Natural Polyphenols for Prevention and Treatment of Urinary Tract Infections
Abstract
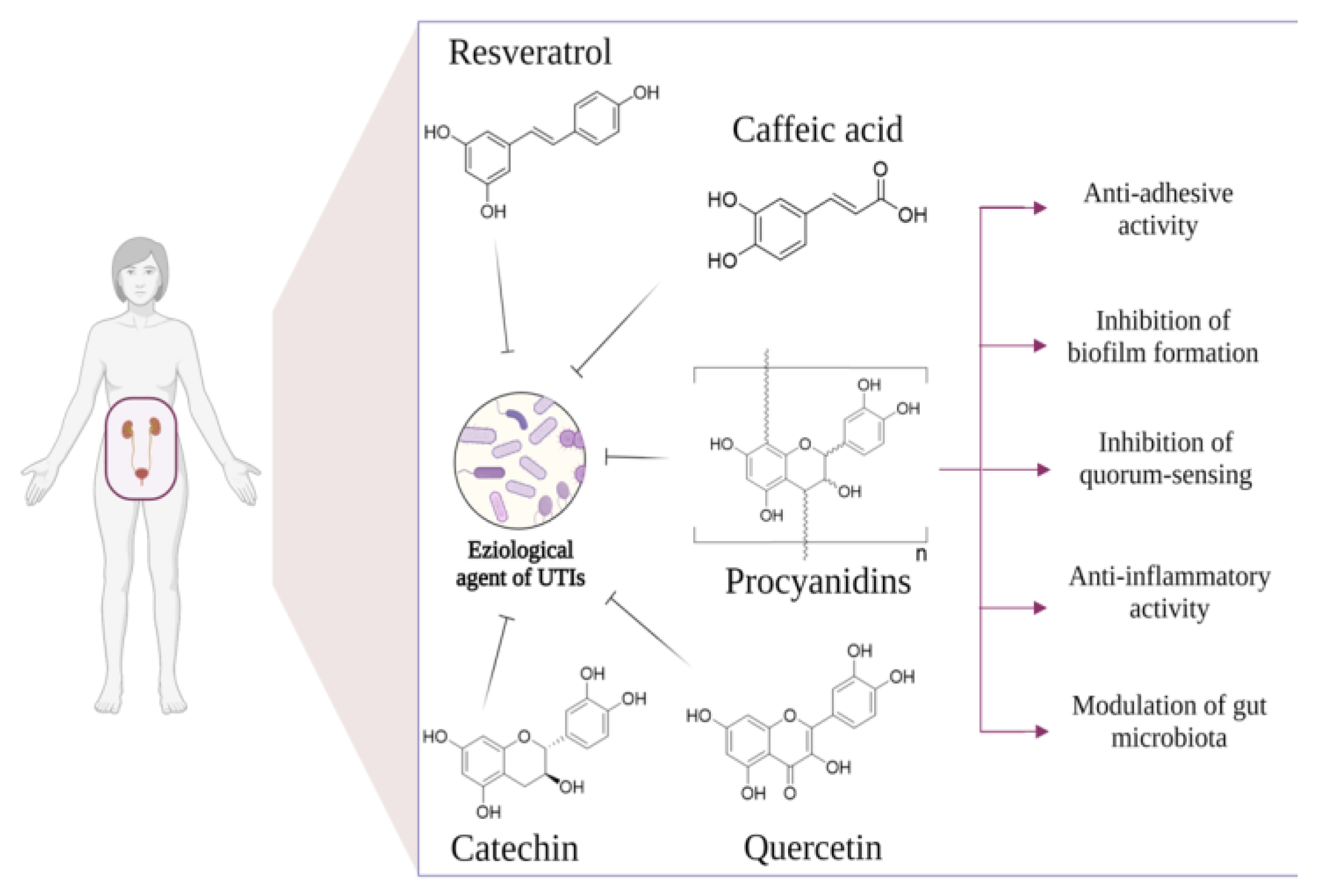
:1. Introduction
2. Proanthocyanidins
2.1. In Vitro Evidence
2.1.1. Antiadhesion Activity
2.1.2. Inhibitory Activity on Biofilm Formation and Quorum Sensing (QS)
2.1.3. PACs Anti-Inflammatory Activity
2.1.4. Relationship between Gut and Urinary Tract Microbiota
2.2. In Vivo Evidence
3. Green Tea Catechins
3.1. In Vitro Evidence
3.2. In Vivo Studies
4. Resveratrol
4.1. In Vitro Evidence
4.2. In Vivo Evidence
5. Caffeic Acid
5.1. In Vitro Evidence
5.2. In Vivo Evidence
6. Quercetin
6.1. In Vitro Evidence
6.2. In Vivo Evidence
7. Other Polyphenols
8. Polyphenolic Toxicity
9. Materials and Methods
Search Strategy
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- de Llano, D.G.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.N.; Hultgren, S.J. Adhesive Pili in UTI Pathogenesis and Drug Development. Pathogens 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Coyle, M.E.; Yang, L.; Liang, J.; Wang, K.; Guo, X.; Zhang, A.L.; Mao, W.; Lu, C.; Xue, C.C.; et al. Acupuncture for Recurrent Urinary Tract Infection in Women: A Systematic Review and Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina 2019, 55, 356. [Google Scholar] [CrossRef]
- Tamadonfar, K.O.; Omattage, N.S.; Spaulding, C.N.; Hultgren, S.J. Reaching the End of the Line: Urinary Tract Infections. Bact. Intracell. 2020, 83–99. [Google Scholar] [CrossRef]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Patterns of Rare Uropathogens: Survey from Central European Urology Department 2011–2019. BMC Urol. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Haider, G.; Zehra, N.; Munir, A.A.; Haider, A. Risk Factors of Urinary Tract Infection in Pregnancy. J. Pak. Med. Assoc. 2010, 60, 213–216. [Google Scholar]
- Petca, R.C.; Negoiță, S.; Mareș, C.; Petca, A.; Popescu, R.I.; Chibelean, C.B. Heterogeneity of Antibiotics Multidrug-Resistance Profile of Uropathogens in Romanian Population. Antibiotics 2021, 10, 523. [Google Scholar] [CrossRef]
- Chibelean, C.B.; Petca, R.C.; Mareș, C.; Popescu, R.I.; Enikő, B.; Mehedințu, C.; Petca, A. A Clinical Perspective on the Antimicrobial Resistance Spectrum of Uropathogens in a Romanian Male Population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef]
- Ngowi, B.N.; Sunguya, B.; Herman, A.; Chacha, A.; Maro, E.; Rugarabamu, L.F.; Bartlett, J.; Balandya, E.; Mteta, K.A.; Mmbaga, B.T. Prevalence Ofmultidrug Resistant Salmonella Spp. Isolated from Environmental Samples of Mosul City. Infect Drug Resist. 2021, 14, 1623–1633. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology and Antibiotic Resistance Profile of Bacterial Uropathogens in Male Patients: A 10-Year Retrospective Study. Farmacia 2021, 69, 530–539. [Google Scholar] [CrossRef]
- Iannuzzo, F.; Piccolo, V.; Novellino, E.; Schiano, E.; Salviati, E.; Summa, V.; Campiglia, P.; Tenore, G.C.; Maisto, M. A Food-Grade Method for Enhancing the Levels of Low Molecular Weight Proanthocyanidins with Potentially High Intestinal Bioavailability. Int. J. Mol. Sci. 2022, 23, 13557. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Krenn, L.; Steitz, M.; Schlicht, C.; Kurth, H.; Gaedcke, F. Anthocyanin- and Proanthocyanidin-Rich Extracts of Berries in Food Supplements-Analysis with Problems. Pharmazie 2007, 62, 803–812. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; van Hoek, M.L. Cranberry Proanthocyanidins Have Anti-Biofilm Properties against Pseudomonas Aeruginosa. BMC Complement Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and Virulence Mechanisms of Uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-Adhesion Therapy of Bacterial Diseases: Prospects and Problems. FEMS Immunol. Med. Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef]
