An Analysis of the Structural Relationship between Thyroid Hormone-Signaling Disruption and Polybrominated Diphenyl Ethers: Potential Implications for Male Infertility
Abstract
1. Introduction
2. Results
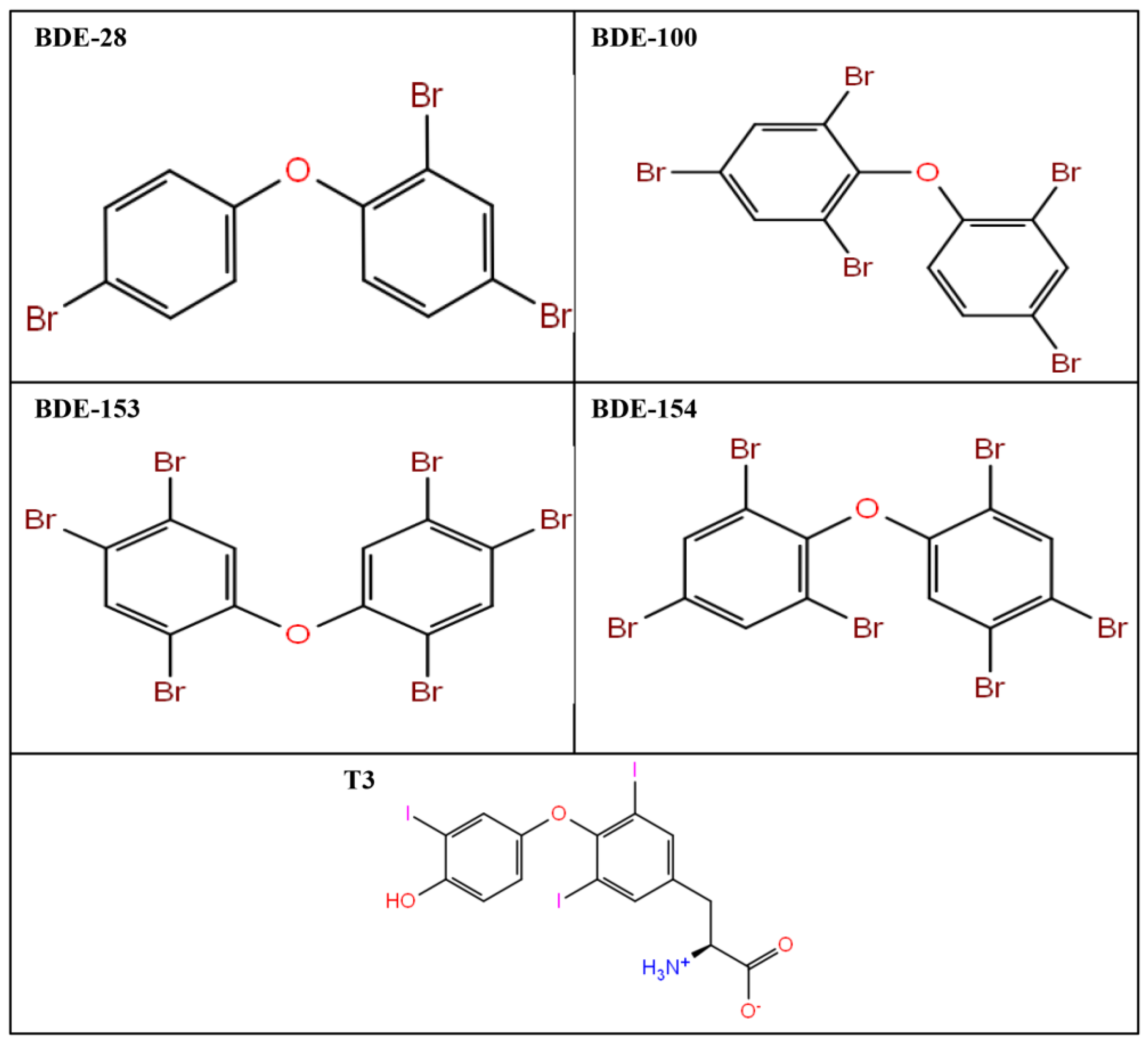
2.1. Induced Fit Docking of BDE-28 with Thyroxine Receptor-α
2.2. Induced Fit Docking of BDE-100 with Thyroxine Receptor-α
2.3. Induced Fit Docking of BDE-153 Ligand with Thyroxine Recepotor-α
2.4. Induced Fit Docking of BDE-154 Ligand with Thyroxine Receptor-α
3. Discussion
4. Materials and Methods
4.1. Protein Preparation
4.2. Ligand Preparation
4.3. Induced Fit Docking
4.4. Binding Affinity Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patisaul, H.B. Endocrine disrupting chemicals (EDCs) and the neuroendocrine system: Beyond estrogen, androgen, and thyroid. Adv. Pharmacol. 2021, 92, 101–150. [Google Scholar] [PubMed]
- Guarnotta, V.; Amodei, R.; Frasca, F.; Aversa, A.; Giordano, C. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710. [Google Scholar] [CrossRef] [PubMed]
- Kurşunoğlu, N.E.; Yurekli, B.P.S. Endocrine disruptor chemicals as obesogen and diabetogen: Clinical and mechanistic evidence. World J. Clin. Cases 2022, 10, 11226–11239. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Health costs in the European Union. How Much Is Related to EDCs? 2014. Available online: https://www.env-health.org/IMG/pdf/heal_policy_statement_en.pdf (accessed on 10 December 2022).
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; DiGangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating Burden and Disease Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- McNabb, F.M.A.; Darras, V.M. Thyroids. Sturkie’s Avian Physiology. Elsevier: Amsterdam, The Netherlands, 2015; pp. 535–547. [Google Scholar]
- Brtko, J. Thyroid hormone and thyroid hormone nuclear receptors: History and present state of art. Endocr. Regul. 2021, 55, 103–119. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone. [Updated 2022 May 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Singh, R.; Hamada, A.J.; Agarwal, A. Thyroid Hormones in Male Reproduction and Fertility. Open Reprod. Sci. J. 2011, 3, 98–104. [Google Scholar]
- Jefferys, A.; Vanderpump, M.; Yasmin, E. Thyroid dysfunction and reproductive health. Obstet. Gynaecol. 2015, 17, 39–45. [Google Scholar] [CrossRef]
- Zavaleta, M.C.; Ibarra, J.L.P.; Yataco, A.R.; Arroyo, J.C.; Urteaga, L.C.; Roseboom, P.J.; Williams, C.A. Assessment of hormonal status in male infertility. An update. Diabetes Metab. Syndr. 2022, 16, 102447. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Martinez, M.E. Thyroid hormone action in the developing testis: Intergenerational epigenetics. J. Endocrinol. 2020, 244, R33–R46. [Google Scholar] [CrossRef] [PubMed]
