Cadmium Exposure Is Associated with Behavioral Deficits and Neuroimmune Dysfunction in BTBR T+ Itpr3tf/J Mice
Abstract
1. Introduction
2. Results
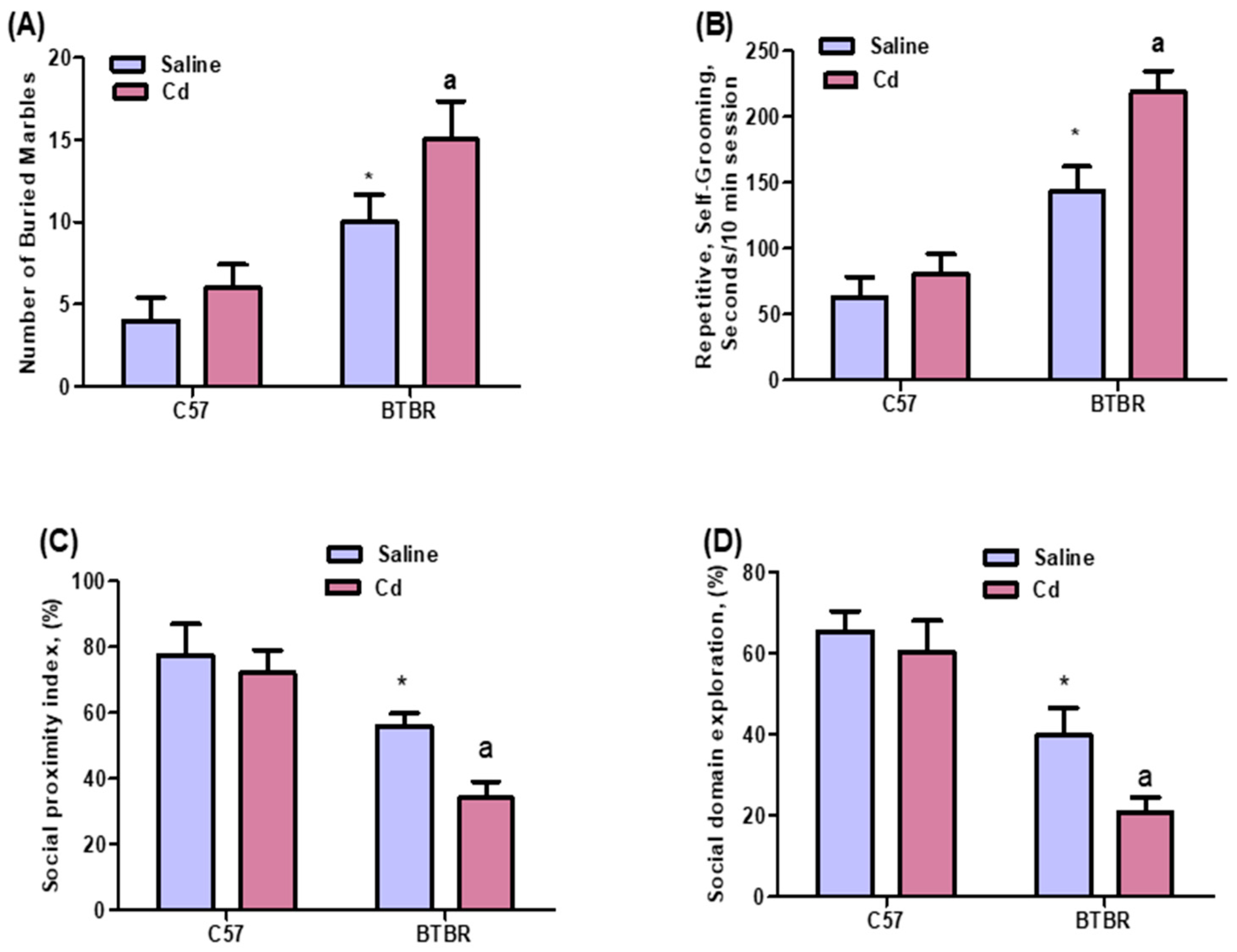