- Bäckhed, F.; Alsén, B.; Roche, N.; Ångström, J.; von Euler, A.; Breimer, M.E.; Westerlund-Wikström, B.; Teneberg, S.; Richter-Dahlfors, A. Identification of Target Tissue Glycosphingolipid Receptors for Uropathogenic, F1C-Fimbriated Escherichia coli and Its Role in Mucosal Inflammation. J. Biol. Chem. 2002, 277, 18198–18205. [Google Scholar] [CrossRef]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 Pilus-Mediated Bacterial Invasion of Bladder Epithelial Cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive Compounds in Cranberries and Their Role in Prevention of Urinary Tract Infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion Activity of A2-Type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143–154. [Google Scholar] [CrossRef] [PubMed]
- de Llano, D.G.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Podgoreanu, P.; Alexandru, I.; Popescu, N.; Măruţescu, L.; Bleotu, C.; Mogoşanu, G.D.; Chifiriuc, M.C.; Gluck, M.; Lazăr, V. Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease. Pathogens 2017, 6, 22. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Totsika, M.; Allsopp, L.P.; Webb, R.I.; Moriel, D.G.; Schembri, M.A. Comparative Proteomics of Uropathogenic Escherichia coli during Growth in Human Urine Identify UCA-like (UCL) Fimbriae as an Adherence Factor Involved in Biofilm Formation and Binding to Uroepithelial Cells. J. Proteom. 2016, 131, 177–189. [Google Scholar] [CrossRef]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.W.; Park, S.C. Impact of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, R.; An, M.M.; Liang, B.B. Iron-Depletion Prevents Biofilm Formation in Pseudomonas Aeruginosa through Twitching Motility and Quorum Sensing. Braz. J. Microbiol. 2010, 41, 37–41. [Google Scholar] [CrossRef]
- Vadekeetil, A.; Saini, H.; Chhibber, S.; Harjai, K. Exploiting the Antivirulence Efficacy of an Ajoene-Ciprofloxacin Combination against Pseudomonas Aeruginosa Biofilm Associated Murine Acute Pyelonephritis. Biofouling 2016, 32, 371–382. [Google Scholar] [CrossRef]
- Laplante, K.L.; Sarkisian, S.A.; Woodmansee, S.; Rowley, D.C.; Seeram, N.P. Effects of Cranberry Extracts on Growth and Biofilm Production of Escherichia coli and Staphylococcus Species. Phytother. Res. 2012, 26, 1371–1374. [Google Scholar] [CrossRef]
- Wojnicz, D.; Tichaczek-Goska, D.; Korzekwa, K.; Kicia, M.; Hendrich, A.B. Study of the Impact of Cranberry Extract on the Virulence Factors and Biofilm Formation by Enterococcus Faecalis Strains Isolated from Urinary Tract Infections. Int. J. Food Sci. Nutr. 2016, 67, 1005–1016. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- O’May, C.; Tufenkji, N. The Swarming Motility of Pseudomonas Aeruginosa Is Blocked by Cranberry Proanthocyanidins and Other Tannin-Containing Materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef]
- Hannan, T.J.; Mysorekar, I.U.; Hung, C.S.; Isaacson-Schmid, M.L.; Hultgren, S.J. Early Severe Inflammatory Responses to Uropathogenic E. coli Predispose to Chronic and Recurrent Urinary Tract Infection. PLoS Pathog. 2010, 6, 29–30. [Google Scholar] [CrossRef]