- Veronelli, A.; Masu, A.; Ranieri, R.; Rognoni, C.; Laneri, M.; Pontiroli, A.E. Prevalence of erectile dysfunction in thyroid disorders: Comparison with control subjects and with obese and diabetic patients. Int. J. Impot. Res. 2006, 18, 111–114. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Segev, O.; Kushmaro, A.; Brenner, A. Environmental impact of flame retardants (persistence and biodegradability). Int. J. Environ. Res. Public Health 2009, 6, 478–491. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Sjodin, A.; Apelberg, B.J.; Witter, F.R.; Halden, R.U.; Patterson, D.G.; Panny, S.R.; Needham, L.L.; Goldman, L.R. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 2008, 116, 1376–1382. [Google Scholar] [CrossRef]
- Meeker, J.D.; Johnson, P.I.; Camann, D.; Hauser, R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009, 407, 3425–3429. [Google Scholar] [CrossRef]
- Chevrier, J.; Harley, K.G.; Bradman, A.; Gharbi, M.; Sjodin, A.; Eskenazi, B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ. Health Perspect. 2010, 118, 1444–1449. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Eagle, S.; Anthopolos, R.; Wolkin, A.; Miranda, M.L. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect. 2011, 119, 1454–1459. [Google Scholar] [CrossRef]
- Zota, A.R.; Park, J.S.; Wang, Y.; Petreas, M.; Zoeller, R.T.; Woodruff, T.J. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in california. Environ. Sci. Technol. 2011, 45, 7896–7905. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Langlois, M.F.; Lavoie, L.; Corbin, F.; Pasquier, J.C.; Takser, L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am. J. Epidemiol. 2013, 178, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Bergman, A.; Becher, G.; Bjerregaard, P.; Bornman, R.; Brandt, I.; Iguchi, T.; Jobling, S.; Kidd, K.A.; Kortenkamp, A.; et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ. Health 2014, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; McClean, M.D.; Stapleton, H.M.; Webster, T.F. Linking pbdes in house dust to consumer products using x-ray fluorescence. Environ. Sci. Technol. 2008, 42, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Gale, S.; Zoeller, R.T.; Spengler, J.D.; Birnbaum, L.; McNeely, E. PBDE Flame Retardants, Thyroid Disease, and Menopausal Status in U.S. Women. Environ. Health 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef]
- Bramwell, L.; Glinianaia, S.V.; Rankin, J.; Rose, M.; Fernandes, A.; Harrad, S.; Pless-Mulolli, T. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environ. Int. 2016, 92–93, 680–694. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, L.; Li, J.; Wu, Y. Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018, 198, 522–536. [Google Scholar] [CrossRef]
- Tay, J.H.; Sellstr¨om, U.; Papadopoulou, E.; Padilla-S´anchez, J.A.; Haug, L.S.; de Wit, C.A. Serum concentrations of legacy and emerging halogenated flame retardants in a Norwegian cohort: Relationship to external exposure. Environ. Res. 2019, 178, 108731. [Google Scholar] [CrossRef]
- Liu, X.; Zhiguo, C.; Gang, Y. Human exposure to emerging halogenated flame retardants. Compr. Anal. Chem. 2020, 88, 215–251. [Google Scholar]
