QTL Mapping and a Transcriptome Integrative Analysis Uncover the Candidate Genes That Control the Cold Tolerance of Maize Introgression Lines at the Seedling Stage
Abstract
1. Introduction
2. Results
2.1. Evaluation and Screening of the Tolerance of MILs to Cold
2.2. Phenotypic Identification and Genetic Analysis of the F2:3 Populations
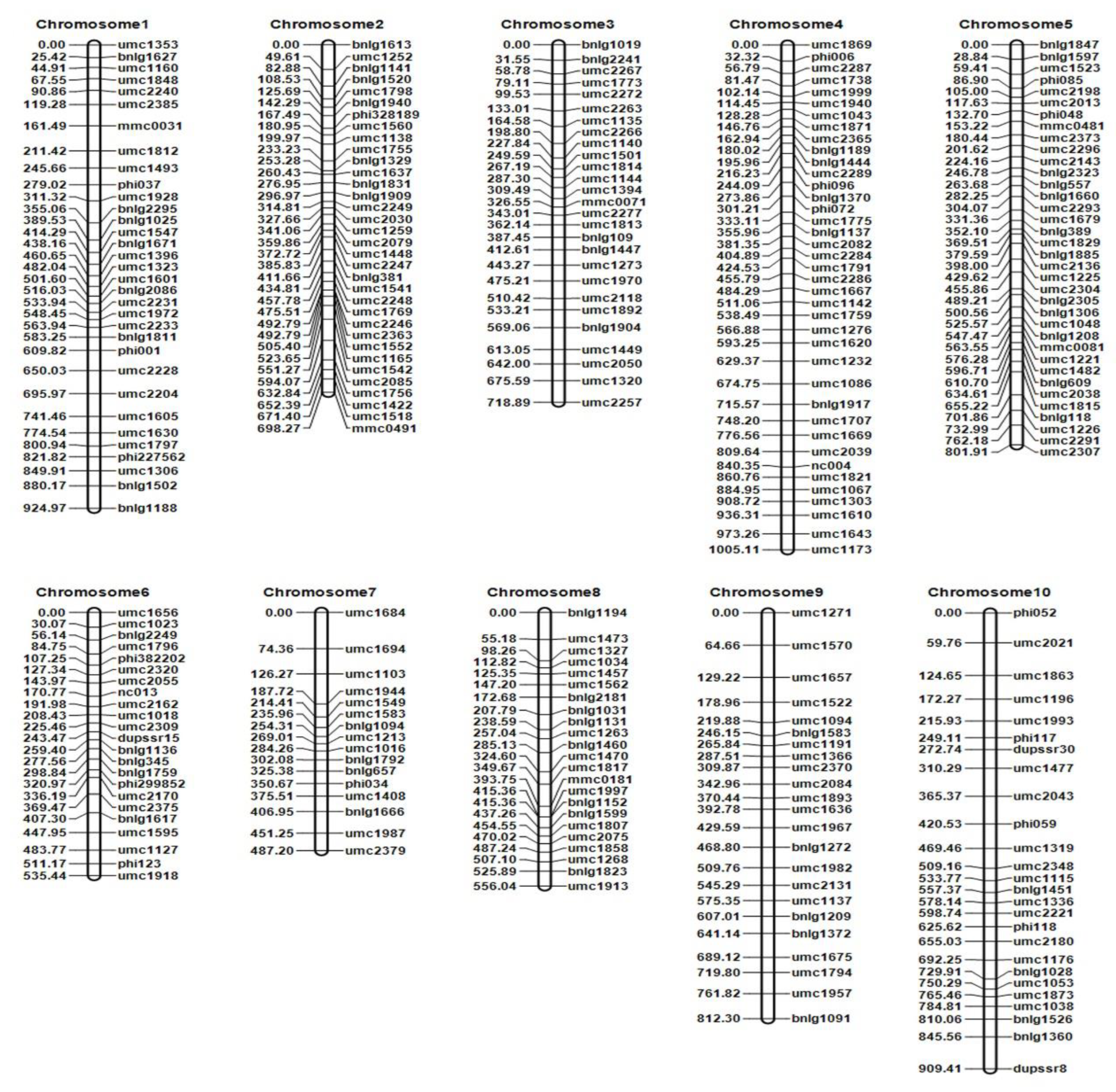
2.3. Construction of High-Density Genetic Linkage Maps and Mapping the QTLs That Controlled Seedling Stage-Related Traits in the F2:3 Populations
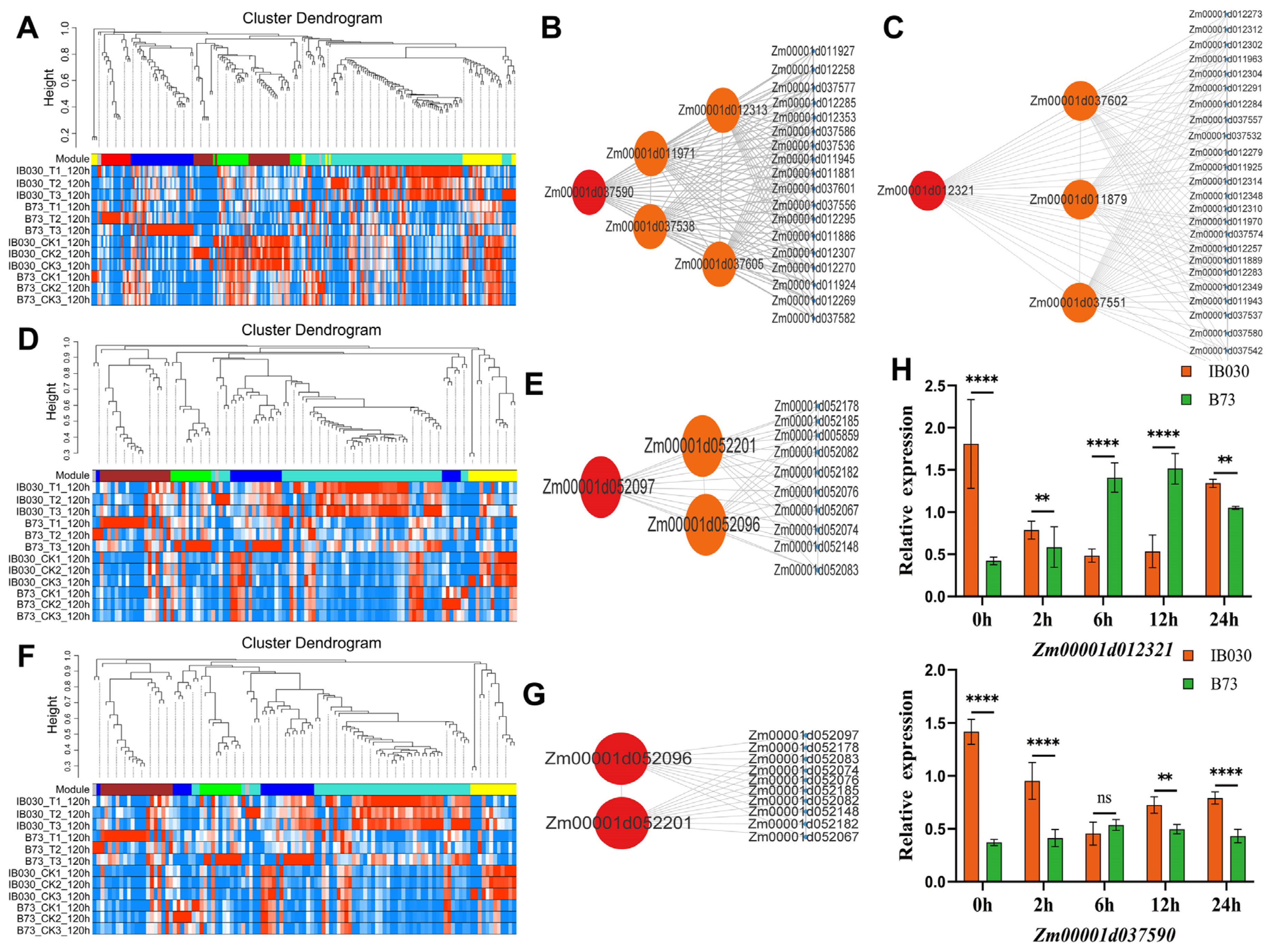
