Vascular Hyper-Reactivity and Hypertension in Pregnancy
Abstract
:1. Introduction
2. Results
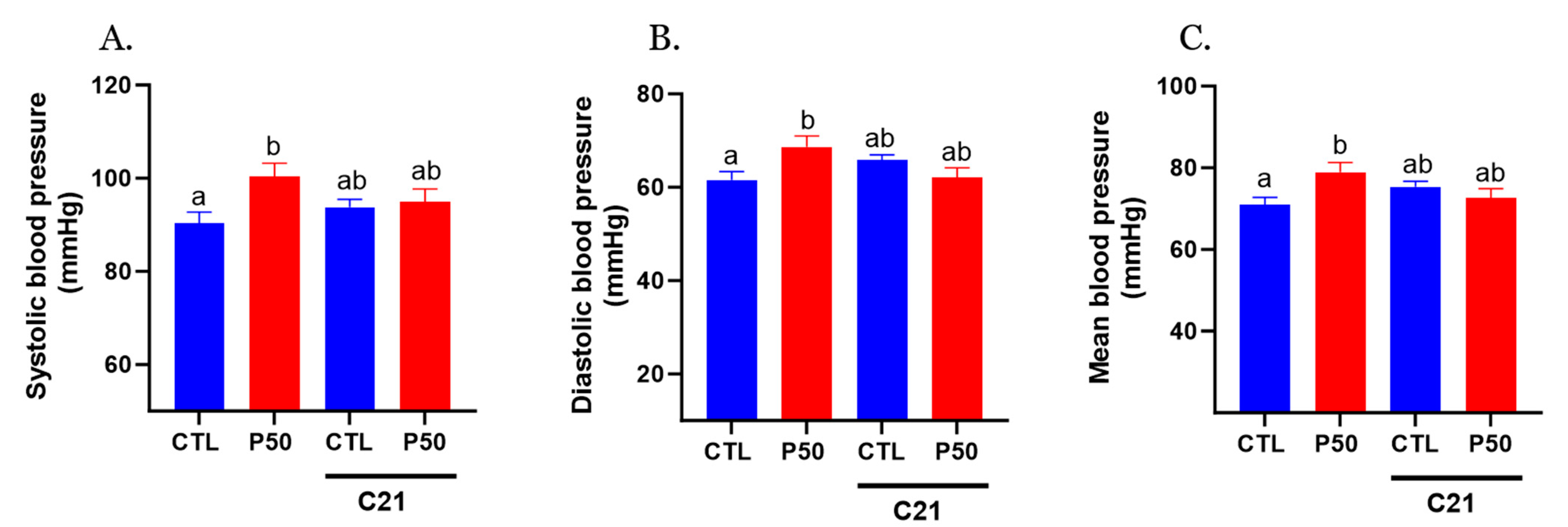
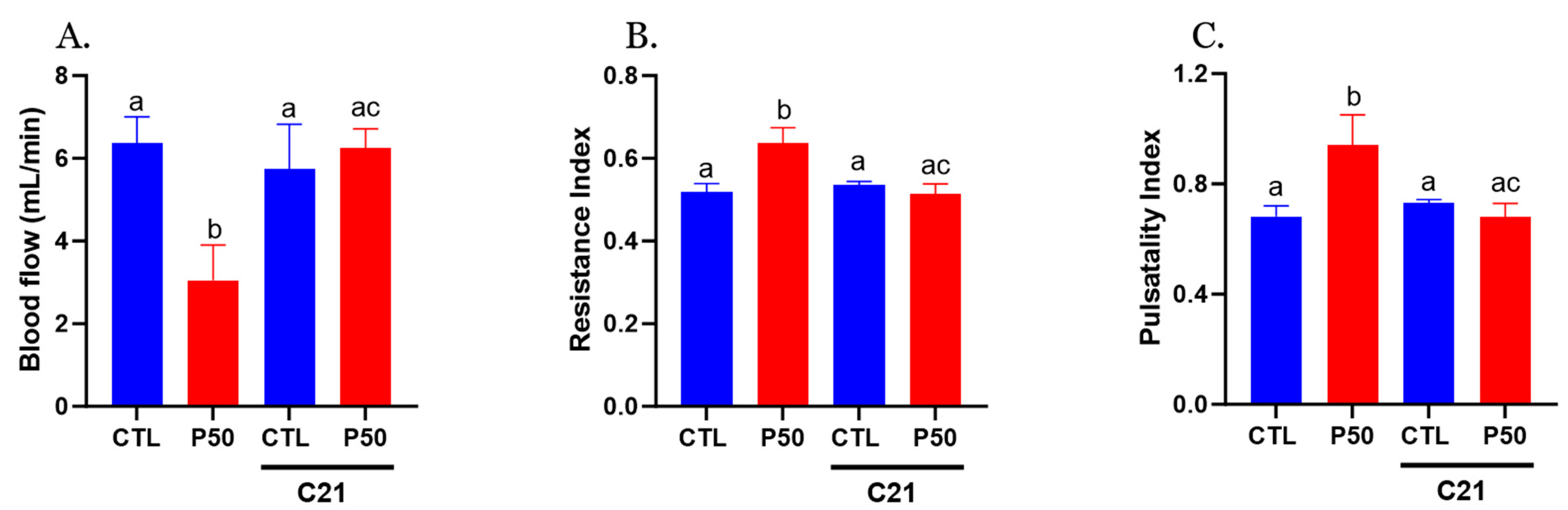
2.1. Blood Pressure and Uterine Artery Blood Flow in Pregnant Rats
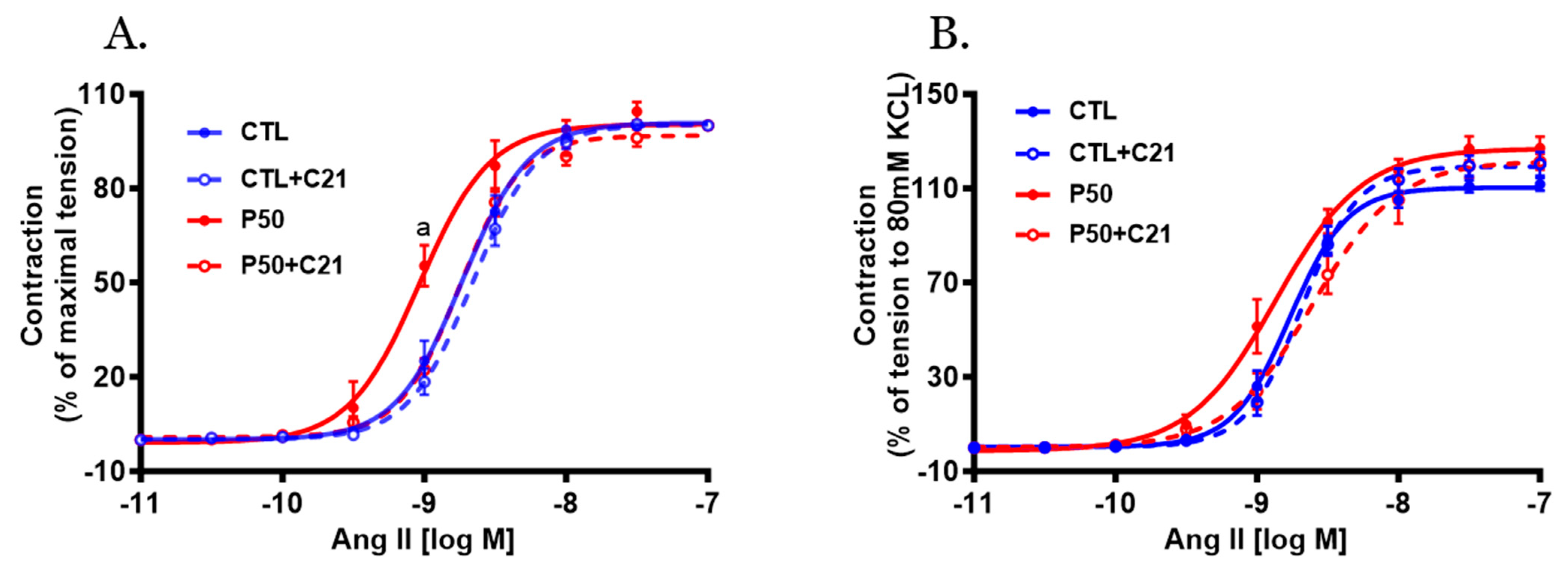
2.2. Vasoconstrictor Response
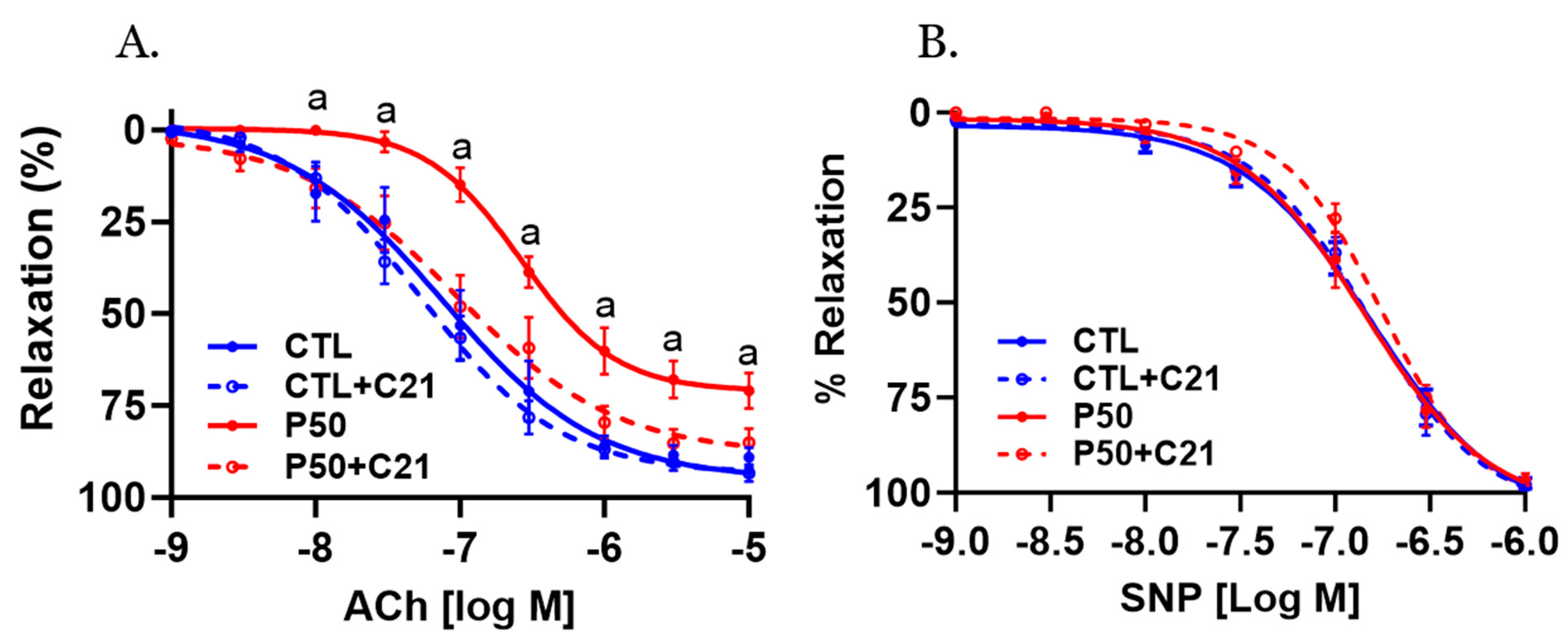
2.3. Vasodilator Response
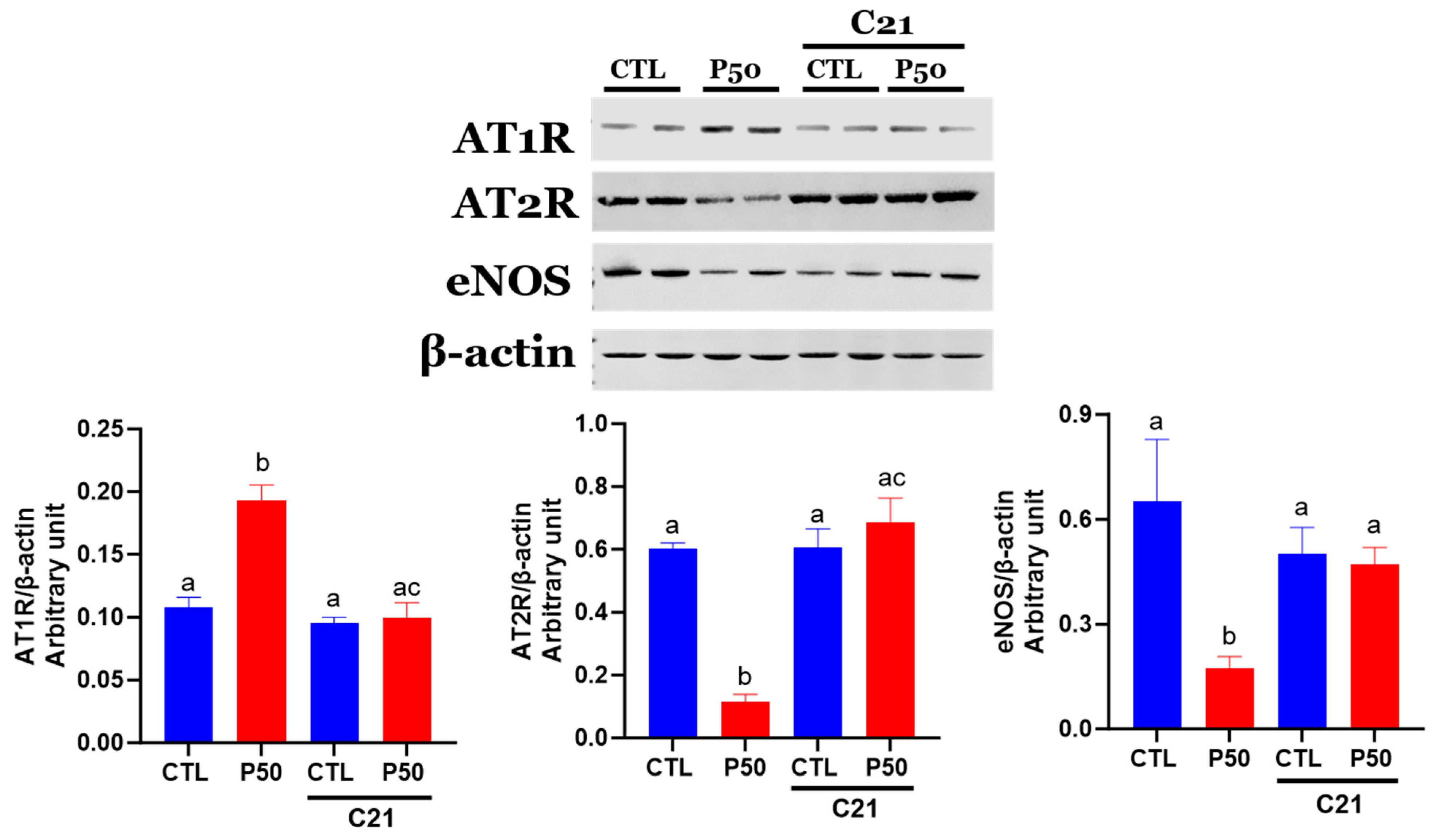
2.4. Ang II receptors and eNOS Protein Levels
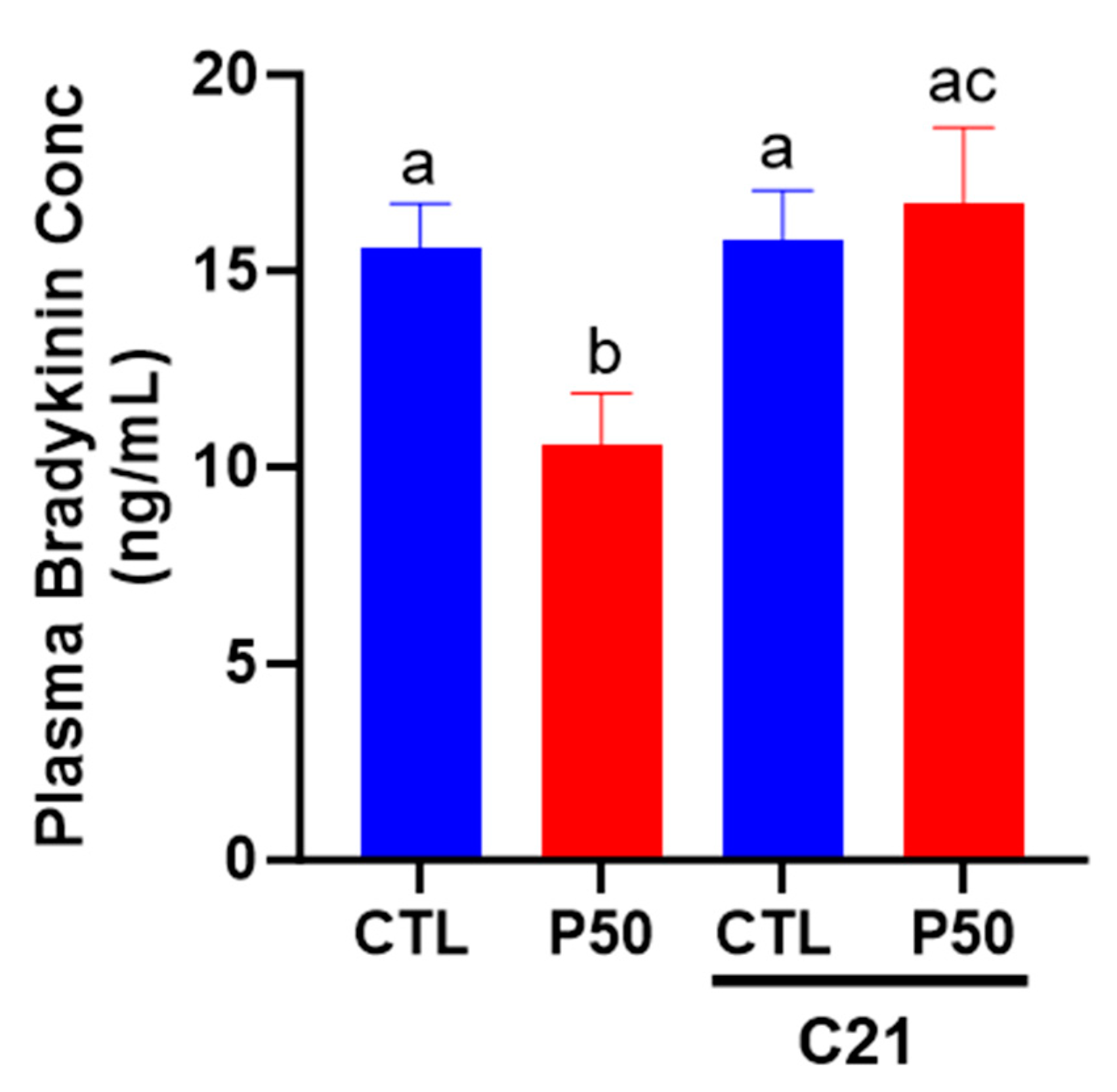
2.5. Plasma Bradykinin Levels
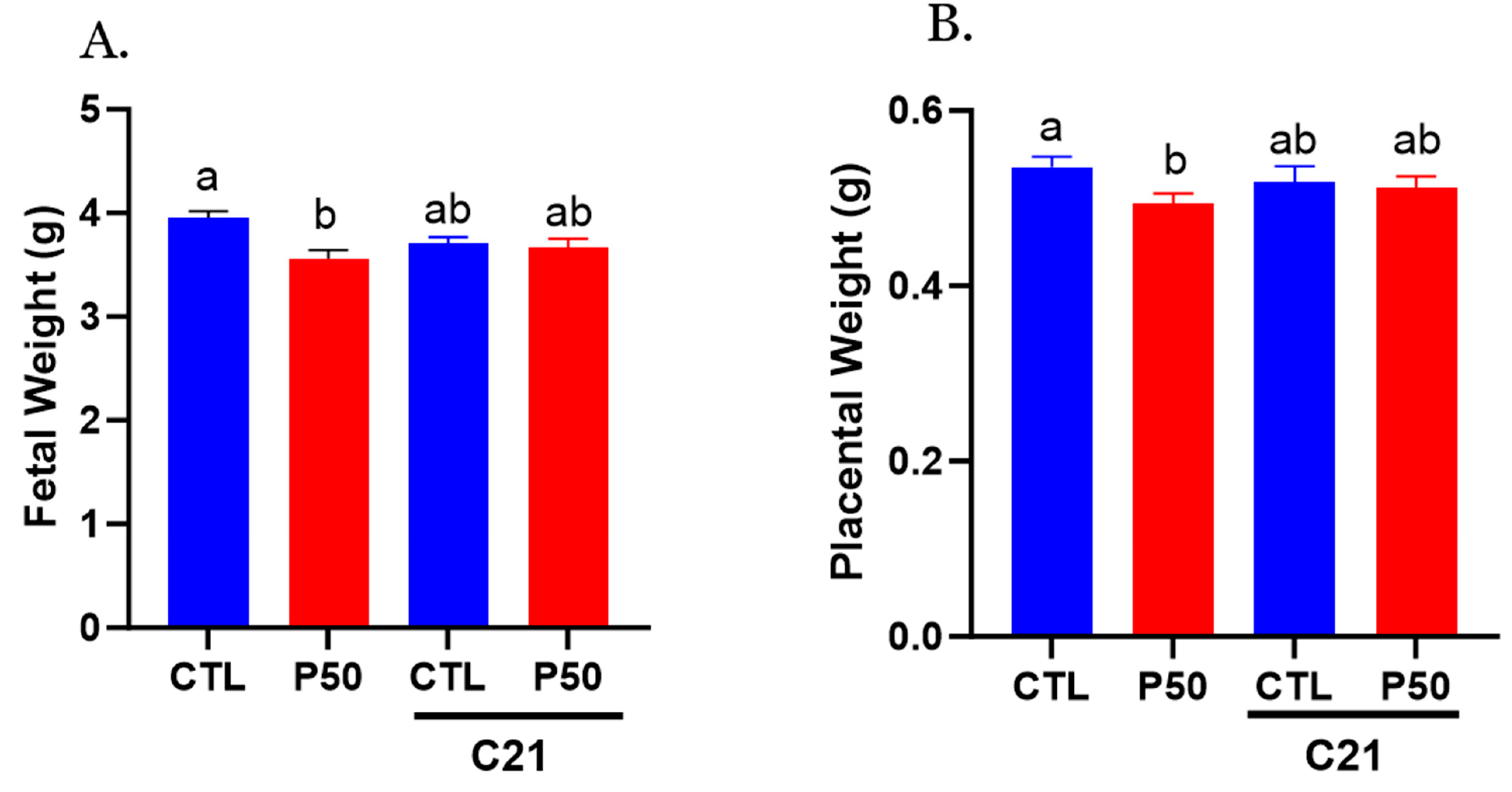
2.6. Placental and Fetal Weight
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Blood Pressure
4.3. Uterine Artery Ultrasound
4.4. Ex-Vivo Vascular Reactivity Studies
4.4.1. Assessment of Vascular Contractile Responses
4.4.2. Assessment of Vascular Relaxation Responses
4.5. Plasma Bradykinin Levels
4.6. Western Blotting
4.7. Placental and Fetal Weights
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, N.D.; Cox, S.; Ko, J.Y.; Ouyang, L.; Romero, L.; Colarusso, T.; Ferre, C.D.; Kroelinger, C.D.; Hayes, D.K.; Barfield, W.D. Hypertensive disorders in pregnancy and mortality at delivery hospitalization—United States, 2017–2019. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Garovic, V.D.; Dechend, R.; Easterling, T.; Karumanchi, S.A.; McMurtry Baird, S.; Magee, L.A.; Rana, S.; Vermunt, J.V.; August, P.; on behalf of the American Heart Association Council on Hypertension; et al. Hypertension in pregnancy: Diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the american heart association. Hypertension 2022, 79, e21–e41. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Lane, S.L.; Doyle, A.S.; Bales, E.S.; Lorca, R.A.; Julian, C.G.; Moore, L.G. Increased uterine artery blood flow in hypoxic murine pregnancy is not sufficient to prevent fetal growth restrictiondagger. Biol. Reprod. 2020, 102, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Lopez, F.; Zhang, H.Y.; Pavlish, K.; Benoit, J.N. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant sprague-dawley rat. Biol. Reprod. 2005, 72, 762–766. [Google Scholar] [CrossRef]
- Konje, J.C.; Howarth, E.S.; Kaufmann, P.; Taylor, D.J. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 2003, 110, 301–305. [Google Scholar] [CrossRef]
- Leon, L.J.; McCarthy, F.P.; Direk, K.; Gonzalez-Izquierdo, A.; Prieto-Merino, D.; Casas, J.P.; Chappell, L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: A caliber study. Circulation 2019, 140, 1050–1060. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The international federation of gynecology and obstetrics (figo) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef]
- Garovic, V.D.; White, W.M.; Vaughan, L.; Saiki, M.; Parashuram, S.; Garcia-Valencia, O.; Weissgerber, T.L.; Milic, N.; Weaver, A.; Mielke, M.M. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J. Am. Coll. Cardiol. 2020, 75, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Borghese, M.M.; Walker, M.; Helewa, M.E.; Fraser, W.D.; Arbuckle, T.E. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the mirec study. Environ. Int. 2020, 141, 105789. [Google Scholar] [CrossRef]
- Wikstrom, S.; Lindh, C.H.; Shu, H.; Bornehag, C.G. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in swedish women. Sci. Rep. 2019, 9, 9179. [Google Scholar] [CrossRef]