- Esplugas, R.; Rovira, J.; Mari, M.; Fern´andez-Arribas, J.; Eljarrat, E.; Domingo, J.L.; Schuhmacher, M. Emerging and legacy flame retardants in indoor air and dust samples of Tarragona Province (Catalonia, Spain). Sci. Total Environ. 2022, 806 Pt 1, 150494. [Google Scholar] [CrossRef] [PubMed]
- Marquès, M.; Nadal, M.; Domingo, J.L. Human exposure to polybrominated diphenyl ethers (PBDEs) through the diet: An update of the scientific literature. Food Chem. Toxicol. 2022, 167, 113322. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2017/227 of 9 February 2017 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(Pentabromophenyl)Ether. Off. J. Legis. 2017, 35, 6, Published on 09-02-2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/16f3bb9e-ef21-11e6-8a35-01aa75ed71a1/language-en (accessed on 26 December 2022).
- Dobslaw, D.; Woiski, C.; Kiel, M.; Kuch, B.; Breuer, J. Plant uptake, translocation and metabolism of PBDEs in plants of food and feed industry: A review. Rev. Environ. Sci. Biotechnol. 2021, 20, 75–142. [Google Scholar] [CrossRef]
- Sahu, R.S.; Peng, Y.H.; Ko, C.F.; Chou, T.H.; Catherine, H.N.; Yang, C.Y.; Tso, C.P.; Su, Y.F.; Shih, Y.H. Processes driving the degradation of polybrominated diphenyl ethers in terrestrial environment. Trends Environ. Anal. Chem. 2021, 30, e00126. [Google Scholar] [CrossRef]
- Sharkey, M.; Harrad, S.; Abdallah, M.A.E.; Drage, D.S.; Berresheim, H. Phasing-out of legacy brominated flame retardants: The UNEP Stockholm convention and other legislative action worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef]
- Hoang, A.C.; Tran, T.M.; Tu, M.B.; Takahashi, S. Polybrominated diphenyl ethers in indoor and outdoor dust from Southeast Asia: An updated review on contamination status, human exposure, and future perspectives. Environ. Pollut. 2021, 272, 116012. [Google Scholar] [CrossRef]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.; Wenjing, X. Polybrominated diphenyl ethers in articles: A review of its applications and legislation. Environ. Sci. Pollut. Res. 2017, 24, 4312–4321. [Google Scholar]
- Wang, M.; Gao, B.; Tang, D.; Sun, H.; Yin, X.; Yu, C. Effects of temperature on aggregation kinetics of graphene oxide in aqueous solutions. Colloids Surf. A 2018, 538, 63–72. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, Y.Y.; Kim, W.I.; Jeon, T.W.; Shin, S.K.; Jeong, M.J.; Kim, J.G. Emission of polybrominated diphenyl ethers (PBDEs) in use of electric/electronic equipment and recycling of e-waste in Korea. Sci. Total Environ. 2014, 470–471, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Sjodin, A.; Jones, R.S.; Caudill, S.P.; Wong, L.Y.; Turner, W.E.; Calafat, A.M. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ. Sci. Technol. 2014, 48, 753–760. [Google Scholar] [CrossRef] [PubMed]
- World Bank. GDP (Constant 2010 US$)—Canada. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.KD?locations=CA (accessed on 1 May 2021).