2.4. RNA-Seq and Identification of the Candidate Genes
2.5. Analyses of Gene Structure and Cis-Control Elements
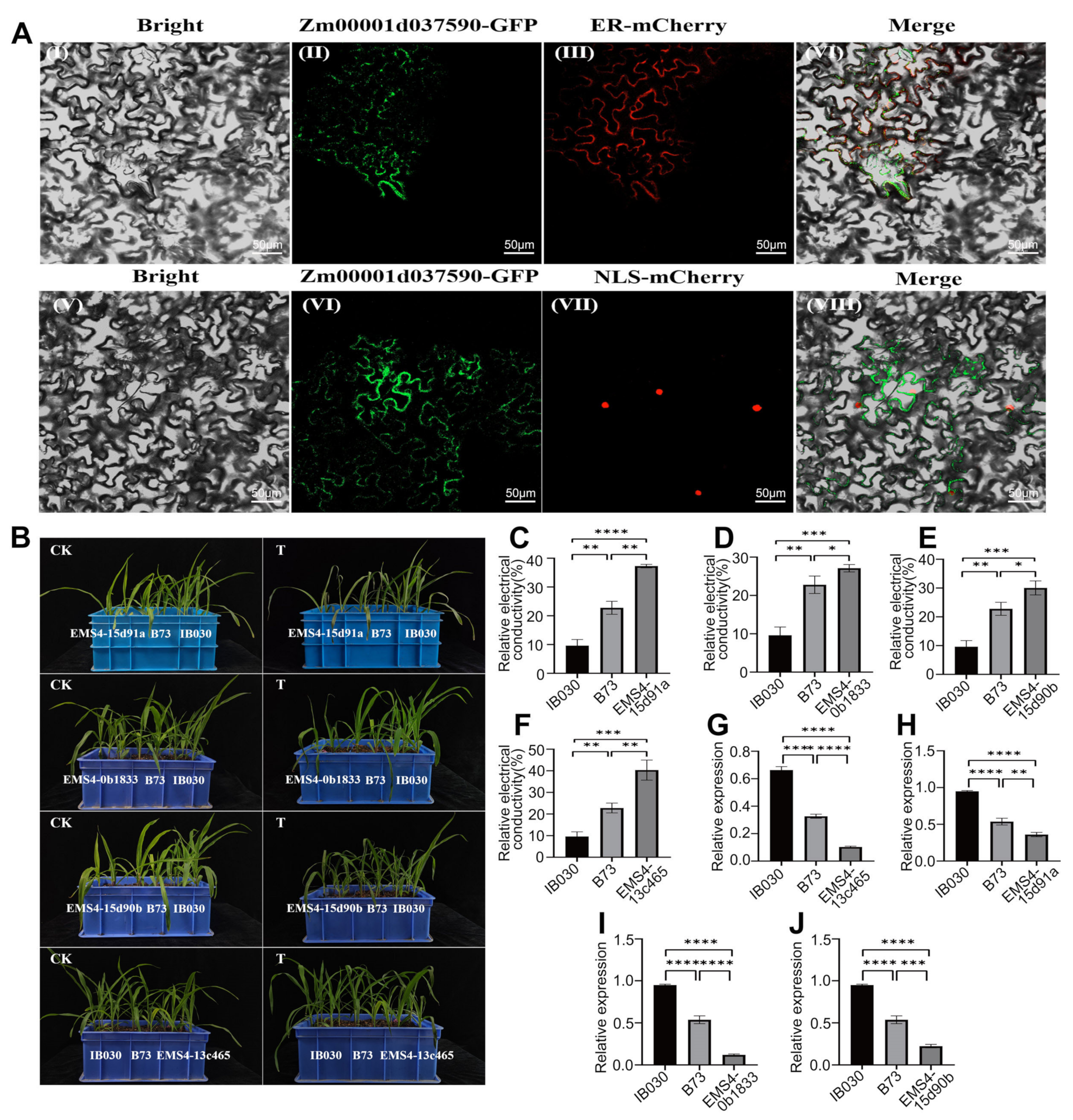
2.6. Candidate Gene Cloning, Expression Pattern Analysis, and Candidate Genes That Were Homologously Cloned in MTP and Z. Perennis
2.7. Subcellular Localization and Functional Verification of the Candidate Genes
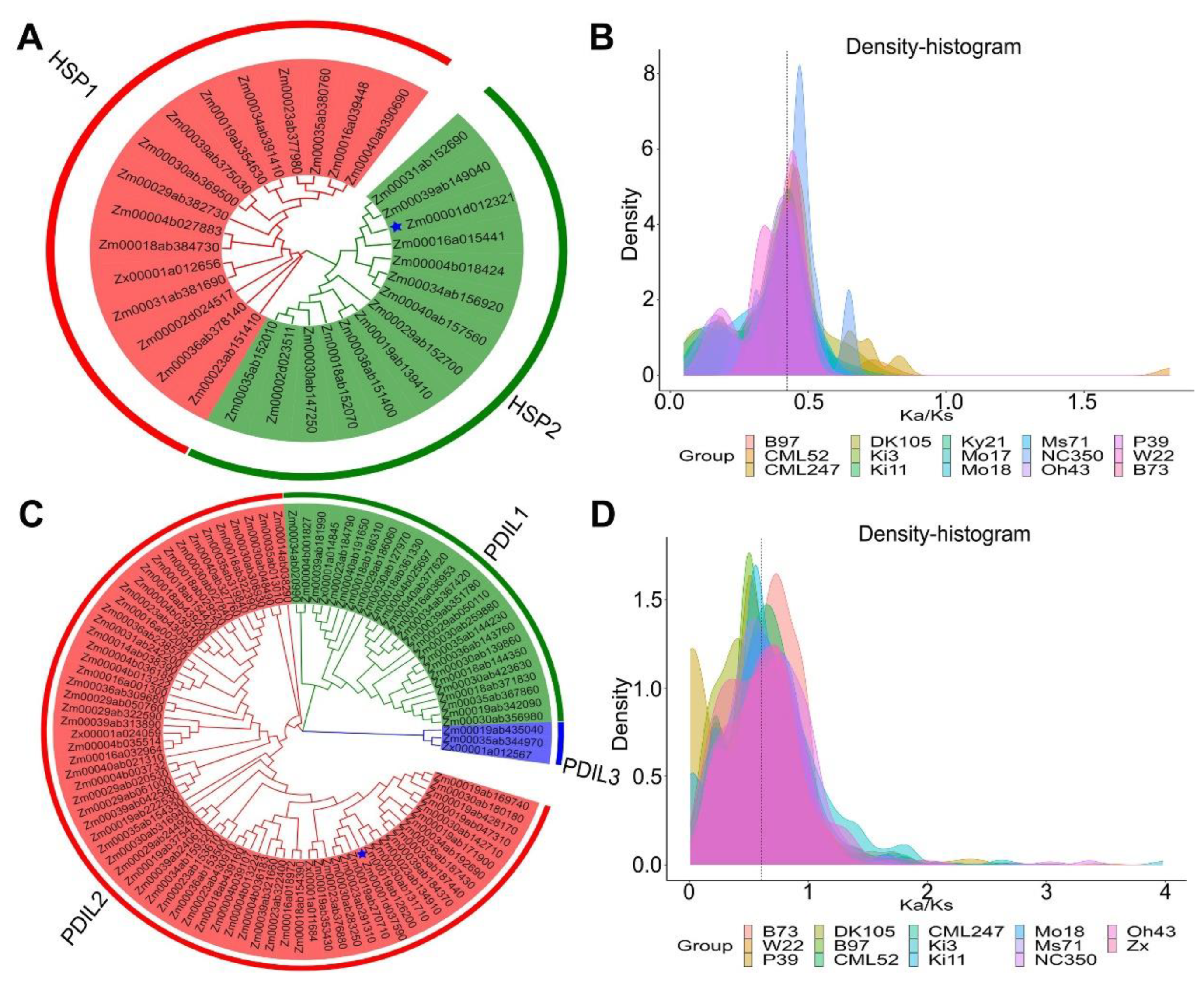
2.8. Identification of the Gene Family among Different Varieties of Maize
3. Discussion
3.1. Excavation of the Cold-Tolerant Resources of Wild Maize Materials Is an Important Strategy
3.2. Major QTLs/Genes for Controlling Cold Tolerance in MILs
3.3. Exploring the Function of Candidate Genes Is Critical
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Phenotypic Data Analysis
- Type with high cold resistance (membership value >0.70);
- Type with moderate cold resistance (membership value between 0.60 and 0.70);
- Cold resistance type (membership value between 0.50 and 0.59);
- Highly sensitive type (membership values between 0.30 and 0.49);
- Highly cold-sensitive type (membership value <0.30).
