Low-Dose Sodium Salicylate Promotes Ovulation by Regulating Steroids via CYP17A1
Abstract
1. Introduction
2. Results
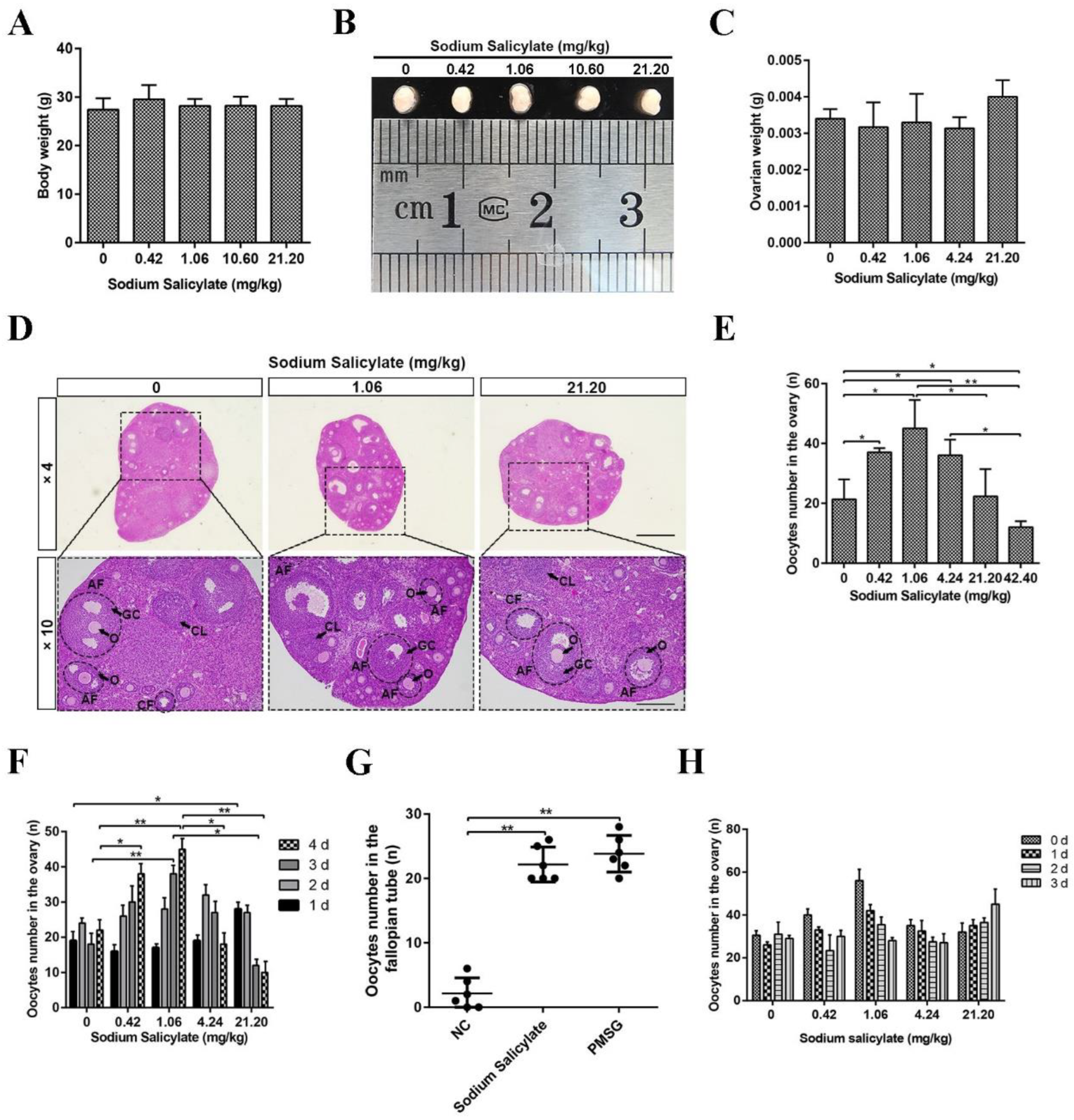
2.1. Low Doses of Sodium Salicylate Promoted Ovulation
2.2. Mouse Oocyte Quality Was Not Affected by Sodium Salicylate
2.3. Sodium Salicylate Affected the Levels of Hormones and Leukocytes in Mice
2.4. Sodium Salicylate Activated P38 Signaling Pathway and the Key Protein CYP17A1
2.5. Abiraterone Acetate Inhibited the Effect of Sodium Salicylate on Excretion
2.6. Superovulation Was Induced by Sodium Salicylate and FSH/PMSG
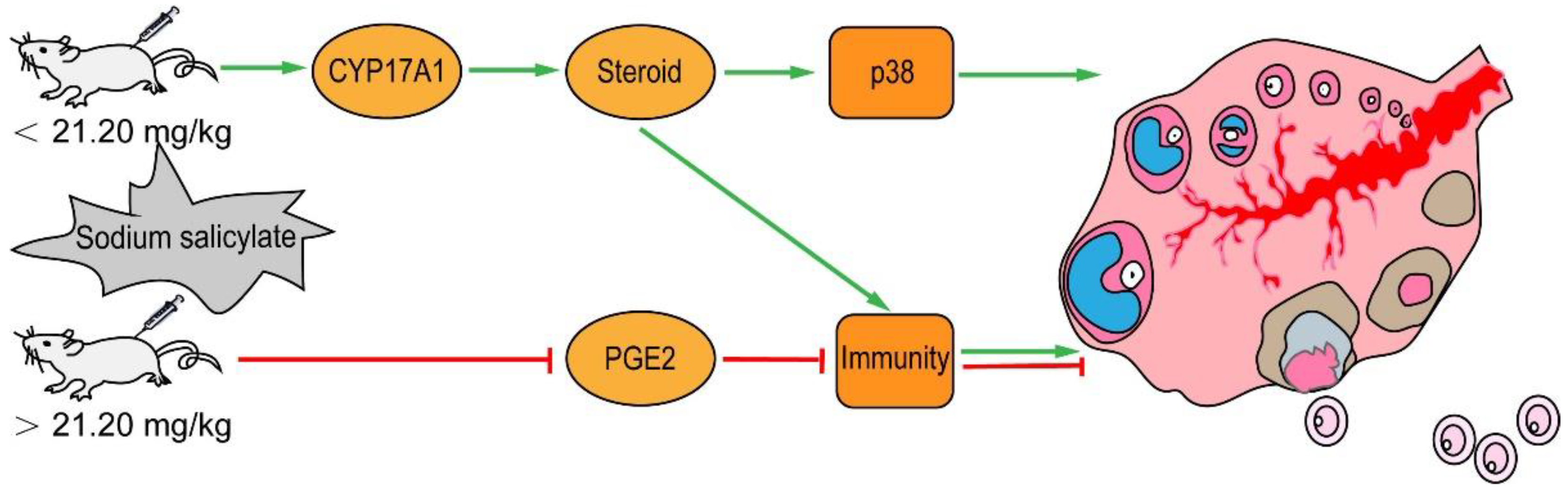
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Treatment
4.3. Collection of Oocytes and Embryo
4.3.1. Collection of Mouse Oocytes
4.3.2. Collection of Embryos from Awang Sheep
4.4. In Vitro Fertilization
4.5. Parthenogenetic Activation
4.6. Detection of ROS Levels in Oocytes
4.7. Detection of GSH Level in Oocyte
4.8. Mitochondrial Membrane Potential Determination in Oocytes
4.9. Cell Culture and Treatment
4.10. RNA Isolation, Reverse Transcription, and Quantitative PCR
4.11. Western Blot Analysis
4.12. Histological Analysis
4.13. Immunofluorescence Analysis
4.14. Immunohistochemistry
4.15. Hormone Determination
4.16. Leukocyte Counts
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Sodium salicylate |
| PMSG | Pregnant horse serum gonadotropin |
| FSH | Follicle-stimulating hormone |
| CYP17A1 | Cytochrome P45017A1 |
| LH | Luteinizing hormone |
| HCG | Human chorionic gonadotropin |
| MAPK | Mitogen-activated protein kinase |
| AMPK | Adenosine 5’-monophosphate (AMP)-activated protein kinase |
| PG | Prostaglandin |
| PGE2 | Prostaglandin E2 |
| COX2 | Cyclooxygenase 2 |
| CAMP | Cyclic adenosine monophosphate |
| NSAID | Non-steroidal anti-inflammatory drug |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| IL-8 | Interleukin-8 |
| AA | Abiraterone acetate |
| ROS | Reactive oxygen species |
| GSH | Glutathione |
| GC | Granulosa cells |
| GV | Germinal vesicle |
| GVBD | Germinal vesicle breakdown |
| PB1 | Polar body first |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PI3K | Phosphatidylinositol 3-kinase |
| JNK | c-Jun N-terminal kinase |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| DAPI | 4’,6-Diamidino-2-phenylindole |
| DAB | 3,3’-Diaminobenzidine |
References
- Savy, V.; Stein, P.; Shi, M.; Williams, C.J. Superovulation does not alter calcium oscillations following fertilization. Front. Cell Dev. Biol. 2021, 9, 762057. [Google Scholar] [CrossRef] [PubMed]