- Malits, J.; Naidu, M.; Trasande, L. Exposure to Endocrine Disrupting Chemicals in Canada: Population-Based Estimates of Disease Burden and Economic Costs. Toxics 2022, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Richardson, V.M.; Staskal, D.F.; Ross, D.G.; Diliberto, J.J.; DeVito, M.J.; Birnbaum, L.S. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol. Appl. Pharmacol. 2008, 226, 244–250. [Google Scholar] [CrossRef]
- Meerts, I.A.; van Zanden, J.J.; Luijks, E.A.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A. Structural studies on the endocrine disrupting role of polybrominated diphenyl ethers (PBDEs) in thyroid diseases. Environ. Sci. Pollut. Res. 2020, 27, 37866–37876. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A. Structural binding perspectives of common plasticizers and a flame retardant, BDE-153, against thyroxine-binding globulin: Potential for endocrine disruption. J. Appl. Toxicol. 2022, 42, 841–851. [Google Scholar] [CrossRef]
- Zhou, T.; Taylor, M.M.; DeVito, M.J.; Crofton, K.M. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002, 66, 105–116. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.; Andersson, P.L.; Legler, J.; Brouwer, A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Marchesini, G.R.; Meimaridou, A.; Haasnoot, W.; Meulenberg, E.; Albertus, F.; Mizuguchi, M.; Takeuchi, M.; Irth, H.; Murk, A.J. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol. Appl. Pharmacol. 2008, 232, 150–160. [Google Scholar] [CrossRef]
- Meijer, L.; Brouwer, B.; De Jong, F.H.J.; Bergman, Å.; Sauer, P.J.J. Influence of prenatal exposure to selected organohalogans on infant sexual and neurological development. Organohal. Compd. 2008, 70, 658–661. [Google Scholar]
- Luan, M.; Liang, H.; Yang, F.; Yuan, W.; Chen, A.; Liu, X.; Ji, H.; Wen, S.; Miao, M. Prenatal polybrominated diphenyl ethers exposure and anogenital distance in boys from a Shanghai birth cohort. Int. J. Hyg. Environ. Health 2019, 222, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Ainmelk, Y.; Takser, L. Polybrominated diphenyl ethers and sperm quality. Reprod. Toxicol. 2011, 31, 546–550. [Google Scholar] [CrossRef]
- Arowolo, O.; Pilsner, J.R.; Sergeyev, O.; Suvorov, A. Mechanisms of Male Reproductive Toxicity of Polybrominated Diphenyl Ethers. Int. J. Mol. Sci. 2022, 23, 14229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Sun, Z.; Dong, H.; Qiu, L.; Gu, J.; Zhou, J.; Wang, X.; Wang, S.L. Cytochrome P450 3A1 mediates 2,2′,4,4′-tetrabromodiphenyl ether-induced reduction of spermatogenesis in adult rats. PLoS ONE 2013, 8, e66301. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, L.T.; van de Kuil, T.; Verhoef, A.; Leonards, P.E.; Slob, W.; Canton, R.F.; Germer, S.; Hamers, T.; Visser, T.J.; Litens, S.; et al. A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (pentaBDE) mixture in Wistar rats. Toxicology 2008, 245, 109–122. [Google Scholar] [CrossRef]
- Patel, N.; Kashanian, J.A. Thyroid Dysfunction and Male Reproductive Physiology. Semin. Reprod. Med. 2016, 34, 356–360. [Google Scholar] [CrossRef]
- Li, F.; Xie, Q.; Li, X.; Li, N.; Chi, P.; Chen, J.; Wang, Z.; Hao, C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: In vitro and in silico investigations. Environ. Health Perspect. 2010, 118, 602–606. [Google Scholar] [CrossRef]
