Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke
Abstract
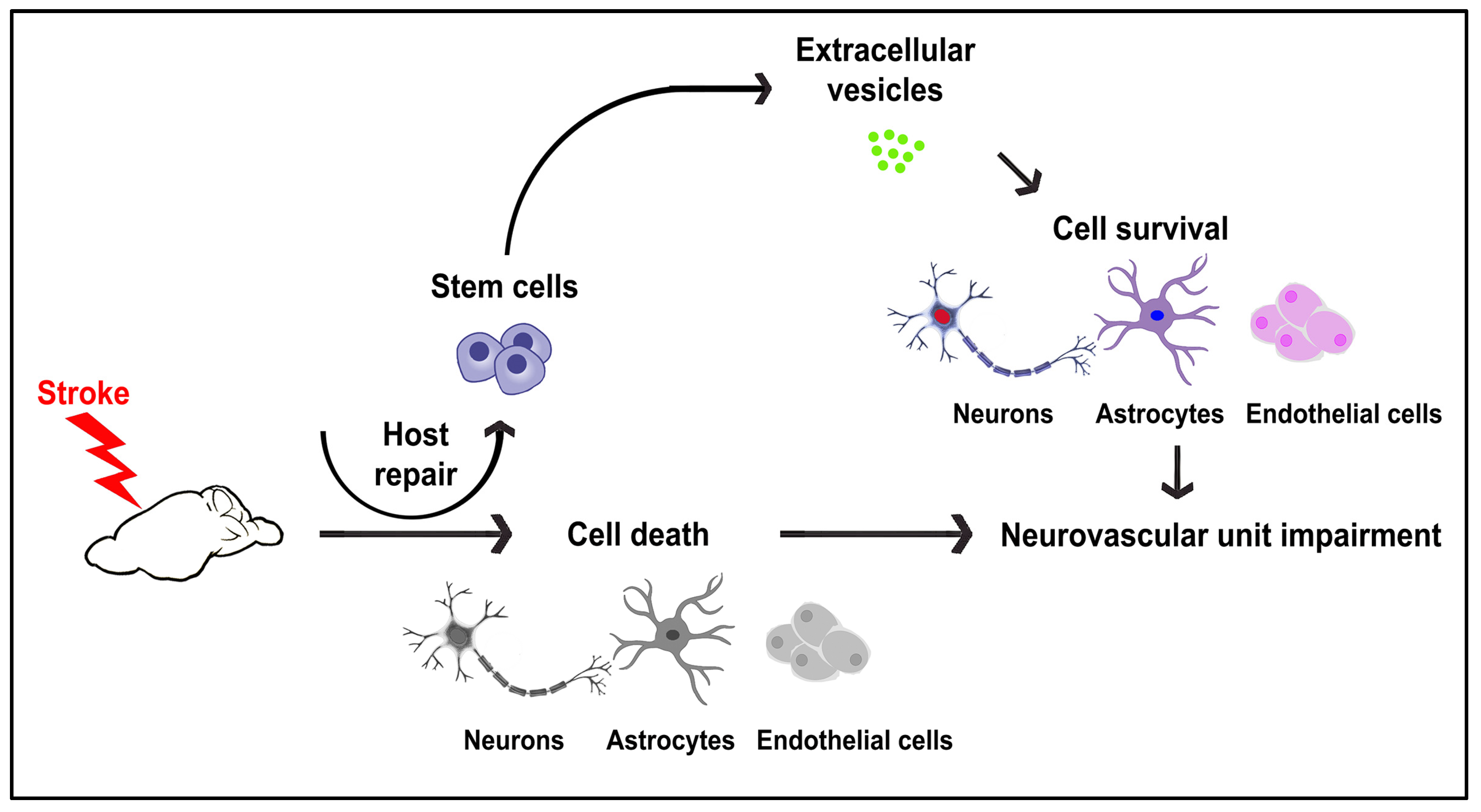
:1. Introduction
2. Results
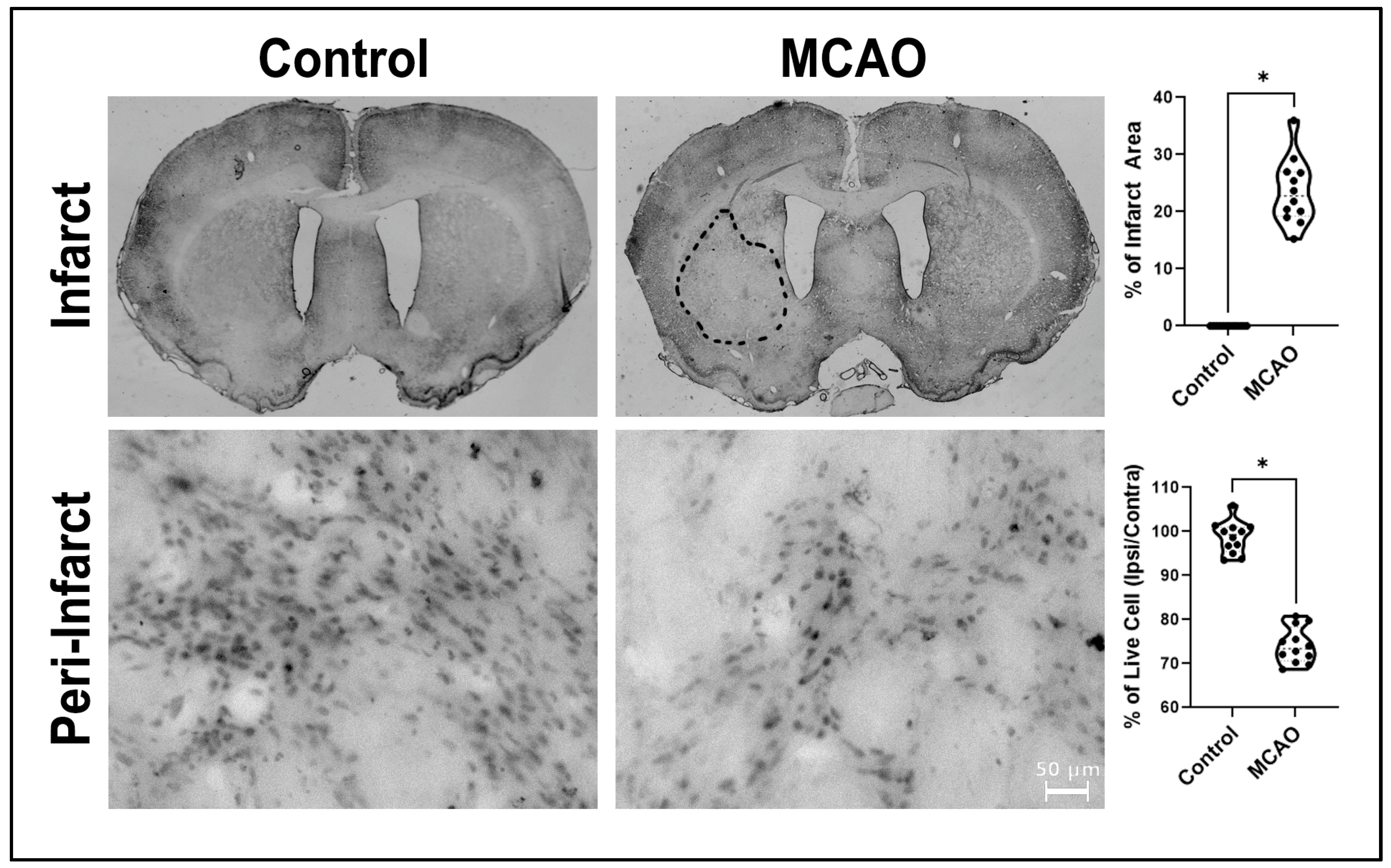
2.1. Infarct Volumes and Peri-Infarct Areas
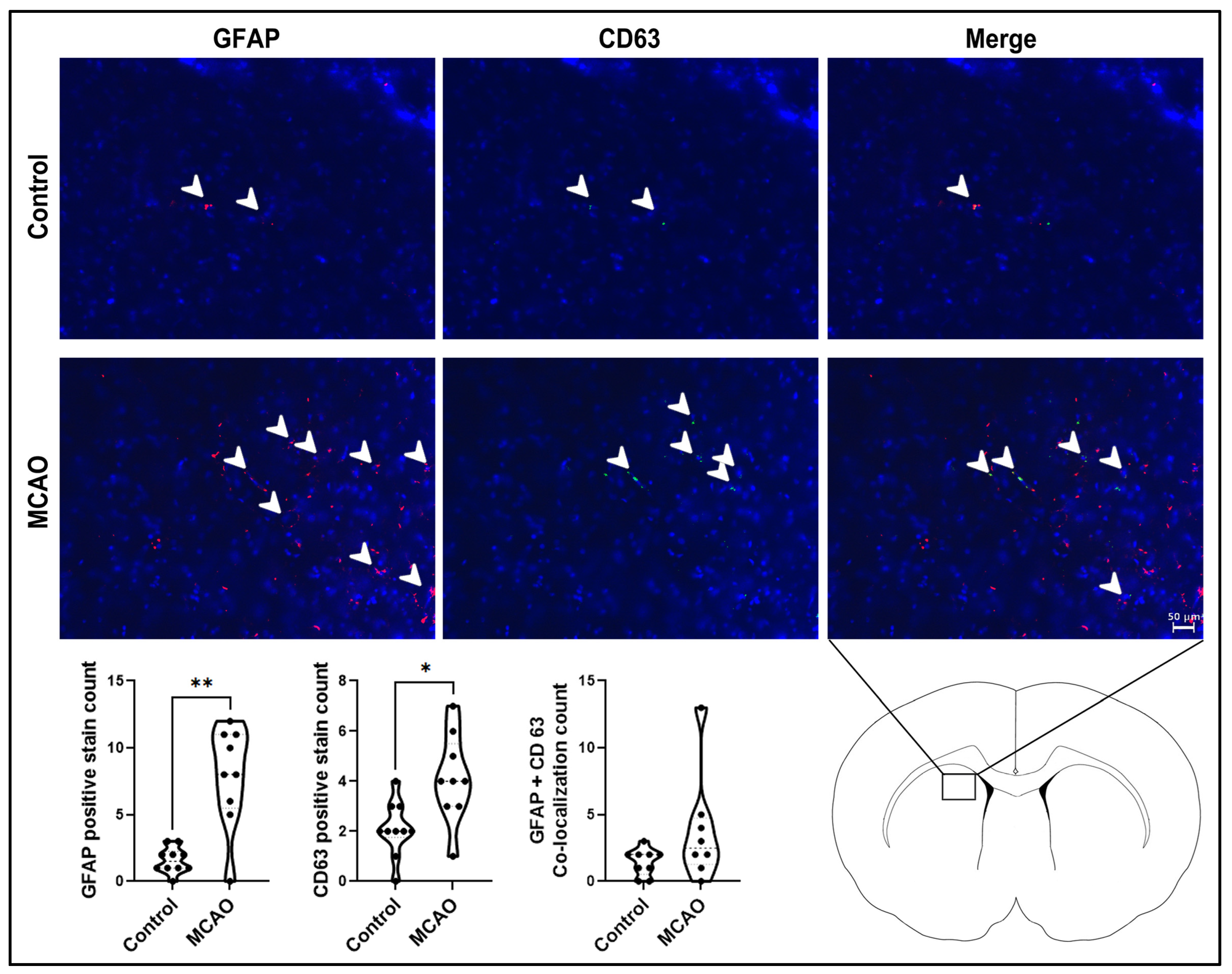
2.2. Glial Activation and Extracellular Vesicle Expression in the Stroke Brain
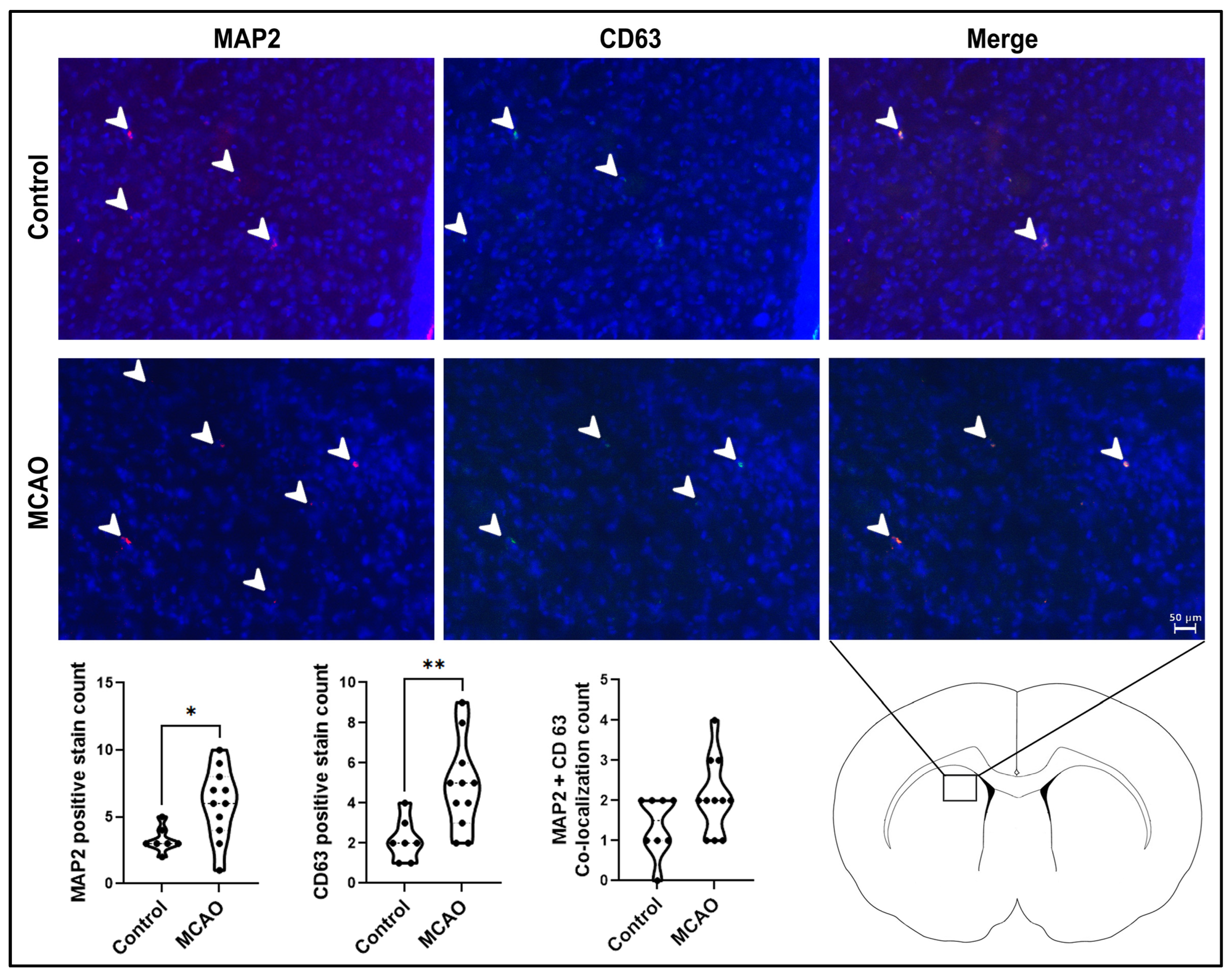
2.3. Neurogenesis and Extracellular Vesicle Expression in the Stroke Brain
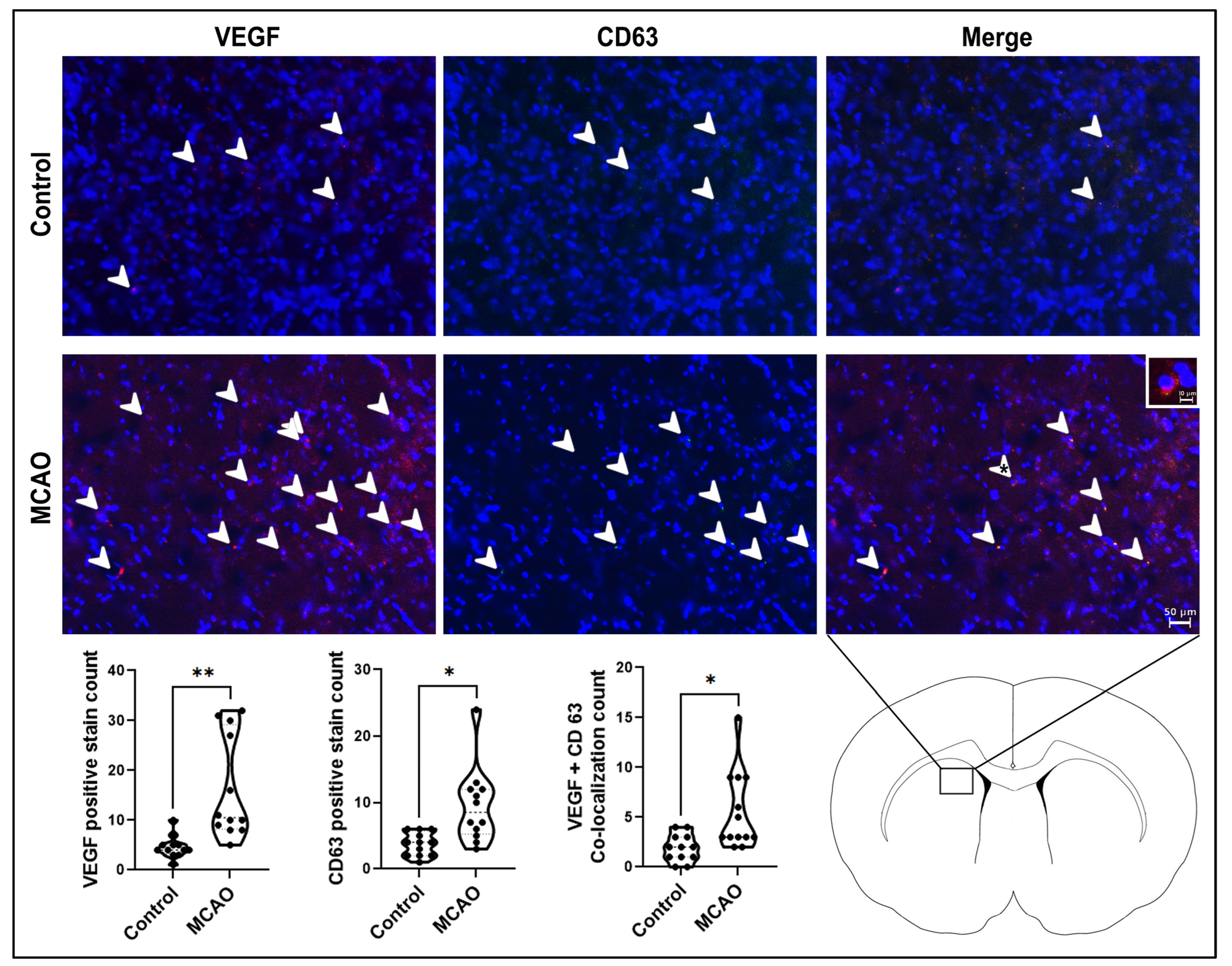
2.4. Angiogenesis and Extracellular Vesicle Expression in the Stroke Brain
3. Discussion
4. Materials and Methods
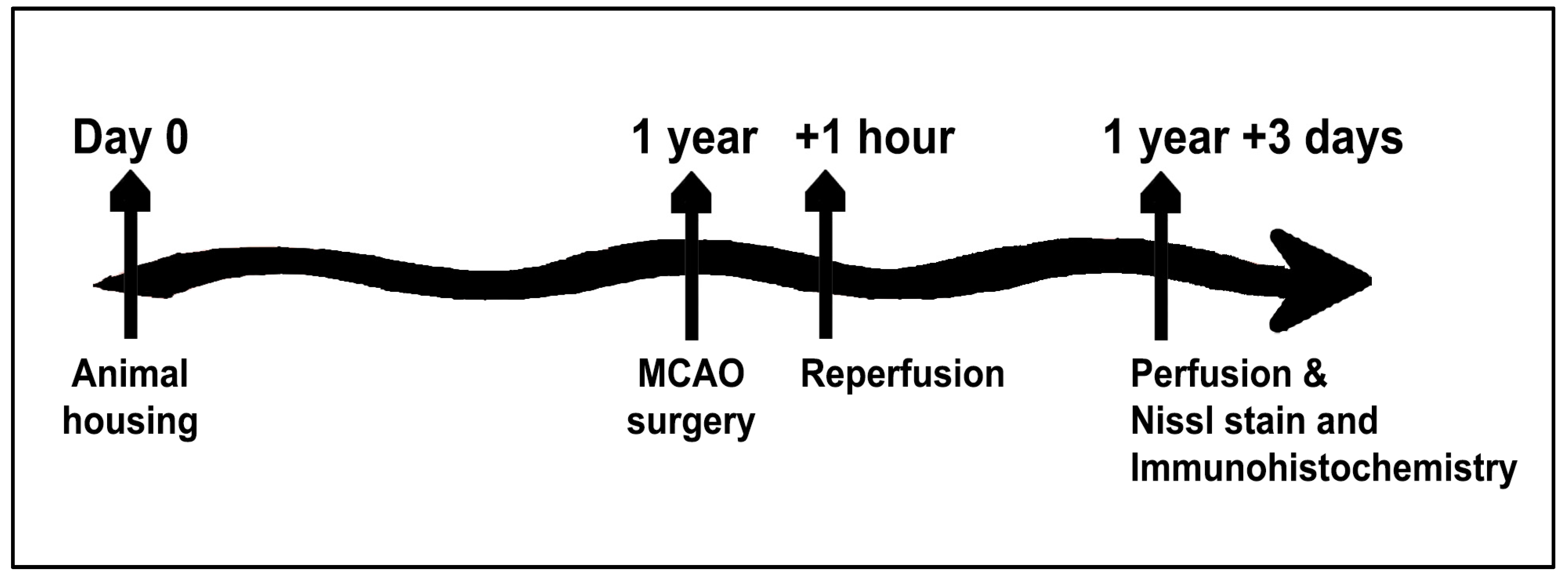
4.1. Subjects
4.2. Stroke Surgical Procedure
4.3. Perfusion
4.4. Nissl Staining
4.5. Immunohistochemistry Protocol
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective Strategies for Ischemic Stroke-Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4334. [Google Scholar] [CrossRef]
- Grossman, A.W.; Broderick, J.P. Advances and challenges in treatment and prevention of ischemic stroke. Ann. Neurol. 2013, 74, 363–372. [Google Scholar] [CrossRef]
- Lo, E.H. A new penumbra: Transitioning from injury into repair after stroke. Nat. Med. 2008, 14, 497–500. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The Science of Stroke: Mechanisms in Search of Treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef]
- Anthony, S.; Cabantan, D.; Monsour, M.; Borlongan, C.V. Neuroinflammation, Stem Cells, and Stroke. Stroke 2022, 53, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, D.; Guérit, S.; Puetz, T.; Khel, M.I.; Armbrust, M.; Dunst, M.; Macas, J.; Zinke, J.; Devraj, G.; Jia, X.; et al. Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke. Acta Neuropathol. 2022, 144, 305–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, F.; Leak, R.K.; Chen, J.; Hu, X. Regulation of Neuroinflammation through Programed Death-1/Programed Death Ligand Signaling in Neurological Disorders. Front. Cell Neurosci. 2014, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Borlongan, C.V. Neurovascular unit permeability in neuroinflammatory diseases: A common pathologic and therapeutic target? Neural Regen. Res. 2023, 18, 1715. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Hess, D.C. New hope for stroke patients: Mobilization of endogenous stem cells. CMAJ 2006, 174, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, T.; Huang, Q.; Liu, W.; Yang, Y.; Jin, Y.; Wu, D.; Yuan, K.; Zhang, P. The roles, mechanism, and mobilization strategy of endogenous neural stem cells in brain injury. Front. Aging Neurosci. 2022, 14, 924262. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Saleki, K.; Javanmehr, N.; Khodaparast, J.; Saadat, P.; Nouri, H.R. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res. Rev. 2020, 62, 101106. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.M.; Kaiser, J.; Schwab, M.E. Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends Neurosci. 2018, 41, 360–372. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front. Cell Dev. Biol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef]
- Ahmad, S.; Srivastava, R.K.; Singh, P.; Naik, U.P.; Srivastava, A.K. Role of Extracellular Vesicles in Glia-Neuron Intercellular Communication. Front. Mol. Neurosci. 2022, 15, 844194. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Zheng, X.; Hermann, D.M.; Bähr, M.; Doeppner, T.R. The role of small extracellular vesicles in cerebral and myocardial ischemia—Molecular signals, treatment targets, and future clinical translation. Stem Cells 2021, 39, 403–413. [Google Scholar] [CrossRef]
- Bang, O.Y.; Kim, E.H. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front. Neurol. 2019, 10, 211. [Google Scholar] [CrossRef]
- Son, J.P.; Kim, E.H.; Shin, E.K.; Kim, D.H.; Sung, J.H.; Oh, M.J.; Cha, J.M.; Chopp, M.; Bang, O.Y. Mesenchymal Stem Cell-Extracellular Vesicle Therapy for Stroke: Scalable Production and Imaging Biomarker Studies. Stem Cells Transl. Med. 2023, 12, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yuan, Q.; Guo, L.; Xiao, G.; Zhang, T.; Liang, B.; Yao, R.; Zhu, Y.; Li, Y.; Hu, L. Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice. Pharmaceuticals 2022, 15, 1287. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Y.; Xue, C.; Ma, B.; Shen, Y.; Guan, J.; Bao, X.; Wu, H.; Han, Q.; Wang, R.; et al. Protection of Nerve Injury with Exosome Extracted from Mesenchymal Stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016, 38, 33–36. [Google Scholar] [CrossRef]
- Harrell, C.R.; Volarevic, V.; Djonov, V.; Volarevic, A. Therapeutic Potential of Exosomes Derived from Adipose Tissue-Sourced Mesenchymal Stem Cells in the Treatment of Neural and Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 4487. [Google Scholar] [CrossRef]
- Brohlin, M.; Mahay, D.; Novikov, L.N.; Terenghi, G.; Wiberg, M.; Shawcross, S.G.; Novikova, L.N. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci. Res. 2009, 64, 41–49. [Google Scholar] [CrossRef]