- Bata, M.S.; Al-Ramahi, M.; Salhab, A.S.; Gharaibeh, M.N.; Schwartz, J. Delay of ovulation by meloxicam in healthy cycling volunteers: A placebo-controlled, double-blind, crossover study. J. Clin. Pharmacol. 2006, 46, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Shao, Y.X.; Wang, P. The progress of research on animals superovulation technology. Lab. Anim. Comp. Med. 2007, 27–32. [Google Scholar]
- Zhang, J.; Wei, H.J.; Chen, X.M.; Song, X.C. Ruminant superovulation technology research and the application in the cervid. China Anim. Husb. Vet. Med. 2012, 39, 39–44. [Google Scholar]
- Ertzeid, G.; Storeng, R. Adverse effects of gonadotrophin treatment on pre- and postimplantation development in mice. J. Reprod. Fertil. 1992, 96, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ingrid, V.D.A.; Hooghe, D. Superovulation of female mice delays embryonic and fetal development. Hum. Reprod. 2001, 16, 1237–1243. [Google Scholar]
- Walton, E.A.; Evans, G.; Armstrong, D.T. Ovulation response and fertilization failure in immature rats induced to superovulate. J. Reprod. Fertil. 1983, 67, 91–96. [Google Scholar] [CrossRef]
- Van, D.; Pijnenborg, R.; Koninckx, P.R. The influence of in-vitro culture versus stimulated and untreated oviductal environment on mouse embryo development and implantation. Hum. Reprod. 1999, 14, 2570–2574. [Google Scholar]
- Walton, E.A. Possible causes of implantation failure in superovulated immature rats. Biol. Reprod. 1982, 27, 847–852. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.M.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with inflammatory processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Choi, Y.; Wilson, K.; Hannon, P.R.; Rosewell, K.L.; Brännström, M.; Akin, J.W.; Curry, T.E., Jr.; Jo, M. Coordinated regulation among progesterone, prostaglandins, and EGF-Like factors in human ovulatory follicles. J. Clin. Endocrinol. Metab. 2017, 102, 1971–1982. [Google Scholar] [CrossRef]
- Robker, R.L.; Russell, D.L.; Espey, L.L.; Lydon, J.P.; O’Malley, B.W.; Richards, J.S. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc. Natl. Acad. Sci. USA 2000, 97, 4689–4694. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sato, M.; Li, Q.X.; Lydon, J.P.; Demayo, F.J.; Bagchi, I.C.; Bagchi, M.K. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol. Cell Biol. 2008, 28, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.M.; Hunzicker-Dunn, M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Dev. Biol. 1995, 172, 72–85. [Google Scholar] [CrossRef]