- Lee, J.B.; Neild, G.H. Urinary Tract Infection. Medicine 2007, 35, 423–428. [Google Scholar] [CrossRef]
- Bjorling, D.E.; Wang, Z.Y.; Bushman, W. Models of Inflammation of the Lower Urinary Tract. Neurourol. Urodyn. 2011, 30, 673–682. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Huang, Y.; Nikolic, D.; Pendland, S.; Doyle, B.J.; Locklear, T.D.; Mahady, G.B. Effects of Cranberry Extracts and Ursolic Acid Derivatives on P-Fimbriated Escherichia coli, COX-2 Activity, pro-Inflammatory Cytokine Release and the NF-Κβ Transcriptional Response In Vitro. Pharm. Biol. 2009, 47, 18–25. [Google Scholar] [CrossRef]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; del Moral, J.S.G.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus 2018, 4, 128–138. [Google Scholar] [CrossRef]
- Magistro, G.; Stief, C.G. The Urinary Tract Microbiome: The Answer to All Our Open Questions? Eur. Urol. Focus 2019, 5, 36–38. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Meudt, J.J.; Shanmuganayagam, D.; Krueger, C.G.; Reed, J.D. Ratio of “a-Type” to “b-Type” Proanthocyanidin Interflavan Bonds Affects Extra-Intestinal Pathogenic Escherichia coli Invasion of Gut Epithelial Cells. J. Agric. Food Chem. 2014, 62, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Polewski, M.A.; Krueger, C.G.; Reed, J.D.; Leyer, G. Ability of Cranberry Proanthocyanidins in Combination with a Probiotic Formulation to Inhibit in Vitro Invasion of Gut Epithelial Cells by Extra-Intestinal Pathogenic E. Coli. J. Funct. Foods 2016, 25, 123–134. [Google Scholar] [CrossRef]
- Nowack, R.; Birck, R. Cranberry Products in the Prevention of Urinary Tract Infections: Examining the Evidence. Botanics 2015, 5, 45–54. [Google Scholar] [CrossRef]
- Haverkorn, M.J. Reduction of Bacteriuria and Pyuria Using Cranberry Juice. JAMA J. Am. Med. Assoc. 1994, 272, 590. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, M.; Pokka, T.; Koskela, M.; Uhari, M. Infections in Women. Clin. Infect. Dis. 2001, 322, 1–5. [Google Scholar]
- Takahashi, S.; Hamasuna, R.; Yasuda, M.; Arakawa, S.; Tanaka, K.; Ishikawa, K.; Kiyota, H.; Hayami, H.; Yamamoto, S.; Kubo, T.; et al. A Randomized Clinical Trial to Evaluate the Preventive Effect of Cranberry Juice (UR65) for Patients with Recurrent Urinary Tract Infection. J. Infect. Chemother. 2013, 19, 112–117. [Google Scholar] [CrossRef]
- Foxman, B.; Cronenwett, A.E.W.; Spino, C.; Berger, M.B.; Morgan, D.M. Cranberry Juice Capsules and Urinary Tract Infection after Surgery: Results of a Randomized Trial. Am. J. Obs. Gynecol. 2015, 213, 194.e1–194.e8. [Google Scholar] [CrossRef]
- Vostalova, J.; Vidlar, A.; Simanek, V.; Galandakova, A.; Kosina, P.; Vacek, J.; Vrbkova, J.; Zimmermann, B.F.; Ulrichova, J.; Student, V. Are High Proanthocyanidins Key to Cranberry Efficacy in the Prevention of Recurrent Urinary Tract Infection? Phytother. Res. 2015, 29, 1559–1567. [Google Scholar] [CrossRef]
- Fu, Z.; Liska, D.A.; Talan, D.; Chung, M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef]
- Gbinigie, O.A.; Spencer, E.A.; Heneghan, C.J.; Lee, J.J.; Butler, C.C. Cranberry Extract for Symptoms of Acute, Uncomplicated Urinary Tract Infection: A Systematic Review. Antibiotics 2021, 10, 12. [Google Scholar] [CrossRef]
- Durham, S.H.; Stamm, P.L.; Eiland, L.S. Cranberry Products for the Prophylaxis of Urinary Tract Infections in Pediatric Patients. Ann. Pharmacother. 2015, 49, 1349–1356. [Google Scholar] [CrossRef]
