Investigating Circular RNAs Using qRT-PCR; Roundup of Optimization and Processing Steps
Abstract
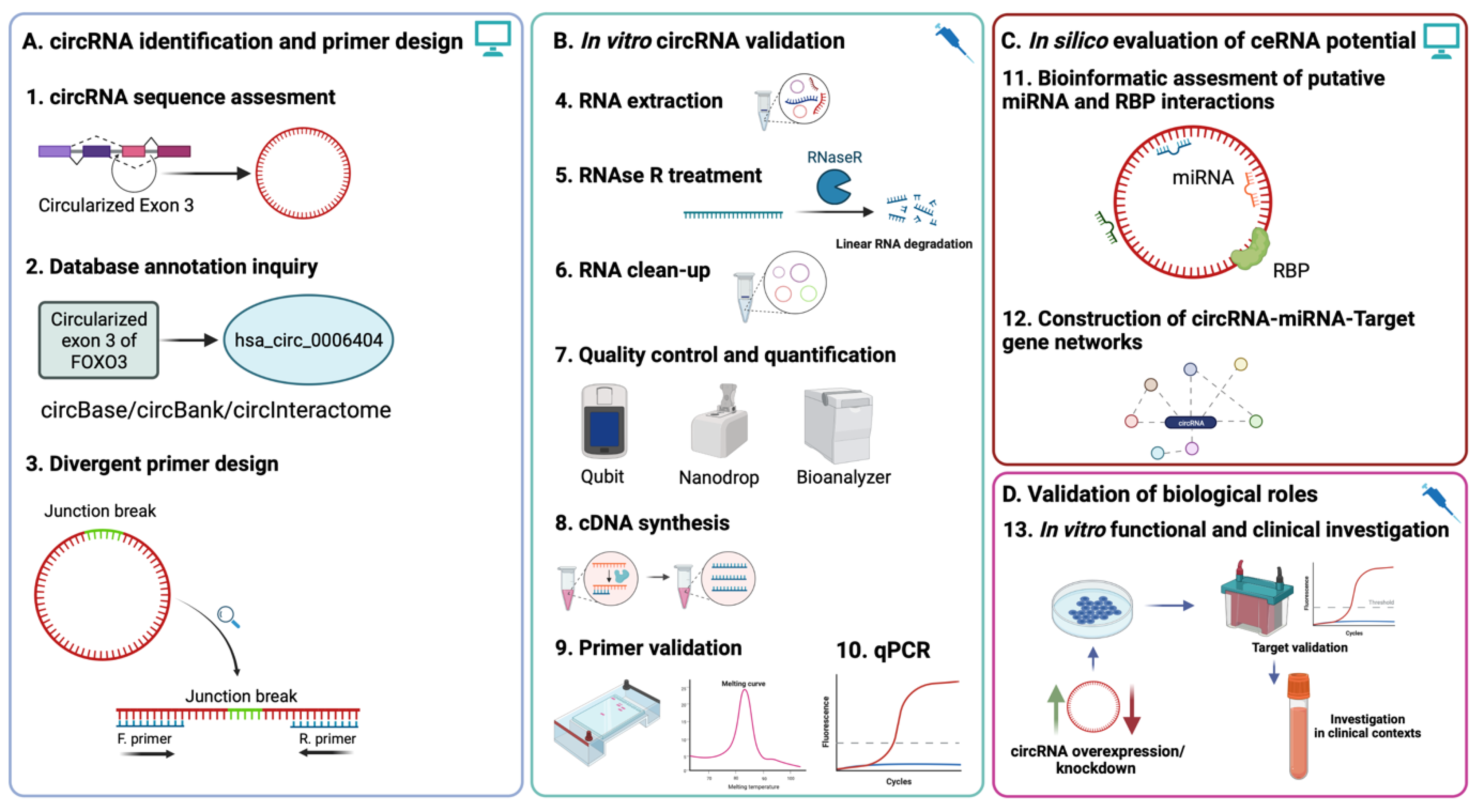
:1. Introduction
2. Results
2.1. Database Annotation, Composition, and Sequence Characterization of circRNAs
2.2. Junction Sequence Mapping and Primer Design
2.3. Primer Validation
2.4. RNAse R Treatment Optimization and RNA Quality Control
2.5. Assessing Enrichment of circRNA following RNAse R Treatment
2.6. Database Prediction of Interacting miRNAs and Construction of circRNA-miRNA-mRNA Networks
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. RNA Extraction
4.3. RNAse R Treatment
4.4. RNA Clean-Up and Quantification
4.5. cDNA Synthesis
4.6. Primer Validation PCR and Agarose Gel Electrophoresis
4.7. qRT-PCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, T.; Bindereif, A. Circular RNAs: Coding or Noncoding? Cell Res. 2017, 27, 724–725. [Google Scholar] [CrossRef] [Green Version]
- Kong, S.; Tao, M.; Shen, X.; Ju, S. Translatable CircRNAs and LncRNAs: Driving Mechanisms and Functions of Their Translation Products. Cancer Lett. 2020, 483, 59–65. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. CircRNA Biogenesis Competes with Pre-MRNA Splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids Are Single-Stranded Covalently Closed Circular RNA Molecules Existing as Highly Base-Paired Rod-like Structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [Green Version]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, Present, and Future of CircRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-O.; Dong, R.; Zhang, Y.; Zhang, J.-L.; Luo, Z.; Zhang, J.; Chen, L.-L.; Yang, L. Diverse Alternative Back-Splicing and Alternative Splicing Landscape of Circular RNAs. Genome Res. 2016, 26, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-O.; Wang, H.-B.; Zhang, Y.; Lu, X.; Chen, L.-L.; Yang, L. Complementary Sequence-Mediated Exon Circularization. Cell 2014, 159, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.; Memczak, S.; Wyler, E.; Torti, F.; Porath, H.T.; Orejuela, M.R.; Piechotta, M.; Levanon, E.Y.; Landthaler, M.; Dieterich, C.; et al. Analysis of Intron Sequences Reveals Hallmarks of Circular RNA Biogenesis in Animals. Cell Rep. 2015, 10, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.-H.; Bindereif, A. Exon Circularization Requires Canonical Splice Signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-L. The Expanding Regulatory Mechanisms and Cellular Functions of Circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient MicroRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Lu, T.; Cui, L.; Zhou, Y.; Zhu, C.; Fan, D.; Gong, H.; Zhao, Q.; Zhou, C.; Zhao, Y.; Lu, D.; et al. Transcriptome-Wide Investigation of Circular RNAs in Rice. RNA 2015, 21, 2076–2087. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhao, F. Computational Strategies for Exploring Circular RNAs. Trends Genet. 2018, 34, 389–400. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, J.; Zhao, F. Accurate Quantification of Circular RNAs Identifies Extensive Circular Isoform Switching Events. Nat. Commun. 2020, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Vromman, M.; Vandesompele, J.; Volders, P.-J. Closing the Circle: Current State and Perspectives of Circular RNA Databases. Brief. Bioinform. 2021, 22, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, C.; Sun, H.; Wang, J.; Liang, Y.; Wang, Y.; Wong, G. The Bioinformatics Toolbox for CircRNA Discovery and Analysis. Brief. Bioinform. 2021, 22, 1706–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, M. Circular RNA Databases. Methods Mol. Biol. 2021, 2362, 109–118. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, P.; Chen, M. Database Resources for Functional Circular RNAs. Methods Mol. Biol. 2021, 2284, 457–466. [Google Scholar] [CrossRef]
- Rahimi, K.; Venø, M.T.; Dupont, D.M.; Kjems, J. Nanopore Sequencing of Brain-Derived Full-Length CircRNAs Reveals CircRNA-Specific Exon Usage, Intron Retention and Microexons. Nat. Commun. 2021, 12, 4825. [Google Scholar] [CrossRef]
- Zhou, R.-M.; Shi, L.-J.; Shan, K.; Sun, Y.-N.; Wang, S.-S.; Zhang, S.-J.; Li, X.-M.; Jiang, Q.; Yan, B.; Zhao, C. Circular RNA-ZBTB44 Regulates the Development of Choroidal Neovascularization. Theranostics 2020, 10, 3293–3307. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shan, G. CircRNA in Cancer: Fundamental Mechanism and Clinical Potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef]
- Drula, R.; Braicu, C.; Harangus, A.; Nabavi, S.M.; Trif, M.; Slaby, O.; Ionescu, C.; Irimie, A.; Berindan-Neagoe, I. Critical Function of Circular RNAs in Lung Cancer. Wiley Interdiscip. Rev. RNA 2020, 11, e1592. [Google Scholar] [CrossRef]
- Pandey, P.R.; Munk, R.; Kundu, G.; De, S.; Abdelmohsen, K.; Gorospe, M. Methods for Analysis of Circular RNAs. Wiley Interdiscip. Rev. RNA 2020, 11, e1566. [Google Scholar] [CrossRef]
- Drula, R.; Pirlog, R.; Trif, M.; Slaby, O.; Braicu, C.; Berindan-Neagoe, I. CircFOXO3: Going around the Mechanistic Networks in Cancer by Interfering with MiRNAs Regulatory Networks. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2021, 1867, 166045. [Google Scholar] [CrossRef]
- Xiang, T.; Jiang, H.-S.; Zhang, B.-T.; Liu, G. CircFOXO3 Functions as a Molecular Sponge for MiR-143-3p to Promote the Progression of Gastric Carcinoma via Upregulating USP44. Gene 2020, 753, 144798. [Google Scholar] [CrossRef]