- Ben-Ami, I.; Freimann, S.; Armon, L.; Dantes, A.; Strassburger, D.; Friedler, S.; Raziel, A.; Seger, R.; Ron-El, R.; Amsterdam, A. PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: New insights into the coordination between PGE2 and LH in ovulation. Mol. Hum. Reprod. 2006, 12, 593–599. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Xu, F.; Stouffer, R.L.; Müller, J.; Hennebold, J.D.; Wright, J.W.; Bahar, A.; Leder, G.; Peters, M.; Thorne, M.; Sims, M.; et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol. Hum. Reprod. 2011, 17, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.C.; Armstrong, D.T. Interactions of steroids and gonadotropins in the control of steroidogenesis in the ovarian follicle. Ann. Rev. Physiol. 1980, 42, 71–82. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Moncada, S.; Vane, J.R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat. New Biol. 1971, 231, 237–239. [Google Scholar] [CrossRef]
- Wu, K.K.; Sanduja, R.; Tsai, A.L.; Ferhanoglu, B.; Loose-Mitchell, D.S. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc. Natl. Acad. Sci. USA 1991, 88, 2384–2387. [Google Scholar] [CrossRef]
- Saunders, M.A.; Sansores-Garcia, L.; Gilroy, D.W.; Wu, K.K. Selective Suppression of CCAAT/Enhancer-binding protein β binding and cyclooxygenase-2 promoter activity by sodium salicylate in quiescent human fibroblasts. J. Biol. Chem. 2001, 276, 18897–18904. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Murphy, S. Inhibition of NF kappa B by sodium salicylate and aspirin. Since 1994, 265, 956–959. [Google Scholar]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Saunders, M.; Barnes, P.J.; Newton, R.; Belvisi, M.G. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (Nuclear Factor κB) activation: Role of arachidonic acid. Mol. Pharmacol. 1997, 51, 907–912. [Google Scholar] [CrossRef]
- Giuliano, F.; Mitchell, J.A.; Warner, T.D. Sodium salicylate inhibits prostaglandin formation without affecting the induction of cyclooxygenase-2 by bacterial lipopolysaccharide in vivo. J. Pharmacol. Exp. Ther. 2001, 299, 894–900. [Google Scholar]
- Steinberg, G.R.; Dandapani, M.; Hardie, D.G. AMPK: Mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol. Metab. 2013, 24, 481–487. [Google Scholar] [CrossRef]
- Andrew, J.; Villani, L.A.; Broadfield, L.A.; Houde, V.P.; Galic, S.; Blandino, G.; Kemp, B.E.; Tsakiridis, T.; Muti, P.; Steinberg, G.R. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem. J. 2015, 469, 177–187. [Google Scholar]