4.3. Construction of the Genetic Map and QTL Mapping
4.4. RNA-Seq and Identification of the Candidate Gene
4.5. Analyses of Gene Structure and Cis-Control Elements
4.6. Cloning and Analysis of the Pattern of Expression of the Candidate Genes
4.7. Subcellular Localization and Functional Verification of the Candidate Genes
4.8. Identification of the Gene Family among Different Maize Varieties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full English Term |
| MIL | Maize Introgression Line |
| WGCNA | Weighted Gene Co-Expression Network Analysis |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| CBF | C-Repeat Binding Factor |
| COR | Cold-Regulated |
| ROS | Reactive Oxygen Species |
| TF | Transcription Factor |
| QTL | Quantitative Trait Locus |
| MTP | Maize, T. dactyloides, and Z. perennis hybrid |
| CTAB | Cetyltrimethyl Ammonium Bromide |
| SSR | Simple Sequence Repeats |
| PH | Plant Height |
| SFW | Seedling Fresh Weight |
| SDW | Seedling Dry Weight |
| RFW | Root Fresh Weight |
| RDW | Root Dry Weight |
| REC | Relative Electrical Conductivity |
| RL | Root Length |
| CDS | Coding Sequence |
References
- Hund, A.; Richner, W.; Soldati, A.; Fracheboud, Y.; Stamp, P. Root morphology and photosynthetic performance of maize inbred lines at low temperature. Eur. J. Agron. 2007, 27, 52–61. [Google Scholar] [CrossRef]
- Farooq, M. Chilling tolerance in maize: Agronomic and physiological approaches. Crop Pasture Sci. 2019, 60, 501–516. [Google Scholar] [CrossRef]
- Pal, M.; Nagy, E. Chilling tolerance of maize. Acta Agron. Hung. 2002, 50, 95–106. [Google Scholar] [CrossRef]
- Revilla, P. EInheritance of cold tolerance at emergence and during early season growth in maize. Crop Sci. 2000, 40, 1579–1585. [Google Scholar] [CrossRef]
- Hu, S.; Lübberstedt, T.; Zhao, G.; Lee, M. QTL mapping of low-temperature germination ability in the maize IBM Syn4 RIL population. PLoS ONE 2016, 11, e0152795. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Leipner, J.; Stamp, P.; Guerra-Peraza, O. Low temperature stress in maize (Zea mays L.) induces genes involved in photosynthesis and signal transduction as studied by suppression subtractive hybridization. Plant Physiol. Biochem. 2009, 47, 116–122. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Li, W.; Hu, W.; Duan, L.; Feng, Y. Genome-wide association analysis of ten chilling tolerance indices at the germination and seedling stages in maize. J. Integr. Plant Biol. 2013, 55, 735–744. [Google Scholar] [CrossRef]
- Strigens, A.; Freitag, N.M.; Gilbert, X.; Grieder, C.; Melchinger, A.E. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant Cell Environ. 2013, 36, 1871–1887. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Fridovich, I. The biology of oxygen radicals. Science 1978, 201, 875–880. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.H.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642. [Google Scholar] [CrossRef]
- Song, J.; Wu, H.; He, F.; Qu, J.; Wang, Y.; Li, C.; Liu, J.H. Citrus sinensis CBF1 functions in cold tolerance by modulating putrescine biosynthesis through regulation of arginine decarboxylase. Plant Cell Physiol. 2021, 63, 19–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev. Cell 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Yang, J.; Ordiz, I.M.; Jaworski, J.G.; Beachy, R.N. Induced accumulation of cuticular waxes enhances drought tolerance in Arabidopsis by changes in development of stomata. Plant Physiol. Biochem. 2011, 49, 1448. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, E.; Yang, J.; Zhou, H.; Liang, H.; Li, J.; Jiang, D.; Wang, Z.; Liu, Z.; Zhuang, C. Rice OsGL1-6 is involved in leaf cuticular wax accumulation and drought resistance. PLoS ONE 2013, 8, e65139. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Cheng, Z.; Lei, C.; Xu, F.; Gao, S.; Ren, Y.; Wang, J.; Zhang, X.; Wang, J.; Wu, F.; et al. Wax crystal-sparse leaf2, a rice homologue of WAX2/GL1, is involved in synthesis of leaf cuticular wax. Planta 2011, 235, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, L. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef] [PubMed]