- Cong, M.; Shen, M.; Wu, X.; Li, Y.; Wang, L.; He, Q.; Shi, H.; Ding, F. Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res. Ther. 2021, 12, 80. [Google Scholar] [CrossRef]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Noroozian, M.; Heidari, M.H.; Piryaei, A. Mesenchymal Stem Cells as an Alternative for Schwann Cells in Rat Spinal Cord Injury. Iran Biomed. J. 2013, 17, 113–122. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Man, A.J.; Kujawski, G.; Burns, T.S.; Miller, E.N.; Fierro, F.A.; Leach, J.K.; Bannerman, P. Neurogenic potential of engineered mesenchymal stem cells overexpressing VEGF. Cell Mol. Bioeng. 2016, 9, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Chang, M.-S.; Koh, S.-H.; Choi, Y.K. Repair Mechanisms of the Neurovascular Unit after Ischemic Stroke with a Focus on VEGF. Int. J. Mol. Sci. 2021, 22, 8543. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chai, Y.; Lv, S.; Ye, M.; Wu, M.; Xie, L.; Fan, Y.; Zhu, X.; Gao, Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 2017, 40, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Schnatz, A.; Müller, C.; Brahmer, A.; Krämer-Albers, E. Extracellular Vesicles in Neural Cell Interaction and CNS Homeostasis. FASEB Bioadv. 2021, 3, 577–592. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell. Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ali Moussa, H.Y.; Manaph, N.; Ali, G.; Maacha, S.; Shin, K.C.; Ltaief, S.M.; Gupta, V.; Tong, Y.; Ponraj, J.; Salloum-Asfar, S.; et al. Single Extracellular Vesicle Analysis Using Flow Cytometry for Neurological Disorder Biomarkers. Front. Integr. Neurosci. 