- Missaghian, E.; Kempná, P.; Dick, B.; Hirsch, A.; Alikhani-Koupaei, R.; Jégou, B.; Mullis, P.E.; Frey, B.M.; Flück, C.E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. [Google Scholar] [CrossRef]
- Duffy, D.M.; Dozier, B.L.; Seachord, C.L. Prostaglandin dehydrogenase and prostaglandin levels767 in periovulatory follicles: Implications for control of primate ovulation by prostaglandin E2. J. Clin. Endocrinol. Metab. 2005, 90, 1021–1027. [Google Scholar] [CrossRef]
- Duffy, D.M.; Stouffer, R.L. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol. Hum. Reprod. 2001, 7, 731–739. [Google Scholar] [CrossRef]
- Murdoch, W.J.; Peterson, T.A.; Kirk, E.A.V.; Vincent, D.L.; Inskeep, E.K. Interactive roles of progesterone, prostaglandins, and collagenase in the ovulatory mechanism of the ewe. Biol. Reprod. 1986, 35, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, M.L.; Stouffer, R.L.; Wolf, D.P. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc. Natl. Acad. Sci. USA 1996, 93, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.; Mikuni, M.; Mitsube, K.; Brnnstrm, M. Time-dependent ovulation inhibition of a selective progesterone-receptor antagonist (Org 31710) and Effects on ovulatory mediators in the in vitro perfused rat ovary. Biol. Reprod. 2000, 63, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Uilenbroek, J.T.; Sánchez-Criado, J.E.; Karels, B. Decreased luteinizing hormone-stimulated progesterone secretion by preovulatory follicles isolated from cyclic rats treated with the progesterone antagonist RU486. Biol. Reprod. 1992, 47, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Robertson, S.A.; Gonzalez, M.B.; Richards, J.S.; Smith, C.W.; Russell, D.L.; Robker, R.L. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am. J. Reprod. Immunol. 2018, 79, e12835. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Martinez, E.; Garcia-Ruiz, G.; Flores-Espinosa, P.; Bermejo-Martinez, L.; Zaga-Clavellina, V. Progesterone suppresses the lipopolysaccharide-induced inflammatory response in mononuclear cells isolated from human term placenta. Immunol. Investig. 2018, 47, 181–195. [Google Scholar] [CrossRef]