- Takáč, T.; Pechan, T.; Šamajová, O.; Šamaj, J. Proteomic analysis of Arabidopsis pldα1 mutants revealed an important role of phospholipase D alpha 1 in chloroplast biogenesis. Front Plant Sic. 2019, 10, 89. [Google Scholar] [CrossRef]
- Cui, D.Z.; Wu, D.; Somarathna, Y.; Xu, C.Y.; Li, S.; Li, P.; Zhang, H.; Chen, H.B.; Zhao, L. QTL mapping for salt tolerance based on snp markers at the seedling stage in maize (Zea mays L.). Euphytica 2015, 203, 273–283. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Mol. Biol. 2004, 56, 241–253. [Google Scholar] [CrossRef]
- Hund, A.; Frascaroli, E.; Leipner, J.; Jompuk, C.; Stamp, P.; Fracheboud, Y. Cold tolerance of the photosynthetic apparatus: Pleiotropic relationship between photosynthetic performance and specific leaf area of maize seedlings. Mol. Breed. 2005, 16, 321–331. [Google Scholar] [CrossRef]
- Jompuk, C.; Fracheboud, Y.; Stamp, P.; Leipner, J. Mapping of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions. J. Exp. Bot. 2005, 56, 1153–1163. [Google Scholar] [CrossRef]
- Hund, A.; Reimer, R.; Messmer, R. A consensus map of QTLs controlling the root length of maize. Plant Soil 2011, 344, 143–158. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Ana, B.; Rady, M.O.A.; Soengas, P.; Revilla, P. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Mol. Breed. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Sukumaran, S.; Dreisigacker, S.; Lopes, M.; Chavez, P.; Reynolds, M.P. Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 2015, 128, 353–363. [Google Scholar] [CrossRef]
- Korte, A.; Farlow, A. The advantages and limitations of trait analysis with GWAS: A review. Plant Methods 2013, 9, 29. [Google Scholar] [CrossRef]
- Revilla, P.; Rodríguez, V.M.; Ordás, A.; Rincent, R.; Charcosset, A.; Giauffret, C.; Melchinger, A.E.; Schön, C.-C.; Bauer, E.; Altmann, T.; et al. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biol. 2016, 16, 127. [Google Scholar] [CrossRef]
- Unterseer, S.; Pophaly, S.D.; Peis, R.; Westermeier, P.; Mayer, M.; Seidel, M.A.; Haberer, G.; Mayer, K.F.; Ordas, B.; Pausch, H.; et al. A comprehensive study of the genomic differentiation between temperate Dent and Flint maize. Genome Biol. 2016, 17, 137. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Fu, J.; Li, L.; Jia, G.; Ren, L.; Lubberstedt, T.; Wang, G.; Wang, J.; Gu, R. QTL mapping in three connected populations reveals a set of consensus genomic regions for low temperature germination ability in Zea mays L. Front. Plant Sci. 2018, 9, 65. [Google Scholar] [CrossRef]
- Han, Z.; Ku, L.; Zhang, Z.; Zhang, J.; Guo, S.; Liu, H.; Zhao, R.; Ren, Z.; Zhang, L.; Su, H.; et al. QTLs for seed vigor-related traits identified in maize seeds germinated under artificial aging conditions. PLoS ONE 2014, 9, e92535. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Fu, Z.; Liu, Z.; Hu, Y.; Tang, J. Comparative QTL analysis of maize seed artificial aging between an immortalized F2 population and its corresponding RILs. Crop J. 2016, 4, 30–39. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, X.; Cao, Y.; Zhou, M.; McNeil, D.; Liang, S.; Yang, C. RNA-seq analysis of cold and drought responsive transcriptomes of Zea mays ssp. mexicana L. Front. Plant Sci. 2017, 8, 136. [Google Scholar] [CrossRef]
- Yi, Q.; Malvar, R.A.; Lvarez-Iglesias, L.; Ordás, B.; Revilla, P. Dissecting the genetics of cold tolerance in a multiparental maize population. Theor. Appl. Genet. 2020, 133, 503–516. [Google Scholar] [CrossRef]
- Goering, R.; Larsen, S.; Jia, T.; Whelan, J.; Makarevitch, I. QTL mapping of seedling tolerance to exposure to low temperature in the maize IBM RIL population. PLoS ONE 2021, 16, e0254437. [Google Scholar] [CrossRef]