2022, 16, 879832. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, W.; Niu, L.; Bao, W.; Wang, Y.; Wang, Y.; Zhu, Y.; Yang, Z.; Chen, J.; Dong, J.; et al. NSC-Derived Exosomes Enhance Therapeutic Effects of NSC Transplantation on Cerebral Ischemia in Mice. eLife 2023, 12, e84493. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.; Ruf, A.; Mansmann, U.; Winter, R.; Schuler, M.; Buggle, F.; Mayer, H.; Grau, A.J. Course of platelet activation markers after ischemic stroke. Stroke 2002, 33, 2570–2574. [Google Scholar] [CrossRef]
- Cha, J.-K.; Jeong, M.-H.; Jang, J.-Y.; Bae, H.-R.; Lim, Y.-J.; Kim, J.S.; Kim, S.-H.; Kim, J.W. Serial measurement of surface expressions of CD63, P-selectin and CD40 ligand on platelets in atherosclerotic ischemic stroke. A possible role of CD40 ligand on platelets in atherosclerotic ischemic stroke. Cerebrovasc. Dis. 2003, 16, 376–382. [Google Scholar] [CrossRef]
- Grau, A.J.; Reiners, S.; Lichy, C.; Buggle, F.; Ruf, A. Platelet Function Under Aspirin, Clopidogrel, and Both After Ischemic Stroke. Stroke 2003, 34, 849–854. [Google Scholar] [CrossRef]
- Cha, J.-K.; Jo, W.-S.; Shin, H.C.; Bae, H.-R.; Ho, J.-M.; Kim, J.W. Increased platelet CD63 and P-selectin expression persist in atherosclerotic ischemic stroke. Platelets 2004, 15, 3–7. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Goodall, S.; Crowther, M.; Jones, L.; Bell, P.R.; Thompson, M.M. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 2000, 31, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.-W.; Chang, W.-N.; Shaw, C.-F.; Jan, C.-R.; Chang, H.-W.; Huang, C.-R.; Chen, S.-D.; Chuang, Y.-C.; Lee, L.