- Salo, J.; Uhari, M.; Helminen, M.; Korppi, M.; Nieminen, T.; Pokka, T.; Kontiokari, T. Cranberry Juice for the Prevention of Recurrences of Urinary Tract Infections in Children: A Randomized Placebo-Controlled Trial. Clin. Infect. Dis. 2012, 54, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Bosmans, J.E.; Beerepoot, M.A.J.; Prins, J.M.; ter Riet, G.; Geerlings, S.E. Cost-Effectiveness of Cranberries vs. Antibiotics to Prevent Urinary Tract Infections in Premenopausal Women: A Randomized Clinical Trial. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Beerepoot, M.A.J. Cranberries vs. Antibiotics to Prevent Urinary Tract Infections. Arch. Intern. Med. 2011, 171, 1270. [Google Scholar] [CrossRef]
- Mcmurdo, M.E.T.; Argo, I.; Phillips, G.; Daly, F.; Davey, P. Cranberry or Trimethoprim for the Prevention of Recurrent Urinary Tract Infections? A Randomized Controlled Trial in Older Women. J. Antimicrob. Chemother. 2009, 63, 389–395. [Google Scholar] [CrossRef]
- Pagonas, N.; Hörstrup, J.; Schmidt, D.; Benz, P.; Schindler, R.; Reinke, P.; van der Giet, M.; Zidek, W.; Westhoff, T.H. Prophylaxis of Recurrent Urinary Tract Infection After Renal Transplantation by Cranberry Juice and L-Methionine. Transpl. Proc. 2012, 44, 3017–3021. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Polyphenols Chemistry of Tea and Coffee: A Century of Progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef]
- Reygaert, W.C. The Antimicrobial Possibilities of Green Tea. Front. Microbiol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Noormandi, A.; Dabaghzadeh, F. Effects of Green Tea on Escherichia coli as a Uropathogen. J. Tradit. Complement. Med. 2015, 5, 15–20. [Google Scholar] [CrossRef]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular Responses and Proteomic Analysis of Escherichia coli Exposed to Green Tea Polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.I.; Ha, U.S.; Sohn, D.W.; Lee, S.J.; Kim, H.W.; Han, C.H.; Lee, C.B.; Cho, Y.H. Anti-Inflammatory and Antimicrobial Effects of Nanocatechin in a Chronic Bacterial Prostatitis Rat Model. J. Infect. Chemother. 2011, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins Are Bioavailable in Men and Women Drinking Black Tea throughout the Day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.; Jusufi, I. Green Tea as an Effective Antimicrobial for Urinary Tract Infections Caused by Escherichia coli. Front. Microbiol. 2013, 4, 1–4. [Google Scholar] [CrossRef]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The Green Tea Polyphenol EGCG Inhibits E. coli Biofilm Formation by Impairing Amyloid Curli Fibre Assembly and Downregulating the Biofilm Regulator CsgD via the ΣE-Dependent SRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef]
- Tükel, Ç.; Nishimori, J.H.; Wilson, R.P.; Winter, M.G.; Keestra, A.M.; van Putten, J.P.M.; Bäumler, A.J. Toll-like Receptors 1 and 2 Cooperatively Mediate Immune Responses to Curli, a Common Amyloid from Enterobacterial Biofilms. Cell Microbiol. 2010, 12, 1495–1505. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic Mode of Action of Catechin, Vanillic and Protocatechuic Acids to Inhibit the Adhesion of Uropathogenic Escherichia coli on Silicone Surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef]