- Yi, Q.; Álvarez-Iglesias, L.; Malvar, R.A.; Romay, M.C.; Revilla, P.A.O. A worldwide maize panel revealed new genetic variation for cold tolerance. Theor. Appl. Genet. 2021, 134, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, C.; Zhang, X.; Li, Y.; Wang, T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2019, 292, 110380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fernie, A.R.; Yan, J. The past, present, and future of maize improvement: Domestication, genomics, and functional genomic routes toward crop enhancement. Plant Commun. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, C.L.; Xia, J.L.; Wu, L.S.; Xu, G.H.; Li, D.; Qin, W.C.; Han, X.; Tian, F. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Wulff, B. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Cheng, M.J.; Su, Y.G.; Li, Y.; Jiang, W.M.; Li, H.F.; Zhao, Y.L.; Wen, X.D.; Zhang, L.; Ali, A.; et al. Allopolyploidization facilitates gene flow and speciation among corn, Zea perennis and Tripsacum dactyloides. Planta 2019, 249, 1949–1962. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Li, Y.Z.; Wu, Z.Z.; Li, Y.; Li, X.F.; He, R.Y.; Yan, C.Y.; Zhao, Y.L.; Tang, Q. Tripsazea, a novel trihybrid of Zea mays, Tripsacum dactyloides and Zea perennis. G3-Genes Genomes Genet. 2019, 10, 839–848. [Google Scholar] [CrossRef]
- Yang, N.; Xu, X.W.; Wang, R.R.; Peng, W.L.; Cai, L.C.; Song, J.M.; Li, W.; Luo, X.; Niu, L.; Wang, Y. Contributions of Zea mays subspecies mexicana haplotypes to modern maize. Nat. Commun. 2017, 8, 1874. [Google Scholar] [CrossRef]
- Rodríguez-Zapata, F.; Barnes, A.C.; Blcher-Juárez, K.A.; Dan, J.G.; Rellán-Lvarez, R. Teosinte introgression modulates phosphatidylcholine levels and induces early maize flowering time. bioRxiv 2021. [Google Scholar]
- Jatimliansky, J.R.; García, M.D.; Molina, M.C. Response to chilling of Zea mays, Tripsacum dactyloides and their hybrid. Biol. Plant. 2004, 48, 561–567. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, W.; Meng, Z.; Braz, G.T.; Jin, W. Megabase-scale presence-absence variation with Tripsacum origin was under selection during maize domestication and adaptation. Genome Biol. 2021, 22, 237. [Google Scholar] [CrossRef]
- Tang, Q.L.; He, R.Y.; Li, X.F.; Yan, X.; Li, Y.; Li, Y.; Zhao, Y.L.; Xie, M.L. A method for cultivating cold-tolerant maize varieties using maize allopolyploids. Chinese Patent CN201710062709.8, 20 June 2017. [Google Scholar]
- Ramekar, R.V.; Sa, K.J.; Park, K.C.; Roy, N.; Kim, N.S.; Lee, J.K. Construction of genetic linkage map and identification of QTLs related to agronomic traits in maize using DNA transposon-based markers. Breed. Sci. 2018, 68, 465–473. [Google Scholar] [CrossRef]
- Choi, J.K.; Sa, K.J.; Park, D.H.; Lim, S.E.; Ryu, S.H.; Park, J.Y.; Park, K.J.; Rhee, H.I.; Lee, M.; Lee, J.K. Construction of genetic linkage map and identification of QTLs related to agronomic traits in DH population of maize (Zea mays L.) using SSR markers. Genes Genom. 2019, 41, 667–678. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Chen, J.; Qing, C.; Shen, Y. GWAS and WGCNA uncover hub genes controlling salt tolerance in maize (Zea mays L.) seedlings. Theor. Appl. Genet. 2021, 134, 3305–3318. [Google Scholar] [CrossRef]
- Ma, L.L.; An, R.; Jiang, L.; Zhang, C.; Li, Z.L.; Zou, C.Y.; Yang, C.; Pan, G.T.; Lübberstedt, T.; Shen, Y.O. Effects of ZmHIPP on lead tolerance in maize seedlings: Novel ideas for soil bioremediation. J. Hazard. Mater. 2022, 430, 128457. [Google Scholar] [CrossRef]
- Goldberger, R.F.; Epstein, C.J.; Anfinsen, C.B. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J. Biol. Chem. 1963, 238, 628–635. [Google Scholar] [CrossRef]
- Narindrasorasak, S.; Yao, P.; Sarkar, B. Protein disulfide isomerase, a multifunctional protein chaperone, shows copper-binding activity. Biochem. Biophys. Res. Commun. 2003, 311, 405–414. [Google Scholar] [CrossRef]
- Ding, X.; Lv, Z.M.; Zhao, Y.; Hang, M.; Yang, W.J. MTH1745, a protein disulfide isomerase-like protein from thermophilic archaea, Methanothermobacter thermoautotrophicum involving in stress response. Cell Stress Chaperones 2008, 13, 239–246. [Google Scholar] [CrossRef]
- Ondzighi, C.A.; Christopher, D.A.; Cho, E.J.; Chang, S.C.; Staehelin, L.A. Arabidopsis protein disulfide isomerase-5 inhibits cysteine proteases during trafficking to vacuoles before programmed cell death of the endothelium in developing seeds. Plant Cell 2008, 20, 2205–2220. [Google Scholar] [CrossRef]