- Kong, Z.; Wan, X.; Lu, Y.; Zhang, Y.; Huang, Y.; Xu, Y.; Liu, Y.; Zhao, P.; Xiang, X.; Li, L.; et al. Circular RNA CircFOXO3 Promotes Prostate Cancer Progression through Sponging MiR-29a-3p. J. Cell. Mol. Med. 2020, 24, 799–813. [Google Scholar] [CrossRef] [Green Version]
- Glažar, P.; Papavasileiou, P.; Rajewsky, N. CircBase: A Database for Circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, Q.; Shen, J.; Yang, B.B.; Ding, X. Circbank: A Comprehensive Database for CircRNA with Standard Nomenclature. RNA Biol. 2019, 16, 899–905. [Google Scholar] [CrossRef]
- Liu, X.; Du, Z.; Yi, X.; Sheng, T.; Yuan, J.; Jia, J. Circular RNA CircANAPC2 Mediates the Impairment of Endochondral Ossification by MiR-874-3p/SMAD3 Signalling Pathway in Idiopathic Short Stature. J. Cell. Mol. Med. 2021, 25, 3408–3426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Zhong, J.; Lin, L. Circ-ACAP2 Facilitates the Progression of Colorectal Cancer through Mediating MiR-143-3p/FZD4 Axis. Eur. J. Clin. Investig. 2021, 51, e13607. [Google Scholar] [CrossRef]
- Zhu, J.; Xiang, X.-L.; Cai, P.; Jiang, Y.-L.; Zhu, Z.-W.; Hu, F.-L.; Wang, J. CircRNA-ACAP2 Contributes to the Invasion, Migration, and Anti-Apoptosis of Neuroblastoma Cells through Targeting the MiRNA-143-3p-Hexokinase 2 Axis. Transl. Pediatr. 2021, 10, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Yang, Z.; Wen, D.; Hu, Z. Circular RNA CircACAP2 Suppresses Ferroptosis of Cervical Cancer during Malignant Progression by MiR-193a-5p/GPX4. J. Oncol. 2022, 2022, 5228874. [Google Scholar] [CrossRef]
- Zhao, B.; Song, X.; Guan, H. CircACAP2 Promotes Breast Cancer Proliferation and Metastasis by Targeting MiR-29a/b-3p-COL5A1 Axis. Life Sci. 2020, 244, 117179. [Google Scholar] [CrossRef]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-S.; Wilusz, J.E. An Improved Method for Circular RNA Purification Using RNAse R That Efficiently Removes Linear RNAs Containing G-Quadruplexes or Structured 3′ Ends. Nucleic Acids Res. 2019, 47, 8755–8769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, A.C.; Gorospe, M. Detection and Analysis of Circular RNAs by RT-PCR. Bio-Protoc. J. 2018, 8, e2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate QPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-H.; Liu, S.; Zhou, H.; Qu, L.-H.; Yang, J.-H. StarBase v2.0: Decoding MiRNA-CeRNA, MiRNA-NcRNA and Protein-RNA Interaction Networks from Large-Scale CLIP-Seq Data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [Green Version]
- CSCD: A Database for Cancer-Specific Circular RNAs—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29036403/ (accessed on 23 August 2022).