-H.; Lu, C.-H. Serial change in platelet activation markers with aspirin and clopidogrel after acute ischemic stroke. Clin. Neuropharmacol. 2010, 33, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Fateh-Moghadam, S.; Htun, P.; Tomandl, B.; Sander, D.; Stellos, K.; Geisler, T.; Langer, H.; Walton, K.; Handschu, R.; Garlichs, C.; et al. Hyperresponsiveness of platelets in ischemic stroke. Thromb. Haemost. 2007, 97, 974–978. [Google Scholar] [PubMed]
- Tsai, N.-W.; Chang, W.-N.; Shaw, C.-F.; Jan, C.-R.; Chang, H.-W.; Huang, C.-R.; Chen, S.-D.; Chuang, Y.-C.; Lee, L.-H.; Wang, H.-C.; et al. Levels and value of platelet activation markers in different subtypes of acute non-cardio-embolic ischemic stroke. Thromb. Res. 2009, 124, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Yamamoto, M.; Takei, N.; Kumazaki, M.; Ungsuparkorn, C.; Hida, H.; Sanberg, P.R.; Nishino, H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000, 14, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Roseborough, A.D.; Zhu, Y.; Zhao, L.; Laviolette, S.R.; Pasternak, S.H.; Whitehead, S.N. Fibrinogen primes the microglial NLRP3 inflammasome and propagates pro-inflammatory signaling via extracellular vesicles: Implications for blood-brain barrier dysfunction. Neurobiol. Dis. 2023, 177, 106001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, A.; Fan, H.; Li, Y.; Yang, N.; Tang, Y. Astrocyte-Derived Extracellular Vesicles for Ischemic Stroke: Therapeutic Potential and Prospective. Aging Dis. 2023. ahead of print. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Shi, Y.; Xiao, Y.; Yin, Y.; Jiang, B.; Ren, H.; Chen, W.; Xue, Q.; Xu, X. Reconstituting neurovascular unit with primary neural stem cells and brain microvascular endothelial cells in three-dimensional matrix. Brain Pathol. 2021, 31, e12940. [Google Scholar] [CrossRef]