- Starling, A.P.; Engel, S.M.; Richardson, D.B.; Baird, D.D.; Haug, L.S.; Stuebe, A.M.; Klungsoyr, K.; Harmon, Q.; Becher, G.; Thomsen, C.; et al. Perfluoroalkyl substances during pregnancy and validated preeclampsia among nulliparous women in the norwegian mother and child cohort study. Am. J. Epidemiol. 2014, 179, 824–833. [Google Scholar] [CrossRef]
- Starling, A.P.; Engel, S.M.; Whitworth, K.W.; Richardson, D.B.; Stuebe, A.M.; Daniels, J.L.; Haug, L.S.; Eggesbo, M.; Becher, G.; Sabaredzovic, A.; et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the norwegian mother and child cohort study. Environ. Int. 2014, 62, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Stein, C.R.; Steenland, K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the mid-ohio valley, 2005–2010. Environ. Health Perspect. 2013, 121, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.R.; Savitz, D.A.; Dougan, M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol. 2009, 170, 837–846. [Google Scholar] [CrossRef]
- Rylander, L.; Lindh, C.H.; Hansson, S.R.; Broberg, K.; Kallen, K. Per- and polyfluoroalkyl substances in early pregnancy and risk for preeclampsia: A case-control study in southern sweden. Toxics 2020, 8, 43. [Google Scholar] [CrossRef]
- Birukov, A.; Andersen, L.B.; Andersen, M.S.; Nielsen, J.H.; Nielsen, F.; Kyhl, H.B.; Jørgensen, J.S.; Grandjean, P.; Dechend, R.; Jensen, T.K. Exposure to perfluoroalkyl substances and blood pressure in pregnancy among 1436 women from the odense child cohort. Environ. Int. 2021, 151, 106442. [Google Scholar] [CrossRef]
- Boronow, K.E.; Brody, J.G.; Schaider, L.A.; Peaslee, G.F.; Havas, L.; Cohn, B.A. Serum concentrations of PFASs and exposure-related behaviors in african american and non-hispanic white women. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated compounds in US Fast food packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Perfluoroalkyls. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 24 July 2023).
- Evans, S. PFAS Contamination of Drinking Water Far More Prevalent Than Previously Reported. Wednesday, January 22, 2020. Available online: https://www.Ewg.Org/research/national-pfas-testing/ (accessed on 9 March 2020).
- EWG. PFAS Contamination Tracker. Available online: https://www.ewg.org/interactive-maps/pfas_contamination/ (accessed on 8 June 2023).