- Santos, S.J.; Aupperlee, M.D.; Xie, J.; Durairaj, S.; Miksicek, R.; Conrad, S.E.; Leipprandt, J.R.; Tan, Y.S.; Schwartz, R.C.; Haslam, S.Z. Progesterone receptor A-regulated gene expression in mammary organoid cultures. J. Steroid Biochem. Mol. Biol. 2009, 115, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.T.; Kou, X.X.; Li, C.S.; Bi, R.Y.; Meng, Z.; Wang, X.D.; Zhou, Y.H.; Gan, Y.H. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-κB pathway in ovariectomized rats. Sci. Rep. 2017, 7, 15334. [Google Scholar] [CrossRef] [PubMed]
- Orhan, B.; Aydin, A. Leukocytes in ovarian function. Hum. Reprod. Update 2000, 6, 1–15. [Google Scholar]
- Ujioka, T. Interleukin-8 as an essential factor in the human chorionic gonadotropin-induced rabbit ovulatory process: Interleukin-8 induces neutrophil accumulation and activation in ovulation. Biol. Reprod. 1998, 58, 526–530. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ward, M.F.; Sama, A.E.; Wang, H. Extracellular HMGB1 as a Proinflammatory Cytokine. J. Interf. Cytokine Res. 2004, 24, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, V.B.; Murphy, L.L.; O’Conner, J.L. Selective Modulation of FSH and LH Secretion by Steroids. Adv Exp Med Biol. 1987, 219, 131–152. [Google Scholar] [PubMed]
- Liva, S.M.; Voskuhl, R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shan, L.; Hardy, M.; Bardin, C.; Sundaram, K. Mechanism of androgen-induced thymolysis in rats. Endocrinology 1995, 136, 4887–4893. [Google Scholar] [CrossRef]
- Olsen, N.J.; Olson, G.; Viselli, S.M.; Gu, X.; Kovacs, W.J. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 2001, 142, 1278–1283. [Google Scholar] [CrossRef]
- Staples, J.E.; Gasiewicz, T.A.; Fiore, N.C.; Lubahn, D.B.; Korach, K.S.; Silverstone, A.E. Estrogen Receptor α Is Necessary in Thymic Development and Estradiol-Induced Thymic Alterations. J. Immunol. 1999, 163, 4168–4174. [Google Scholar] [CrossRef]
- Altuwaijri, S.; Chuang, K.-H.; Lai, K.-P.; Lai, J.-J.; Lin, H.-Y.; Young, F.M.; Bottaro, A.; Tsai, M.-Y.; Zeng, W.-P.; Chang, H.-C.; et al. Susceptibility to Autoimmunity and B Cell Resistance to Apoptosis in Mice Lacking Androgen Receptor in B Cells. Mol. Endocrinol. 2009, 23, 444–453. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar]
- Brown, M.A.; Su, M.A. An Inconvenient Variable: Sex Hormones and Their Impact on T Cell Responses. J. Immunol. 2019, 202, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Hwang, J.T.; Kwak, D.W.; Lee, Y.K.; Park, O.J. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann. New York Acad. Sci. 2007, 1095, 496–503. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Primer (5’→3’) | Reverse Primer (5’→3’) | Product Size, bp |
|---|---|---|---|