- Houston, N.L.; Fan, C.; Xiang, J.Q.Y.; Schulze, J.M.; Jung, R.; Boston, R.S. Phylogenetic analyses identify 10 classes of the protein disulfide isomerase family in plants, including single-domain protein disulfide isomerase-related proteins. Plant Physiol. 2005, 137, 762–778. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Heazlewood, J.L.; Herald, V.; Holtzapffel, R.H.; Millar, A.H. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2010, 32, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009, 11, 2807–2850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Chi, W.C.; Huang, T.L.; Lin, C.Y.; Huang, H.J. Mercury-induced biochemical and proteomic changes in rice roots. Plant Physiol. Biochem. 2012, 55, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.A.; Pan, Y.; Wang, S.; Ding, Y.; Yang, W.; Zhu, C. Overexpression of a protein disulfide isomerase-like protein from Methanothermobacter thermoautotrophicum enhances mercury tolerance in transgenic rice. Plant Sci. 2012, 197, 10–20. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Hu, X.L.; Yang, Y.F.; Gong, F.P.; Zhang, D.Y.; Zhang, L.; Wu, L.J.; Li, C.H.; Wang, W. Protein sHSP26 improves chloroplast performance under heat stress by interacting with specific chloroplast proteins in maize (Zea mays). J. Proteom. 2015, 115, 81–92. [Google Scholar] [CrossRef]
- Li, Z.; Long, R.; Zhang, T.; Wang, Z.; Zhang, F.; Yang, Q.; Kang, J.; Sun, Y. Molecular cloning and functional analysis of the drought tolerance gene MsHSP70 from alfalfa (Medicago sativa L.). J. Plant Res. 2017, 130, 387–396. [Google Scholar] [CrossRef]
- Hashimoto, M.; Komatsu, S. Proteomic analysis of rice seedlings during cold stress. Proteomics 2007, 7, 1293–1302. [Google Scholar] [CrossRef]
- Hlaváčková, I.; Vítámvás, P.; Santrůček, J.; Kosová, K.; Zelenková, S.; Prášil, I.T.; Ovesná, J.; Hynek, R.; Kodíček, M. Proteins involved in distinct phases of cold hardening process in frost resistant winter barley (Hordeum vulgare L.) cv Luxor. Int. J. Mol. Sic. 2013, 14, 8000–8024. [Google Scholar] [CrossRef]
- Klára, K.; Pavel, V.; Ilja Tom, P.; Renaut, J. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar]
- Vítámvás, P.; Prášil, I.T.; Kosová, K.; Planchon, S.; Renaut, J. Analysis of proteome and frost tolerance in chromosome 5A and 5B reciprocal substitution lines between two winter wheats during long-term cold acclimation. Proteomics 2012, 12, 68–85. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef]
- Bae, M.S.; Cho, E.J.; Choi, E.Y.; Park, O.K. Analysis of the Arabidopsis nuclear proteome and its response to cold stress. Plant J. 2010, 36, 652–663. [Google Scholar] [CrossRef]
- Renaut, J.; Lutts, S.; Hoffmann, L.; Hausman, J.F. Responses of poplar to chilling temperatures: Proteomic and physiological aspects. Plant Biol. 2004, 6, 81–90. [Google Scholar]
- Cui, S.; Huang, F.; Wang, J.; Ma, X.; Cheng, Y.S.; Liu, J. A proteomic analysis of cold stress responses in rice seedlings. Proteomics 2005, 5, 3162–3172. [Google Scholar] [CrossRef]
- Balbuena, T.S.; Salas, J.J.; Martínez-Force, E.; Garcés, R.; Thelen, J.J. Proteome analysis of cold acclimation in sunflower. J. Proteome Res. 2011, 10, 2330–2346. [Google Scholar] [CrossRef]
- Rinalducci, S.; Egidi, M.G.; Mahfoozi, S.; Godehkahriz, S.J.; Zolla, L. The influence of temperature on plant development in a vernalization-requiring winter wheat: A 2-DE based proteomic investigation. J. Proteom. 2011, 74, 643–659. [Google Scholar] [CrossRef]
- Adnan, S.; Susan, L.; David, W. Expression of small heat-shock proteins at low temperatures: A possible role in protecting against chilling injuries. Plant Physiol. 1998, 117, 651–658. [Google Scholar]
- Yu, J.J.; Nou, I.S.; Kang, K.K. Overexpression of Oshsp16.9 gene encoding small heat shock protein enhances tolerance to abiotic stresses in rice. Plant Breed. Biotechnol. 2014, 2, 370–379. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1995, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Itoh, M. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 00341. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Wen, R.; Yang, Q.; Chai, Z.; Chen, R.; Lei, W.; Zhao, J.; Lang, Z.; Wang, H. Gene-Indexed mutations in maize. Mol. Plant 2018, 11, 496–504. [Google Scholar] [CrossRef]