- Lin, R.; Cai, J.; Kenyon, L.; Iozzo, R.; Rosenwasser, R.; Iacovitti, L. Systemic Factors Trigger Vasculature Cells to Drive Notch Signaling and Neurogenesis in Neural Stem Cells in the Adult Brain. Stem Cells 2019, 37, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Sekine, H.; Homma, J.; Kobayashi, T.; Kobayashi, E.; Kawamata, T.; Shimizu, T. Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J. Neurosurg. 2019, 132, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Xin, W.; Bähr, M.; Giebel, B.; Doeppner, T.R. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics 2022, 12, 5776–5802. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Hira, K.; Miyamoto, N.; Kijima, C.; Inaba, T.; Hattori, N. Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery. Int. J. Mol. Sci. 2020, 21, 6894. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, L.; Hashemi, S.M.; Saadatnia, M.; Zali, A.; Oraee-Yazdani, S.; Heidari Keshel, S.; Khojasteh, A.; Soleimani, M. Stem Cell-Derived Exosomes as Treatment for Stroke: A Systematic Review. Stem Cell Rev. Rep. 2021, 17, 428–438. [Google Scholar] [CrossRef]
- Kawauchi, S.; Yasuhara, T.; Kin, K.; Yabuno, S.; Sugahara, C.; Nagase, T.; Hosomoto, K.; Okazaki, Y.; Tomita, Y.; Umakoshi, M.; et al. Transplantation of modified human bone marrow-derived stromal cells affords therapeutic effects on cerebral ischemia in rats. CNS Neurosci. Ther. 2022, 28, 1974–1985. [Google Scholar] [CrossRef]