- DeLuca, N.M.; Thomas, K.; Mullikin, A.; Slover, R.; Stanek, L.W.; Pilant, A.N.; Cohen Hubal, E.A. Geographic and demographic variability in serum PFAS concentrations for pregnant women in the united states. J. Expo. Sci. Environ. Epidemiol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Worley, R.R.; Moore, S.M.; Tierney, B.C.; Ye, X.; Calafat, A.M.; Campbell, S.; Woudneh, M.B.; Fisher, J. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ. Int. 2017, 106, 135–143. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Beesoon, S.; Martin, J.W. Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ. Sci. Technol. 2015, 49, 5722–5731. [Google Scholar] [CrossRef]
- Butenhoff, J.L.; Chang, S.C.; Olsen, G.W.; Thomford, P.J. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in sprague dawley rats. Toxicology 2012, 293, 1–15. [Google Scholar] [CrossRef]
- Harada, K.H.; Hashida, S.; Kaneko, T.; Takenaka, K.; Minata, M.; Inoue, K.; Saito, N.; Koizumi, A. Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environ. Toxicol. Pharmacol. 2007, 24, 134–139. [Google Scholar] [CrossRef]
- Bogdanska, J.; Borg, D.; Sundstrom, M.; Bergstrom, U.; Halldin, K.; Abedi-Valugerdi, M.; Bergman, A.; Nelson, B.; Depierre, J.; Nobel, S. Tissue distribution of 35s-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology 2011, 284, 54–62. [Google Scholar] [CrossRef]
- Narizzano, A.M.; Lent, E.M.; Hanson, J.M.; East, A.G.; Bohannon, M.E.; Quinn, M.J. Reproductive and developmental toxicity of perfluorooctane sulfonate (PFOS) in the white-footed mouse (peromyscus leucopus). Reprod. Toxicol. 2022, 113, 120–127. [Google Scholar] [CrossRef]
- Chambers, W.S.; Hopkins, J.G.; Richards, S.M. A review of per- and polyfluorinated alkyl substance impairment of reproduction. Front. Toxicol. 2021, 3, 732436. [Google Scholar] [CrossRef]
- Meneguzzi, A.; Fava, C.; Castelli, M.; Minuz, P. Exposure to perfluoroalkyl chemicals and cardiovascular disease: Experimental and epidemiological evidence. Front. Endocrinol. 2021, 12, 706352. [Google Scholar] [CrossRef]
- Cordner, A.; Goldenman, G.; Birnbaum, L.S.; Brown, P.; Miller, M.F.; Mueller, R.; Patton, S.; Salvatore, D.H.; Trasande, L. The true cost of PFAS and the benefits of acting now. Environ. Sci. Technol. 2021, 55, 9630–9633. [Google Scholar] [CrossRef]
- Huang, J.; Sun, L.; Mennigen, J.A.; Liu, Y.; Liu, S.; Zhang, M.; Wang, Q.; Tu, W. Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: An emphasis on cilia disruption. J. Hazard. Mater. 2021, 409, 124491. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Huang, Q.S.; Fang, C.; Kiyama, R.; Shen, H.; Dong, S. Evaluation of cellular response to perfluorooctane sulfonate in human umbilical vein endothelial cells. Toxicol. In Vitro 2012, 26, 421–428. [Google Scholar] [CrossRef]
- Qian, Y.; Ducatman, A.; Ward, R.; Leonard, S.; Bukowski, V.; Lan Guo, N.; Shi, X.; Vallyathan, V.; Castranova, V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J. Toxicol. Environ. Health A 2010, 73, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Hao, F.; Sun, Z.; Long, Y.; Zhou, Q.; Jiang, G. Perfluorohexadecanoic acid increases paracellular permeability in endothelial cells through the activation of plasma kallikrein-kinin system. Chemosphere 2018, 190, 191–200. [Google Scholar] [CrossRef]
- Forsthuber, M.; Widhalm, R.; Granitzer, S.; Kaiser, A.M.; Moshammer, H.; Hengstschläger, M.; Dolznig, H.; Gundacker, C. Perfluorooctane sulfonic acid (PFOS) inhibits vessel formation in a human 3d co-culture angiogenesis model (NCFS/HUVECS). Environ. Pollut. 2022, 293, 118543. [Google Scholar] [CrossRef]
- Li, J.; Quan, X.; Lei, S.; Huang, Z.; Wang, Q.; Xu, P. PFOS inhibited normal functional development of placenta cells via PPARγ Signaling. Biomedicines 2021, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Dangudubiyyam, S.V.; Mishra, J.S.; Song, R.; Kumar, S. Maternal perfluorooctane sulfonic acid exposure during rat pregnancy causes hypersensitivity to angiotensin ii and attenuation of endothelium-dependent vasodilation in the uterine arteries. Biol. Reprod. 2022, 107, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Świątkowska-Stodulska, R.; Kmieć, P.; Stefańska, K.; Sworczak, K. Renin-angiotensin-aldosterone system in the pathogenesis of pregnancy-induced hypertension. Exp. Clin. Endocrinol. Diabetes 2018, 126, 362–366. [Google Scholar] [CrossRef]
- Verdonk, K.; Visser, W.; Van Den Meiracker, A.H.; Danser, A.H. The renin-angiotensin-aldosterone system in pre-eclampsia: The delicate balance between good and bad. Clin. Sci. 2014, 126, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Touyz, R.; Dominiczak, A.F.; Webb, R.C.; Johns, D.G. Angiotensin receptors: Signaling, vascular pathophysiology, and interactions with ceramide. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2337–H2365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.S.; Kumar, S. Activation of angiotensin type 2 receptor attenuates testosterone-induced hypertension and uterine vascular resistance in pregnant rats. Biol. Reprod. 2021, 105, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Stennett, A.K.; Qiao, X.; Falone, A.E.; Koledova, V.V.; Khalil, R.A. Increased vascular angiotensin type 2 receptor expression and nos-mediated mechanisms of vascular relaxation in pregnant rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H745–H755. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Fleisch, A.F.; Webster, T.F.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: Confounded by pregnancy hemodynamics? Am. J. Epidemiol. 2018, 187, 793–802. [Google Scholar] [CrossRef]
- Chen, M.H.; Ng, S.; Hsieh, C.J.; Lin, C.C.; Hsieh, W.S.; Chen, P.C. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci. Total Environ. 2017, 607–608, 669–675. [Google Scholar] [CrossRef]