| Cox2 Cyp17a1 VEGF Star Cyp19a1 | CAGCCAGGCAGCAAATCCTT TGGAGGCCACTATCCGAGAA GCACTGGACCCTGGCTTTACT AACGGGGACGAAGTGCTAAG ATCCGGTTTTTAAACGGCTGC | GTCCGGGTACAGTCACACTT GAAGCGCTCAGGCATAAACC TCTCAATCGGACGGCAGTAG CCTCTGCAGGACCTTGATCTC TCTTGCGCTATTTGGCCTGG | 99 194 143 168 100 |
| β-actin | TCTTTTCCAGCCTTCCTTCTTG | GTTGGCATAGAGGTCTTTACGGA | 109 |
| Antibody | Species Source | Supplier | Identifier | Dilution | |
|---|---|---|---|---|---|
| WB | IF/IHC | ||||
| P-AMPK | Rabbit | Abcam | ab133448 | 1:1000 | |
| CYP17A1 | Rabbit | Proteintech | 14447-1-AP | 1:1000 | 1:200 |
| COX2 | Rabbit | Proteintech | 12375-1-AP | 1:1000 | |
| PKA | Rabbit | Proteintech | 55388-1-AP | 1:1000 | |
| TNF-α | Mouse | Proteintech | 60291-1-Ig | 1:1000 | |
| HMGB1 | Rabbit | Proteintech | 10829-1-AP | 1:1000 | |
| H3K4me3 | Rabbit | Cell Signaling Technology | 9751 | 1:1000 | |
| H3K9me3 | Rabbit | Cell Signaling Technology | 13969 | 1:1000 | |
| H3K27me3 | Rabbit | Cell Signaling Technology | 9733 | 1:1000 | |
| H3 | Rabbit | Proteintech | 17168-1-AP | 1:1000 | |
| AMPK | Mouse | Santa Cruz Biotechnology | sc-74461 | 1:500 | |
| JNK | Mouse | Cell Signaling Technology | 9252 | 1:1000 | |
| P-JNK | Rabbit | Cell Signaling Technology | 4668 | 1:1000 | |
| p38 | Mouse | Santa Cruz Biotechnology | sc-7972 | 1:500 | |
| P-p38 | Mouse | Santa Cruz Biotechnology | sc-166182 | 1:500 | |
| β-actin | Mouse | CWBIO | CW0096 | 1:2000 | |
| CD45 | Mouse | Santa Cruz Biotechnology | sc-1178 | 1:500 | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ren, X.; Li, T.; Yu, L.; Teng, M.; Zheng, Y.; Lei, A. Low-Dose Sodium Salicylate Promotes Ovulation by Regulating Steroids via CYP17A1. Int. J. Mol. Sci. 2023, 24, 2579. https://doi.org/10.3390/ijms24032579
Li T, Ren X, Li T, Yu L, Teng M, Zheng Y, Lei A. Low-Dose Sodium Salicylate Promotes Ovulation by Regulating Steroids via CYP17A1. International Journal of Molecular Sciences. 2023; 24(3):2579. https://doi.org/10.3390/ijms24032579
Chicago/Turabian StyleLi, Tao, Xuehua Ren, Tianjiao Li, Lian Yu, Mingming Teng, Yi Zheng, and Anmin Lei. 2023. "Low-Dose Sodium Salicylate Promotes Ovulation by Regulating Steroids via CYP17A1" International Journal of Molecular Sciences 24, no. 3: 2579. https://doi.org/10.3390/ijms24032579
APA StyleLi, T., Ren, X., Li, T., Yu, L., Teng, M., Zheng, Y., & Lei, A. (2023). Low-Dose Sodium Salicylate Promotes Ovulation by Regulating Steroids via CYP17A1. International Journal of Molecular Sciences, 24(3), 2579. https://doi.org/10.3390/ijms24032579