- Schriks, M.; Roessig, J.M.; Murk, A.J.; Furlow, J.D. Thyroid hormone receptor isoform selectivity of thyroid hormone disrupting compounds quantified with an in vitro reporter gene assay. Environ. Toxicol. Pharmacol. 2007, 23, 302–307. [Google Scholar] [CrossRef]
- Czerska, M.; Zieliński, M.; Kamińska, J.; Ligocka, D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int. J. Occup. Med. Environ. Health 2013, 26, 498–510. [Google Scholar] [CrossRef]
- Mazdai, A.; Dodder, N.G.; Abernathy, M.P.; Hites, R.A.; Bigsby, R.M. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003, 111, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Meijer, L.; Bakker, A.; Van Braeckel, K.N.; Sauer, P.J.; Bos, A.F. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009, 117, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, Y.J.; Lee, E.; Patra, N.; Lee, J.; Kwack, S.J.; Kim, K.B.; Chung, K.K.; Han, S.Y.; Han, J.Y.; et al. Exposure assessment of polybrominated diphenyl ethers (PBDE) in umbilical cord blood of Korean infants. J. Toxicol. Environ. Health A 2009, 72, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, Y.; Zhou, J.; Wu, B.; Liang, Y.; Peng, Z.; Fang, D.; Liu, B.; Huang, H.; He, C.; et al. Elevated body burdens of PBDEs, dioxins, and PCBs on thyroid hormone homeostasis at an electronic waste recycling site in China. Environ. Sci. Technol. 2010, 44, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Chen, F.A.; Huang, Y.F.; Hsing, L.L.; Chen, L.L.; Wu, L.S.; Liu, T.S.; Chang-Chien, G.P.; Chen, K.C.; Chao, H.R. Negative associations between PBDE levels and thyroid hormones in cord blood. Int. J. Hyg. Environ. Health 2011, 214, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lignell, S.; Aune, M.; Darnerud, P.O.; Stridsberg, M.; Hanberg, A.; Larsson, S.C.; Glynn, A. Maternal body burdens of PCDD/Fs and PBDEs are associated with maternal serum levels of thyroid hormones in early pregnancy: A crosssectional study. Environ. Health 2016, 15, 55. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Mall, J.K. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep. 2014, 1, 101–112. [Google Scholar] [CrossRef]
- Vuong, A.M.; Webster, G.M.; Romano, M.E.; Braun, J.M.; Zoeller, R.T.; Hoofnagle, A.N.; Sjödin, N.; Yolton, K.; Lanphear, B.P.; Chen, A. Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA. Environ. Health Perspect. 2015, 123, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Hagmar, L.; Bjork, J.; Sjodin, A.; Bergman, A.; Erfurth, E.M. Plasma levels of persistent organohalogens and hormone levels in adult male humans. Arch. Environ. Health 2001, 56, 138–143. [Google Scholar] [CrossRef]
- Turyk, M.E.; Persky, V.W.; Imm, P.; Knobeloch, L.; Chatterton, R.; Anderson, H.A. Hormone disruption by PBDEs in adult male sport fish consumers. Environ. Health Perspect. 2008, 116, 1635–1641. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.M.; Lin, Y.P.; Kass, P.H.; Puschner, B. Association of Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) with Hyperthyroidism in Domestic Felines, Sentinels for Thyroid Hormone Disruption. BMC Vet. Res. 2017, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Guigueno, M.F.; Fernie, K.J. Birds and flame retardants: A review of the toxic effects on birds of historical and novel flame retardants. Environ. Res. 2017, 154, 398–424. [Google Scholar] [CrossRef] [PubMed]
- Szabo, D.T.; Richardson, V.M.; Ross, D.G.; Diliberto, J.J.; Kodavanti, P.R.; Birnbaum, L.S. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol. Sci. 2009, 107, 27–39. [Google Scholar] [CrossRef]