- Starling, A.P.; Adgate, J.L.; Hamman, R.F.; Kechris, K.; Calafat, A.M.; Ye, X.; Dabelea, D. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: Examining mediation by maternal fasting glucose in the healthy start study. Environ. Health Perspect. 2017, 125, 067016. [Google Scholar] [CrossRef]
- Maisonet, M.; Terrell, M.L.; McGeehin, M.A.; Christensen, K.Y.; Holmes, A.; Calafat, A.M.; Marcus, M. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in british girls. Environ. Health Perspect. 2012, 120, 1432–1437. [Google Scholar] [CrossRef]
- Chen, M.H.; Ha, E.H.; Wen, T.W.; Su, Y.N.; Lien, G.W.; Chen, C.Y.; Chen, P.C.; Hsieh, W.S. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS ONE 2012, 7, e42474. [Google Scholar] [CrossRef]
- Halldorsson, T.I.; Rytter, D.; Haug, L.S.; Bech, B.H.; Danielsen, I.; Becher, G.; Henriksen, T.B.; Olsen, S.F. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ. Health Perspect. 2012, 120, 668–673. [Google Scholar] [CrossRef]
- Andersen, C.S.; Fei, C.; Gamborg, M.; Nohr, E.A.; Sorensen, T.I.; Olsen, J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am. J. Epidemiol. 2010, 172, 1230–1237. [Google Scholar] [CrossRef]
- Washino, N.; Saijo, Y.; Sasaki, S.; Kato, S.; Ban, S.; Konishi, K.; Ito, R.; Nakata, A.; Iwasaki, Y.; Saito, K.; et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Perspect. 2009, 117, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Apelberg, B.J.; Witter, F.R.; Herbstman, J.B.; Calafat, A.M.; Halden, R.U.; Needham, L.L.; Goldman, L.R. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 2007, 115, 1670–1676. [Google Scholar] [CrossRef]
- Rogers, J.M.; Ellis-Hutchings, R.G.; Grey, B.E.; Zucker, R.M.; Norwood, J., Jr.; Grace, C.E.; Gordon, C.J.; Lau, C. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol. Sci. 2014, 137, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Padia, S.H.; Carey, R.M. At₂ receptor activation induces natriuresis and lowers blood pressure. Circ. Res. 2014, 115, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Patel, S.; Hussain, T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese zucker rats. Am. J. Physiol. Renal Physiol. 2015, 308, F1379–F1385. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Morriss, F.H.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol. Invest. 1974, 5, 252–268. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef]
- Julian, C.G.; Galan, H.L.; Wilson, M.J.; Desilva, W.; Cioffi-Ragan, D.; Schwartz, J.; Moore, L.G. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R906–R915. [Google Scholar] [CrossRef]
- Zamudio, S.; Palmer, S.K.; Dahms, T.E.; Berman, J.C.; Young, D.A.; Moore, L.G. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J. Appl. Physiol. 1995, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Jones, E.S.; Steckelings, U.M.; Unger, T.; Widdop, R.E.; Denton, K.M. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: A novel therapeutic target for hypertension. Hypertension 2012, 59, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R. Distribution of cardiac output in ovine pregnancy. Am. J. Physiol. 1977, 232, H231–H235. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef]
- Spradley, F.T.; Ge, Y.; Granger, J.P.; Chade, A.R. Utero-placental vascular remodeling during late gestation in sprague-dawley rats. Pregnancy Hypertens. 2020, 20, 36–43. [Google Scholar] [CrossRef]
- Chen, G.; Xu, L.L.; Huang, Y.F.; Wang, Q.; Wang, B.H.; Yu, Z.H.; Shi, Q.M.; Hong, J.W.; Li, J.; Xu, L.C. Prenatal exposure to perfluorooctane sulfonate impairs placental angiogenesis and induces aberrant expression of LncRNA Xist. Biomed. Environ. Sci. 2018, 31, 843–847. [Google Scholar]
- Jiang, W.; Deng, Y.; Song, Z.; Xie, Y.; Gong, L.; Chen, Y.; Kuang, H. Gestational perfluorooctanoic acid exposure inhibits placental development by dysregulation of labyrinth vessels and unk cells and apoptosis in mice. Front. Physiol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Mishra, J.S.; Chen, D.B.; Kumar, S. AT2R activation increases in vitro angiogenesis in pregnant human uterine artery endothelial cells. PLoS ONE 2022, 17, e0267826. [Google Scholar] [CrossRef]
- Jin, X.Q.; Fukuda, N.; Su, J.Z.; Lai, Y.M.; Suzuki, R.; Tahira, Y.; Takagi, H.; Ikeda, Y.; Kanmatsuse, K.; Miyazaki, H. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension 2002, 39, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Hayashida, W.; Akishita, M.; Tamura, K.; Daviet, L.; Lehtonen, J.Y.; Dzau, V.J. Stimulation of different subtypes of angiotensin II receptors, AT1 and AT2 receptors, regulates stat activation by negative crosstalk. Circ. Res. 1999, 84, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tsuchida, S.; Imai, T.; Fujii, N.; Miyazaki, H.; Ichiki, T.; Naruse, M.; Inagami, T. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem. Biophys. Res. Commun. 1999, 258, 194–198. [Google Scholar] [CrossRef]
- Savoia, C.; Tabet, F.; Yao, G.; Schiffrin, E.L.; Touyz, R.M. Negative regulation of rhoa/rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: Role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J. Hypertens. 2005, 23, 1037–1045. [Google Scholar] [CrossRef]
- De Paolis, P.; Porcellini, A.; Gigante, B.; Giliberti, R.; Lombardi, A.; Savoia, C.; Rubattu, S.; Volpe, M. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J. Hypertens. 1999, 17, 1873–1877. [Google Scholar] [CrossRef]
- Rehman, A.; Leibowitz, A.; Yamamoto, N.; Rautureau, Y.; Paradis, P.; Schiffrin, E.L. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2012, 59, 291–299. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Alcalá, M.; Fernández-Alfonso, M.S.; Steckelings, U.M.; Lorenzo, M.P.; Viana, M.; Boisvert, W.A.; Unger, T.; Gil-Ortega, M.; Somoza, B. C21 preserves endothelial function in the thoracic aorta from dio mice: Role for AT2, mas and B2 receptors. Clin. Sci. 2021, 135, 1145–1163. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Jin, X.; Wang, Z.; Siragy, H.M. Nitric oxide: A physiological mediator of the type 2 (AT2) angiotensin receptor. Acta Physiol. Scand. 2000, 168, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yayama, K.; Okamoto, H. Angiotensin ii-induced vasodilation via type 2 receptor: Role of bradykinin and nitric oxide. Int. Immunopharmacol. 2008, 8, 312–318. [Google Scholar] [CrossRef]