- Lyu, Y.; Caudron-Herger, M.; Diederichs, S. Circ2GO: A Database Linking Circular RNAs to Gene Function. Cancers 2020, 12, E2975. [Google Scholar] [CrossRef]
- Dudekula, D.B.; Panda, A.C.; Grammatikakis, I.; De, S.; Abdelmohsen, K.; Gorospe, M. CircInteractome: A Web Tool for Exploring Circular RNAs and Their Interacting Proteins and MicroRNAs. RNA Biol. 2016, 13, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targeting Circular RNAs as a Therapeutic Approach: Current Strategies and Challenges|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-021-00569-5 (accessed on 4 September 2022).
- Danac, J.M.C.; Garcia, R.L. CircPVT1 Attenuates Negative Regulation of NRAS by Let-7 and Drives Cancer Cells towards Oncogenicity. Sci. Rep. 2021, 11, 9021. [Google Scholar] [CrossRef]
- Shi, J.; Lv, X.; Zeng, L.; Li, W.; Zhong, Y.; Yuan, J.; Deng, S.; Liu, B.; Yuan, B.; Chen, Y.; et al. CircPVT1 Promotes Proliferation of Lung Squamous Cell Carcinoma by Binding to MiR-30d/e. J. Exp. Clin. Cancer Res. 2021, 40, 193. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Taheri, M.; Jamali, E. A Concise Review on the Role of CircPVT1 in Tumorigenesis, Drug Sensitivity, and Cancer Prognosis. Front. Oncol. 2021, 11, 762960. [Google Scholar] [CrossRef]
- Vromman, M.; Yigit, N.; Verniers, K.; Lefever, S.; Vandesompele, J.; Volders, P.-J. Validation of Circular RNAs Using RT-QPCR After Effective Removal of Linear RNAs by Ribonuclease R. Curr. Protoc. 2021, 1, e181. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.R.; Rout, P.K.; Das, A.; Gorospe, M.; Panda, A.C. RPAD (RNAse R Treatment, Polyadenylation, and Poly(A)+ RNA Depletion) Method to Isolate Highly Pure Circular RNA. Methods 2019, 155, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, B.; Huang, S. Identification of CircRNAs for MiRNA Targets by Argonaute2 RNA Immunoprecipitation and Luciferase Screening Assays. Methods Mol. Biol. 2018, 1724, 209–218. [Google Scholar] [CrossRef]
- Das, A.; Das, D.; Panda, A.C. Validation of Circular RNAs by PCR. Methods Mol. Biol. 2022, 2392, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Schreiner, S.; Preußer, C.; Bindereif, A.; Rossbach, O. Northern Blot Analysis of Circular RNAs. Methods Mol. Biol. 2018, 1724, 119–133. [Google Scholar] [CrossRef] [PubMed]





| CircRNA | Parental Gene | CircBase ID | Structure | Genomic Location | Length |
|---|---|---|---|---|---|
| circFOXO3 | FOXO3 | hsa_circ_0006404 | Exon 2 | chr6:108984657-108986092 | 1435 bp |
| circPVT1 | PVT1 lncRNA | hsa_circ_0009143 | Exon 2 | chr8:128867400-128903244 | 410 bp |
| circANAPC2 | ANAPC2 | Unannotated variant | Exon 1 | chr9:137188416-137188560 | 145 bp |
| circACAP2 | ACAP2 | Unannotated variant | Exon 3-1 | Chr3:195442795-195381903 | 404 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drula, R.; Braicu, C.; Chira, S.; Berindan-Neagoe, I. Investigating Circular RNAs Using qRT-PCR; Roundup of Optimization and Processing Steps. Int. J. Mol. Sci. 2023, 24, 5721. https://doi.org/10.3390/ijms24065721
Drula R, Braicu C, Chira S, Berindan-Neagoe I. Investigating Circular RNAs Using qRT-PCR; Roundup of Optimization and Processing Steps. International Journal of Molecular Sciences. 2023; 24(6):5721. https://doi.org/10.3390/ijms24065721
Chicago/Turabian StyleDrula, Rares, Cornelia Braicu, Sergiu Chira, and Ioana Berindan-Neagoe. 2023. "Investigating Circular RNAs Using qRT-PCR; Roundup of Optimization and Processing Steps" International Journal of Molecular Sciences 24, no. 6: 5721. https://doi.org/10.3390/ijms24065721
APA StyleDrula, R., Braicu, C., Chira, S., & Berindan-Neagoe, I. (2023). Investigating Circular RNAs Using qRT-PCR; Roundup of Optimization and Processing Steps. International Journal of Molecular Sciences, 24(6), 5721. https://doi.org/10.3390/ijms24065721