- Passat, D.N. Interactions of Black and Green Tea Water Extracts with Antibiotics Activity in Local Urinary Isolated Escherichia coli. J. Al-Nahrain Univ. Sci. 2012, 15, 134–142. [Google Scholar] [CrossRef]
- Taylor, P.W.; Hamilton-Miller, J.M.T.; Stapleton, P.D. Antimicrobial Properties of Green Tea Catechins. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 71–81. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, S.; Tyagi, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development of Nanoemulsion Based Gel Loaded with Phytoconstituents for the Treatment of Urinary Tract Infection and in Vivo Biodistribution Studies. Adv. Pharm. Bull. 2017, 7, 611–619. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, C.H.; Kang, S.H.; Lee, S.J.; Kim, S.W.; Shin, O.R.; Sim, Y.C.; Lee, S.J.; Cho, Y. Synergistic Effect between Catechin and Ciprofloxacin on Chronic Bacterial Prostatitis Rat Model Title. Int. J. Urol. 2005, 12, 383–389. [Google Scholar] [CrossRef]
- Kheirabadi, Z.; Mehrabani, M.; Sarafzadeh, F.; Dabaghzadeh, F.; Ahmadinia, N. Green Tea as an Adjunctive Therapy for Treatment of Acute Uncomplicated Cystitis in Women: A Randomized Clinical Trial. Complement. Clin. Pr. 2019, 34, 13–16. [Google Scholar] [CrossRef]
- Ivanov, D.; Abramov-Sommariva, D.; Moritz, K.; Eskötter, H.; Kostinenko, T.; Martynyuk, L.; Kolesnik, N.; Naber, K.G. An Open Label, Non-Controlled, Multicentre, Interventional Trial to Investigate the Safety and Efficacy of Canephron® N in the Management of Uncomplicated Urinary Tract Infections (UUTIs). Clin. Phytosci. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharm. 2012, 3, 1–18. [Google Scholar] [CrossRef]
- Jeandet, P.; Douillet-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.; Tsai, M. Resveratrol Selectively Inhibits Neisseria Gonorrhoeae and Neisseria Meningitidis. J. Antimicrob. Chemother. 2001, 47, 243–244. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L. Resveratrol Inhibits the Growth of Helicobacter Pylori in Vitro Re: Marker of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2000, 95, 1849. [Google Scholar]
- Hwang, D.; Lim, Y.H. Resveratrol Antibacterial Activity against Escherichia coli Is Mediated by Z-Ring Formation Inhibition via Suppression of FtsZ Expression. Sci. Rep. 2015, 5, 2–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, J. Carvacrol-Rich Oregano Oil and Thymol-Rich Thyme Red Oil Inhibit Biofilm Formation and the Virulence of Uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Raorane, C.J.; Ryu, S.Y.; Shim, J.J.; Lee, J. The Anti-Biofilm and Anti-Virulence Activities of Trans-Resveratrol and Oxyresveratrol against Uropathogenic Escherichia coli. Biofouling 2019, 35, 758–767. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, J.; He, Y.; Wang, W.; Shen, C.; Yang, B. Resveratrol Improves Urinary Dysfunction in Rats with Chronic Prostatitis and Suppresses the Activity of the Stem Cell Factor/c-Kit Signaling Pathway. Mol. Med. Rep. 2017, 16, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; He, Y.; Yu, Y.; Zhang, J.; Zeng, X.; Gong, F.; Liu, Q.; Yang, B. Resveratrol Improves Prostate Fibrosis during Progression of Urinary Dysfunction in Chronic Prostatitis by Mast Cell Suppression. Mol. Med. Rep. 2018, 17, 918–924. [Google Scholar] [CrossRef]