- Lee, E.; Kim, T.H.; Choi, J.S.; Nabanata, P.; Kim, N.Y.; Ahn, M.Y.; Jung, K.K.; Kang, I.H.; Kim, T.S.; Kwack, S.J.; et al. Evaluation of liver and thyroid toxicity in SpragueDawley rats after exposure to polybrominated diphenyl ether BDE209. J. Toxicol. Sci. 2010, 35, 535–545. [Google Scholar] [CrossRef]
- Kuriyama, S.N.; Wanner, A.; Fidalgo-Neto, A.A.; Talsness, C.E.; Koerner, W.; Chahoud, I. Developmental exposure to low-dose PBDE-99: Tissue distribution and thyroid hormone levels. Toxicology 2007, 242, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, Y.J.; Lee, E.; Kim, M.S.; Kwack, S.J.; Kim, K.B.; Chung, K.K.; Kang, T.S.; Han, S.Y.; Lee, J.; et al. Effects of gestational exposure to decabromodiphenyl ether on reproductive parameters, thyroid hormone levels, and neuronal development in Sprague-Dawley rats offspring. J. Toxicol. Environ. Health A 2009, 72, 1296–1303. [Google Scholar] [CrossRef]
- Han, Z.; Li, Y.; Zhang, S.; Song, N.; Xu, H.; Dang, Y.; Liu, C.; Giesy, J.P.; Yu, H. Prenatal transfer of decabromodiphenyl ether (BDE209) results in disruption of the thyroid system and developmental toxicity in zebrafish offspring. Aquat. Toxicol. 2017, 190, 46–52. [Google Scholar] [CrossRef]
- Rajender, S.; Monica, M.G.; Walter, L.; Agarwal, A. Thyroid, spermatogenesis, and male infertility. Front. Biosci. 2011, E3, 843–855. [Google Scholar] [CrossRef]
- Harley, K.G.; Marks, A.R.; Chevrier, J.; Bradman, A.; Sjödin, A.; Eskenazi, B. PBDE concentrations in women’s serum and fecundability. Environ. Health Perspect. 2010, 118, 699–704. [Google Scholar] [CrossRef]
- Akutsu, K.; Takatori, S.; Nozawa, S.; Yoshiike, M.; Nakazaw, H.; Hayakawa, K.; Makino, T.; Iwamoto, T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol. 2008, 80, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Wang, S.Q. Updated reproductive toxicology of polybrominated diphenyl ether. Zhonghua Nan Ke Xue 2020, 26, 1140–1144. [Google Scholar] [PubMed]
- Kuriyama, S.N.; Talsness, C.E.; Grote, K.; Chahoud, I. Developmental exposure to low-dose PBDE-99: Effects on male fertility and neurobehavior in rat offspring. Environ. Health Perspect. 2005, 113, 149–154. [Google Scholar] [CrossRef]
- Tseng, L.H.; Lee, C.W.; Pan, M.H.; Tsai, S.S.; Li, M.H.; Chen, J.R.; Lay, J.J.; Hsu, P.C. Postnatal exposure of male mouse to 2,2′,3,3′,4,4′,5,5′,6,6′-decabrominated diphenyl ether: Decreased epididymal sperm functions without alterations in DNA content and histology in testis. Toxicology 2006, 224, 33–43. [Google Scholar] [CrossRef]
- van der Ven, L.T.; van de Kuil, T.; Leonards, P.E.; Slob, W.; Canton, R.F.; Germer, S.; Visser, T.J.; Litens, S.; Hakansson, H.; Schrenk, D.; et al. A 28-day oral dose toxicity study in Wistar rats enhanced to detect endocrine effects of decabromodiphenyl ether (decaBDE). Toxicol. Lett. 2008, 179, 6–14. [Google Scholar] [CrossRef]
- Khalil, A.; Parker, M.; Brown, S.E.; Cevik, S.E.; Guo, L.W.; Jensen, J.; Olmsted, A.; Portman, D.; Wu, H.; Suvorov, A. Perinatal exposure to 2,2′,4′4′ −Tetrabromodiphenyl ether induces testicular toxicity in adult rats. Toxicology 2017, 389, 21–30. [Google Scholar] [CrossRef] [PubMed]
- National Biomonitoring Program. Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyls (PBBs) Factsheet. Centers for Disease Control and Prevention. 2017. Available online: https://www.cdc.gov/biomonitoring/PBDEs_FactSheet.html (accessed on 24 January 2023).