- Carey, R.M.; Jin, X.H.; Siragy, H.M. Role of the angiotensin AT2 receptor in blood pressure regulation and therapeutic implications. Am. J. Hypertens. 2001, 14, 98S–102S. [Google Scholar] [CrossRef]
- Dangudubiyyam, S.V.; Mishra, J.S.; Zhao, H.; Kumar, S. Perfluorooctane sulfonic acid (PFOS) exposure during pregnancy increases blood pressure and impairs vascular relaxation mechanisms in the adult offspring. Reprod. Toxicol. 2020, 98, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Luebker, D.J.; York, R.G.; Hansen, K.J.; Moore, J.A.; Butenhoff, J.L. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in sprague-dawley rats: Dose-response, and biochemical and pharamacokinetic parameters. Toxicology 2005, 215, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Luebker, D.J.; Case, M.T.; York, R.G.; Moore, J.A.; Hansen, K.J.; Butenhoff, J.L. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 2005, 215, 126–148. [Google Scholar] [CrossRef]
- Thibodeaux, J.R.; Hanson, R.G.; Rogers, J.M.; Grey, B.E.; Barbee, B.D.; Richards, J.H.; Butenhoff, J.L.; Stevenson, L.A.; Lau, C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol. Sci. 2003, 74, 369–381. [Google Scholar] [CrossRef]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Rogers, J.M.; Grey, B.E.; Stanton, M.E.; Butenhoff, J.L.; Stevenson, L.A. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: Postnatal evaluation. Toxicol. Sci. 2003, 74, 382–392. [Google Scholar] [CrossRef]
- Li, X.; Ye, L.; Ge, Y.; Yuan, K.; Zhang, Y.; Liang, Y.; Wei, J.; Zhao, C.; Lian, Q.Q.; Zhu, X.; et al. In utero perfluorooctane sulfonate exposure causes low body weights of fetal rats: A mechanism study. Placenta 2016, 39, 125–133. [Google Scholar] [CrossRef]
- Yahia, D.; Tsukuba, C.; Yoshida, M.; Sato, I.; Tsuda, S. Neonatal death of mice treated with perfluorooctane sulfonate. J. Toxicol. Sci. 2008, 33, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef]
- Wan, H.T.; Wong, A.Y.; Feng, S.; Wong, C.K. Effects of in utero exposure to perfluorooctane sulfonate on placental functions. Environ. Sci. Technol. 2020, 54, 16050–16061. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS); Office of Water Document 822-r-16-004; U.S. EPA: Washington, DC, USA, 2016. [Google Scholar]
- Zhou, Z.; Shi, Y.; Vestergren, R.; Wang, T.; Liang, Y.; Cai, Y. Highly elevated serum concentrations of perfluoroalkyl substances in fishery employees from tangxun lake, china. Environ. Sci. Technol. 2014, 48, 3864–3874. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [PubMed]
- Bosnyak, S.; Welungoda, I.K.; Hallberg, A.; Alterman, M.; Widdop, R.E.; Jones, E.S. Stimulation of angiotensin at2 receptors by the non-peptide agonist, compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br. J. Pharmacol. 2010, 159, 709–716. [Google Scholar] [CrossRef]
- Gelosa, P.; Pignieri, A.; Fändriks, L.; de Gasparo, M.; Hallberg, A.; Banfi, C.; Castiglioni, L.; Turolo, L.; Guerrini, U.; Tremoli, E.; et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: Focus on renal damage. J. Hypertens. 2009, 27, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Elkins, R.; Yallampalli, U.; Yallampalli, C. Protein restriction during pregnancy induces hypertension in adult female rat offspring—Influence of oestradiol. Br. J. Nutr. 2012, 107, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thatcher, S.E.; Cassis, L.A. Measuring blood pressure using a noninvasive tail cuff method in mice. Methods Mol. Biol. 2017, 1614, 69–73. [Google Scholar] [PubMed]
- Gopalakrishnan, K.; Mishra, J.S.; Chinnathambi, V.; Vincent, K.L.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Hankins, G.D.; Sathishkumar, K. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 2016, 67, 630–639. [Google Scholar] [CrossRef] [PubMed]
| Variable | Control | Control + C21 | PFOS | PFOS + C21 |
|---|---|---|---|---|
| Ang II pD2 | 8.73 ± 0.03 a | 8.65 ± 0.02 a | 9.03 ± 0.05 b | 8.75 ± 0.02 ac |
| Ang II Emax | 111.76 ± 2.73 | 120.33 ± 5.09 | 127.14 ± 4.84 | 121.39 ± 3.44 |
| ACh pD2 | 7.12 ± 0.11 a | 7.26 ± 0.06 a | 6.58 ± 0.07 b | 7.04 ± 0.12 ac |
| ACh Emax | 98.68 ± 0.73 a | 93.50 ± 2.20 a | 70.90 ± 4.72 b | 85.01 ± 3.72 ac |
| SNP pD2 | 6.80 ± 0.06 | 6.82 ± 0.05 | 6.84 ± 0.07 | 6.75 ± 0.03 |
| SNP Emax | 97.45 ± 4.13 | 97.79 ± 4.12 | 96.45 ± 4.64 | 97.89 ± 4.19 |
| Control | PFOS | Control + C21 | PFOS + C21 | |
|---|---|---|---|---|
| Litter size | 12.3 ± 2.6 | 11.9 ± 1.8 | 13.4 ± 0.37 | 13.4 ± 0.91 |
| Sex ratio (percent males per litter) | 47 ± 3.9% | 50 ± 4.5% | 48 ± 4.6% | 44 ± 4.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dangudubiyyam, S.V.; Bosse, B.; Yadav, P.; Song, R.; Hofmann, A.; Mishra, J.S.; Kumar, S. Restoring Angiotensin Type 2 Receptor Function Reverses PFOS-Induced Vascular Hyper-Reactivity and Hypertension in Pregnancy. Int. J. Mol. Sci. 2023, 24, 14180. https://doi.org/10.3390/ijms241814180
Dangudubiyyam SV, Bosse B, Yadav P, Song R, Hofmann A, Mishra JS, Kumar S. Restoring Angiotensin Type 2 Receptor Function Reverses PFOS-Induced Vascular Hyper-Reactivity and Hypertension in Pregnancy. International Journal of Molecular Sciences. 2023; 24(18):14180. https://doi.org/10.3390/ijms241814180
Chicago/Turabian StyleDangudubiyyam, Sri Vidya, Bradley Bosse, Pankaj Yadav, Ruolin Song, Alissa Hofmann, Jay S. Mishra, and Sathish Kumar. 2023. "Restoring Angiotensin Type 2 Receptor Function Reverses PFOS-Induced Vascular Hyper-Reactivity and Hypertension in Pregnancy" International Journal of Molecular Sciences 24, no. 18: 14180. https://doi.org/10.3390/ijms241814180