- Calmasini, F.B.; Silva, F.H.; Alexandre, E.C.; Antunes, E. Efficacy of Resveratrol in Male Urogenital Tract Dysfunctions: An Evaluation of Preclinical Data. Nutr. Res. Rev. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.C.; Calmasini, F.B.; de Oliveira, M.G.; Silva, F.H.; da Silva, C.P.V.; André, D.M.; Leonardo, F.C.; Delbin, M.A.; Antunes, E. Chronic Treatment with Resveratrol Improves Overactive Bladder in Obese Mice via Antioxidant Activity. Eur. J. Pharm. 2016, 788, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Şehirli, Ö.; Şakarcan, A.; Velioǧlu-Öǧünç, A.; Çetinel, Ş.; Gedik, N.; Yeǧen, B.Ç.; Şener, G. Resveratrol Improves Ifosfamide-Induced Fanconi Syndrome in Rats. Toxicol. Appl. Pharm. 2007, 222, 33–41. [Google Scholar] [CrossRef]
- Tsuda, Y.; Nakahara, T.; Mori, A.; Sakamoto, K.; Ishii, K. Resveratrol Prevents Bradykinin-Induced Contraction of Rat Urinary Bladders by Decreasing Prostaglandin Production and Calcium Influx. Eur. J. Pharm. 2011, 666, 189–195. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Micek, A.; Castellano, S.; D’amico, E.; Paladino, N.; Ferri, R.; Galvano, F.; Grosso, G. Dietary Phenolic Acids and Their Major Food Sources Are Associated with Cognitive Status in Older Italian Adults. Antioxidants 2021, 10, 700. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Celik, S.; Gorur, S.; Aslantas, O.; Erdogan, S.; Ocak, S.; Hakverdi, S. Caffeic Acid Phenethyl Ester Suppresses Oxidative Stress in Escherichia coli-Induced Pyelonephritis in Rats. Mol. Cell Biochem. 2007, 297, 131–138. [Google Scholar] [CrossRef]
- Sarshar, S.; Brandt, S.; Asadi Karam, M.R.; Habibi, M.; Bouzari, S.; Lechtenberg, M.; Dobrindt, U.; Qin, X.; Goycoolea, F.M.; Hensel, A. Aqueous Extract from Orthosiphon Stamineus Leaves Prevents Bladder and Kidney Infection in Mice. Phytomedicine 2017, 28, 1–9. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G. Quantification of Quercetin in Different Parts of Onion and Its DPPH Radical Scavenging and Antibacterial Activity. Food Sci. Biotechnol. 2006, 15, 39–43. [Google Scholar]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharm. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of Quercetin Binding Site on DNA Gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential Synergistic Activity of Quercetin with Antibiotics against Multidrug-Resistant Clinical Strains of Pseudomonas Aeruginosa. PLoS ONE 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Minardi, D.; D’Anzeo, G.; Cantoro, D.; Conti, A.; Muzzonigro, G. Urinary Tract Infections in Women: Etiology and Treatment Options. Int. J. Gen. Med. 2011, 4, 333–343. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Sharma, N.K.; Bharti, S.K.; Krishnan, S.; Kumar, A.; Prakash, O.; Kumar, P.; Kumar, A.; Gupta, A.K. Phytochemicals As Uropathognic Escherichia coli FimH Antagonist: In Vitro And In Silico Approach. Curr. Mol. Med. 2019, 18, 640–653. [Google Scholar] [CrossRef]
- Hamzah, H.; Hertiani, T.; Pratiwi, S.U.T.; Nuryastuti, T. Efficacy of Quercetin against Polymicrobial Biofilm on Catheters. Res. J. Pharm. Technol. 2020, 13, 5277–5282. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative in Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Schutte, M.E.; Alink, G.M.; Freidig, A.P.; Spenkelink, B.; Vaessen, J.C.H.; van de Sandt, J.J.M.; Groten, J.P.; Rietjens, I.M.C.M. Quercetin Increases the Bioavailability of 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP) in Rats. Food Chem. Toxicol. 2008, 46, 3422–3428. [Google Scholar] [CrossRef]