- Sheikh, I.A. Stereoselectivity and potential endocrine disrupting activity of Bis-(2-ethylhexyl) phthalate (DEHP) against human progesterone receptor: A computational perspective. J. Appl. Toxicol. 2016, 36, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Zughaibi, T.A.; Sheikh, I.A.; Beg, M.A. Insights into the Endocrine Disrupting Activity of Emerging Non-Phthalate Alternate Plasticizers against Thyroid Hormone Receptor: A Structural Perspective. Toxics 2022, 10, 263. [Google Scholar] [CrossRef]


| Ligand | Number of Interacting TRα Residues | Percentage of Interacting Residues Common with Native Ligand (%) | IFD Score | Docking Score (Kcal/mol) | Glide Score (Kcal/mol) | MMGB-SA (Kcal/mol) |
|---|---|---|---|---|---|---|
| BDE-28 | 22 | 81.81 | −558.04 | −7.24 | −7.24 | −93.19 |
| BDE-100 | 21 | 85.75 | −558.16 | −7.03 | −7.03 | −114.81 |
| BDE-153 | 19 | 73.68 | −560.99 | −8.78 | −8.78 | −146.89 |
| BDE-154 | 18 | 94.44 | −561.93 | −8.85 | −8.85 | −133.29 |
| T3 | 23 | 100 | −564.42 | −9.44 | −9.44 | −133.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, I.A.; Beg, M.A.; Hamoda, T.A.-A.A.-M.; Mandourah, H.M.S.; Memili, E. An Analysis of the Structural Relationship between Thyroid Hormone-Signaling Disruption and Polybrominated Diphenyl Ethers: Potential Implications for Male Infertility. Int. J. Mol. Sci. 2023, 24, 3296. https://doi.org/10.3390/ijms24043296
Sheikh IA, Beg MA, Hamoda TA-AA-M, Mandourah HMS, Memili E. An Analysis of the Structural Relationship between Thyroid Hormone-Signaling Disruption and Polybrominated Diphenyl Ethers: Potential Implications for Male Infertility. International Journal of Molecular Sciences. 2023; 24(4):3296. https://doi.org/10.3390/ijms24043296
Chicago/Turabian StyleSheikh, Ishfaq Ahmad, Mohd Amin Beg, Taha Abo-Almagd Abdel-Meguid Hamoda, Hammam Mahmoud Siraj Mandourah, and Erdogan Memili. 2023. "An Analysis of the Structural Relationship between Thyroid Hormone-Signaling Disruption and Polybrominated Diphenyl Ethers: Potential Implications for Male Infertility" International Journal of Molecular Sciences 24, no. 4: 3296. https://doi.org/10.3390/ijms24043296
APA StyleSheikh, I. A., Beg, M. A., Hamoda, T. A.-A. A.-M., Mandourah, H. M. S., & Memili, E. (2023). An Analysis of the Structural Relationship between Thyroid Hormone-Signaling Disruption and Polybrominated Diphenyl Ethers: Potential Implications for Male Infertility. International Journal of Molecular Sciences, 24(4), 3296. https://doi.org/10.3390/ijms24043296