- Pianta, S.; Lee, J.Y.; Tuazon, J.P.; Castelli, V.; Mantohac, L.M.; Tajiri, N.; Borlongan, C.V. A Short Bout of Exercise Prior to Stroke Improves Functional Outcomes by Enhancing Angiogenesis. Neuromol. Med. 2019, 21, 517–528. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, J.; Vignon, C.; D’Egidio, F.; Streefkerk, H.; de Kalbermatten, M.; Garitaonandia, I.; Borlongan, C.V. Probing multiple transplant delivery routes of CD+34 stem cells for promoting behavioral and histological benefits in experimental ischemic stroke. Stem Cells Transl. Med. 2023, in press. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, M.M.; Salazar, F.E.; Rodriguez, T.; D’Egidio, F.; Borlongan, C.V.; Lee, J.-Y. Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke. Int. J. Mol. Sci. 2023, 24, 16857. https://doi.org/10.3390/ijms242316857
Alvarez MM, Salazar FE, Rodriguez T, D’Egidio F, Borlongan CV, Lee J-Y. Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke. International Journal of Molecular Sciences. 2023; 24(23):16857. https://doi.org/10.3390/ijms242316857
Chicago/Turabian StyleAlvarez, Mauricio Muleiro, Felipe Esparza Salazar, Thomas Rodriguez, Francesco D’Egidio, Cesar V. Borlongan, and Jea-Young Lee. 2023. "Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke" International Journal of Molecular Sciences 24, no. 23: 16857. https://doi.org/10.3390/ijms242316857
APA StyleAlvarez, M. M., Salazar, F. E., Rodriguez, T., D’Egidio, F., Borlongan, C. V., & Lee, J.-Y. (2023). Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke. International Journal of Molecular Sciences, 24(23), 16857. https://doi.org/10.3390/ijms242316857