- Umathe, S.N.; Dixit, P.V.; kumar, V.; Bansod, K.U.; Wanjari, M.M. Quercetin Pretreatment Increases the Bioavailability of Pioglitazone in Rats: Involvement of CYP3A Inhibition. Biochem. Pharm. 2008, 75, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Katske, F.; A Shoskes, D.; Sender, M.; Poliakin, R.; Gagliano, K.; Rajfer, J. Treatment of Interstitial Cystitis with a Quercetin Supplement. Tech. Urol. 2001, 7, 44–46. [Google Scholar] [PubMed]
- Torella, M.; del Deo, F.; Grimaldi, A.; Iervolino, S.A.; Pezzella, M.; Tammaro, C.; Gallo, P.; Rappa, C.; de Franciscis, P.; Colacurci, N. Efficacy of an Orally Administered Combination of Hyaluronic Acid, Chondroitin Sulfate, Curcumin and Quercetin for the Prevention of Recurrent Urinary Tract Infections in Postmenopausal Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 125–128. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Carmen Garcia-Parrilla, M.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; di Daniele, F.; Campo, M.; di Lauro, M.; Zaitseva, A.P.; di Daniele, N.; Marrone, G.; Romani, A. Effect of Hydrolysable Tannins and Anthocyanins on Recurrent Urinary Tract Infections in Nephropathic Patients: Preliminary Data. Nutrients 2021, 13, 591. [Google Scholar] [CrossRef]
- Murakami, A. Dose-Dependent Functionality and Toxicity of Green Tea Polyphenols in Experimental Rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef]
- Vaz-da-Silva, M.; Loureiro, A.I.; Falcao, A.; Nunes, T.; Rocha, J.F.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-da-Silva, P. Effect of Food on the Pharmacokinetic Profile of Trans-Resveratrol. Int. J. Clin. Pharm. 2008, 46, 564–570. [Google Scholar] [CrossRef]
- Lluís, L.; Muñoz, M.; Rosa Nogués, M.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Valls, J.; Solà, R. Toxicology Evaluation of a Procyanidin-Rich Extract from Grape Skins and Seeds. Food Chem. Toxicol. 2011, 49, 1450–1454. [Google Scholar] [CrossRef]
- Badolati, N.; Masselli, R.; Maisto, M.; di Minno, A.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Genotoxicity Assessment of Three Nutraceuticals Containing Natural Antioxidants Extracted from Agri-Food Waste Biomasses. Foods 2020, 9, 1461. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of in Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
| Trial Type | Subjects | No. of Subjects | Age (Years) | Treatment(s) | Duration of Treatment | Main Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Procyanidins | |||||||
| R, DB, PC | Elderly Woman | 153 | 78.1 ± 8.3 (Cranberry group) 79.0 ± 9.4 (Placebo group) |
| 6 months | 42% of the pathologic bacteriuria (p < 0.01) compared to the control group and 27% odds of remaining bacteriuric-pyuric (p < 0.01) compared to the control group | [44] |
| R, DB, PC | Premenopausal women | 150 | 32 ± 9.8 (Cranberry group) 30.0 ± 11.8 (Lactobacillus group) 29.0 ± 10.5 (Control group) |
| 6 months | −20% of recurrence (p < 0.05) compared to the control group | [45] |
| R, DB, PC | Women | 300 | Over 50 |
| 24 weeks | 29.1% of patients showed UTI relapse in group A 49.2% of patients showed UTI relapse in group P, (log-rank test; p = 0.0425) | [46] |
| R, DB, PC | Subjects undergoing elective gynecologic surgery | 160 | >18 years old |
| 6 weeks after surgery | Lower UTI occurrence in the cranberry treatment group compared to the placebo group (15/80 patients (19%) versus 30/80 (38%) | [47] |
| R, DB, PC | Woman with recurrent UTIs | 182 | 55.3 ± 13.3 year (active group) 55.1 ± 10.9 year (placebo group) |