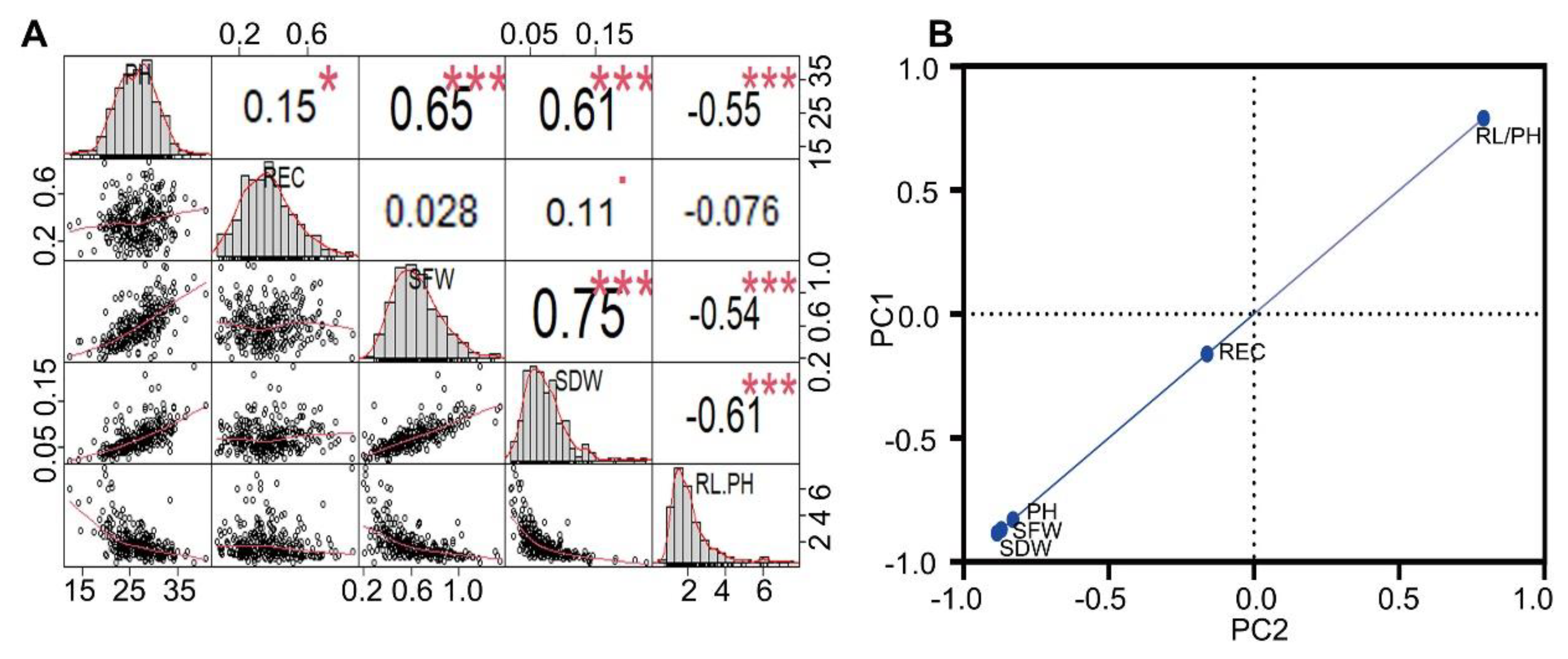

| Trait | Range | Mean | SE | CV/% | Kurtosis | Skewness | Sig |
|---|---|---|---|---|---|---|---|
| PH(cm) | 12.40–40.93 | 26.9065 | 0.2546 | 0.0095 | 0.3284 | −0.1786 | ** |
| REC | 0.06–0.87 | 0.3726 | 0.0096 | 0.0256 | −0.0803 | 0.5014 | ** |
| SFW(g) | 0.20–1.35 | 0.6162 | 0.0130 | 0.0211 | 0.2186 | 0.5765 | ** |
| SDW(g) | 0.02–0.23 | 0.0733 | 0.0018 | 0.0240 | 3.9244 | 1.3964 | ** |
| RL/PH | 0.40–7.63 | 1.8908 | 0.0682 | 0.0361 | 5.6565 | 2.1093 | ** |
| Trait | QTL | Physical Distance/Mb | Chromosome | Marker Interval | LOD | PVE/% | Additive Effect |
|---|---|---|---|---|---|---|---|
| REC | qTREC3-1 | 2.10 | 3 | umc1892–umc2118 | 3.08 | 2.09 | 0.0383 |
| REC | qTREC6-1 | 3.41 | 6 | bnlg2249–umc1918 | 4.82 | 3.46 | 0.0035 |
| REC | qTREC8-1 | 9.09 | 8 | umc1268–bnlg1152 | 3.57 | 6.03 | 0.0046 |
| PH | qTPH3-1 | – | 3 | umc1892–umc2118 | 5.65 | 8.48 | −0.5848 |
| PH | qTPH5-1 | – | 5 | umc2296–umc2143 | 3.25 | 6.62 | −1.6670 |
| SFW | qTSFW4-1 | 5.52 | 4 | umc1620–umc1667 | 6.51 | 8.77 | 0.0304 |
| SDW | qTSDW2-1 | 0.06 | 2 | bnlg1329–umc1637 | 3.72 | 1.00 | 0.0055 |
| SDW | qTSDW4-1 | 5.52 | 4 | umc1620–umc1667 | 3.99 | 1.41 | 0.0076 |
| RL/PH | qTRLRPH2-1 | 1.25 | 2 | umc1798–bnlg1940 | 7.41 | 10.57 | 0.6498 |
| Trait | Module | Gene | KME | TOM | Gene Description |
|---|---|---|---|---|---|
| SDW | yellow | Zm00001d052201 | 0.96 | 0.25 | probable magnesium transporter NIPA8 |
| SDW | yellow | Zm00001d052096 | 0.94 | 0.27 | uncharacterized LOC103654201 |
| SDW | yellow | Zm00001d052097 | 0.99 | 0.25 | uncharacterized LOC103654201 |
| REC | green | Zm00001d012313 | 0.9 | 0.39 | uncharacterized LOC100285836 |
| REC | green | Zm00001d037538 | −0.98 | 0.33 | arginyl-tRNA synthetase |
| REC | green | Zm00001d037590 | 0.91 | 0.29 | protein disulfide isomerase 7 |
| REC | green | Zm00001d037605 | 0.92 | 0.26 | GATA transcription factor 23 |
| REC | green | Zm00001d011971 | 0.96 | 0.21 | delta3,5-delta2,4-dienoyl-CoA isomerase |
| REC | yellow | Zm00001d012321 | −0.97 | 0.43 | nematode resistance protein-like HSPRO1 |
| REC | yellow | Zm00001d037551 | 0.96 | 0.35 | putative GTP diphosphokinase CRSH chloroplastic |
| REC | yellow | Zm00001d011879 | −0.95 | 0.31 | uncharacterized LOC100275154 |
| REC | yellow | Zm00001d037602 | 0.92 | 0.27 | U1 small nuclear ribonucleoprotein A |
| SFW | yellow | Zm00001d052201 | 0.97 | 0.52 | probable magnesium transporter NIPA8 |
| SFW | yellow | Zm00001d052096 | 0.94 | 0.51 | uncharacterized LOC103654201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.-y.; Yang, T.; Zheng, J.-j.; Pan, Z.-y.; Chen, Y.; Zhou, Y.; Li, X.-f.; Li, Y.-z.; Iqbal, M.-Z.; Yang, C.-y.; et al. QTL Mapping and a Transcriptome Integrative Analysis Uncover the Candidate Genes That Control the Cold Tolerance of Maize Introgression Lines at the Seedling Stage. Int. J. Mol. Sci. 2023, 24, 2629. https://doi.org/10.3390/ijms24032629
He R-y, Yang T, Zheng J-j, Pan Z-y, Chen Y, Zhou Y, Li X-f, Li Y-z, Iqbal M-Z, Yang C-y, et al. QTL Mapping and a Transcriptome Integrative Analysis Uncover the Candidate Genes That Control the Cold Tolerance of Maize Introgression Lines at the Seedling Stage. International Journal of Molecular Sciences. 2023; 24(3):2629. https://doi.org/10.3390/ijms24032629
Chicago/Turabian StyleHe, Ru-yu, Tao Yang, Jun-jun Zheng, Ze-yang Pan, Yu Chen, Yang Zhou, Xiao-feng Li, Ying-zheng Li, Muhammad-Zafar Iqbal, Chun-yan Yang, and et al. 2023. "QTL Mapping and a Transcriptome Integrative Analysis Uncover the Candidate Genes That Control the Cold Tolerance of Maize Introgression Lines at the Seedling Stage" International Journal of Molecular Sciences 24, no. 3: 2629. https://doi.org/10.3390/ijms24032629
APA StyleHe, R.-y., Yang, T., Zheng, J.-j., Pan, Z.-y., Chen, Y., Zhou, Y., Li, X.-f., Li, Y.-z., Iqbal, M.-Z., Yang, C.-y., He, J.-m., Rong, T.-z., & Tang, Q.-l. (2023). QTL Mapping and a Transcriptome Integrative Analysis Uncover the Candidate Genes That Control the Cold Tolerance of Maize Introgression Lines at the Seedling Stage. International Journal of Molecular Sciences, 24(3), 2629. https://doi.org/10.3390/ijms24032629