| 6 months | Cranberry group, the UTIs were significantly fewer (10.8% vs. 25.8%, p = 0.04) Cranberry group experienced a longer time to first UTI than the placebo group (p = 0.04) | [48] |
| R, DB, PC | Children with normal urinary anatomy or grade I or II VUR | 263 | 1–16 years old |
| 6 months | −6 days on antimicrobials per patient-year; 95% CI, −7 to −5; p < 0.001) | [52] |
| R, DB | Premenopausal women with recurrent UTIs | 221 | 18 years or older |
| 12 months | 78.2% vs. 71.1% patients with at least 1 symptomatic UTI, cranberry vs. TMP-SMX group | [53] |
| R, DB | Women with two or more antibiotic-treated UTIs in the previous 12 months | 137 | ≥45 years |
| 6 months | Time to first recurrence of UTI was not significantly different (log-rank test: Δ = 2.7, χ2 (2.7, 1) p = 0.100) 84.5 days median time to UTI recurrence (cranberry group), 91 days for trimethoprim group (U = 166, p = 0.479). | [55] |
| Catechins | |||||||
| R, SB, PC | Premenopausal nonpregnant women with acute uncomplicated cystitis | 70 | 18–50 years |
| 3 days | Green tea group exhibited significant improvement in urinalysis data (abnormal urine color, pyuria, and bacteriuria) among with Placebo group, except for hematuria After 4 weeks, 2.86% of patients in the green tea group, and after 6 weeks, 15.38% of patients in the placebo group had the symptoms of recurrent cystitis | [72] |
| Quercetin | |||||||
| CT | Subjects with documented interstitial cystitis | 22 | 53.1 years |
| 4 weeks | From 11.3 +/− 0.6 to 5.1 +/− 0.7 (p = 0.000001) improved the mean problem index From 11.9 +/− 0.9 to 4.5 +/− 0.5 (p = 0.000001) mitigated the mean symptom index From 8.2 +/− 0.4 to 3.5 +/− 0.4 (p = 0.000001) the mean global assessment score ameliorated | [102] |
| MC, CT, PS | Postmenopausal women with recurrent UTIs during the last year | 145 |
|
| 12 months | After 6 months, in group 3 was reduced the recurrent UTIs episodes compared to those receiving single treatments (group 1 and group 2) | [103] |
| Tannins | |||||||
| CT, PS | Nephropathic patients affected by recurrent UTIs | 26 | >18 years old |
| 6 weeks | In supplemented group a significant reduction in urine leukocyte content was observed. Urinary bacterial flora decreased significant untreated weeks of treatment vs. untreated subjects | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisto, M.; Iannuzzo, F.; Novellino, E.; Schiano, E.; Piccolo, V.; Tenore, G.C. Natural Polyphenols for Prevention and Treatment of Urinary Tract Infections. Int. J. Mol. Sci. 2023, 24, 3277. https://doi.org/10.3390/ijms24043277
Maisto M, Iannuzzo F, Novellino E, Schiano E, Piccolo V, Tenore GC. Natural Polyphenols for Prevention and Treatment of Urinary Tract Infections. International Journal of Molecular Sciences. 2023; 24(4):3277. https://doi.org/10.3390/ijms24043277
Chicago/Turabian StyleMaisto, Maria, Fortuna Iannuzzo, Ettore Novellino, Elisabetta Schiano, Vincenzo Piccolo, and Gian Carlo Tenore. 2023. "Natural Polyphenols for Prevention and Treatment of Urinary Tract Infections" International Journal of Molecular Sciences 24, no. 4: 3277. https://doi.org/10.3390/ijms24043277
APA StyleMaisto, M., Iannuzzo, F., Novellino, E., Schiano, E., Piccolo, V., & Tenore, G. C. (2023). Natural Polyphenols for Prevention and Treatment of Urinary Tract Infections. International Journal of Molecular Sciences, 24(4), 3277. https://doi.org/10.3390/ijms24043277