2.1. Cd Exposure Alters Repetitive and Social Behaviors
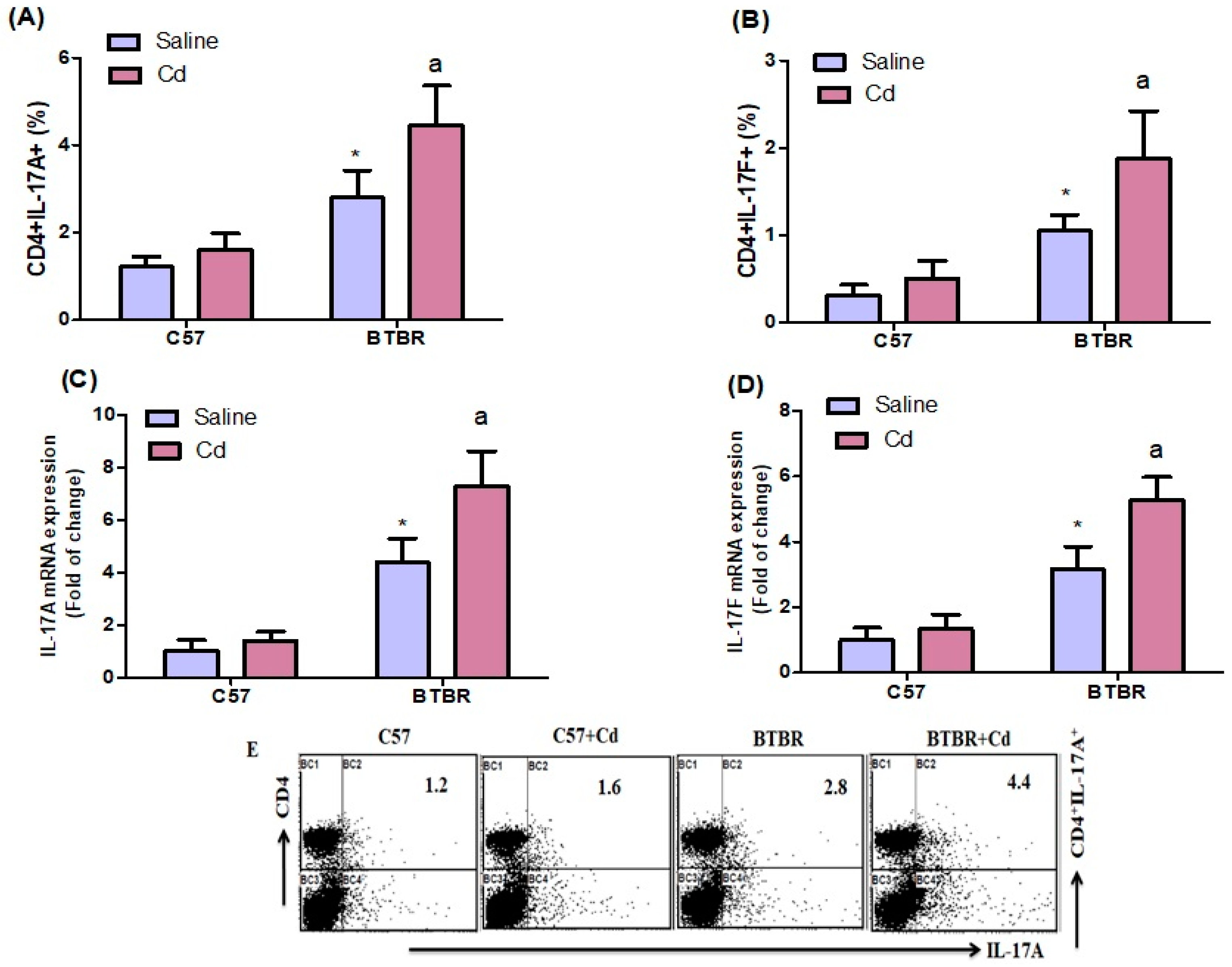
2.2. Cd Exposure Increases IL-17A/IL-17F Cytokine Expression
2.3. Cd Exposure Upregulates IL-21 Expression
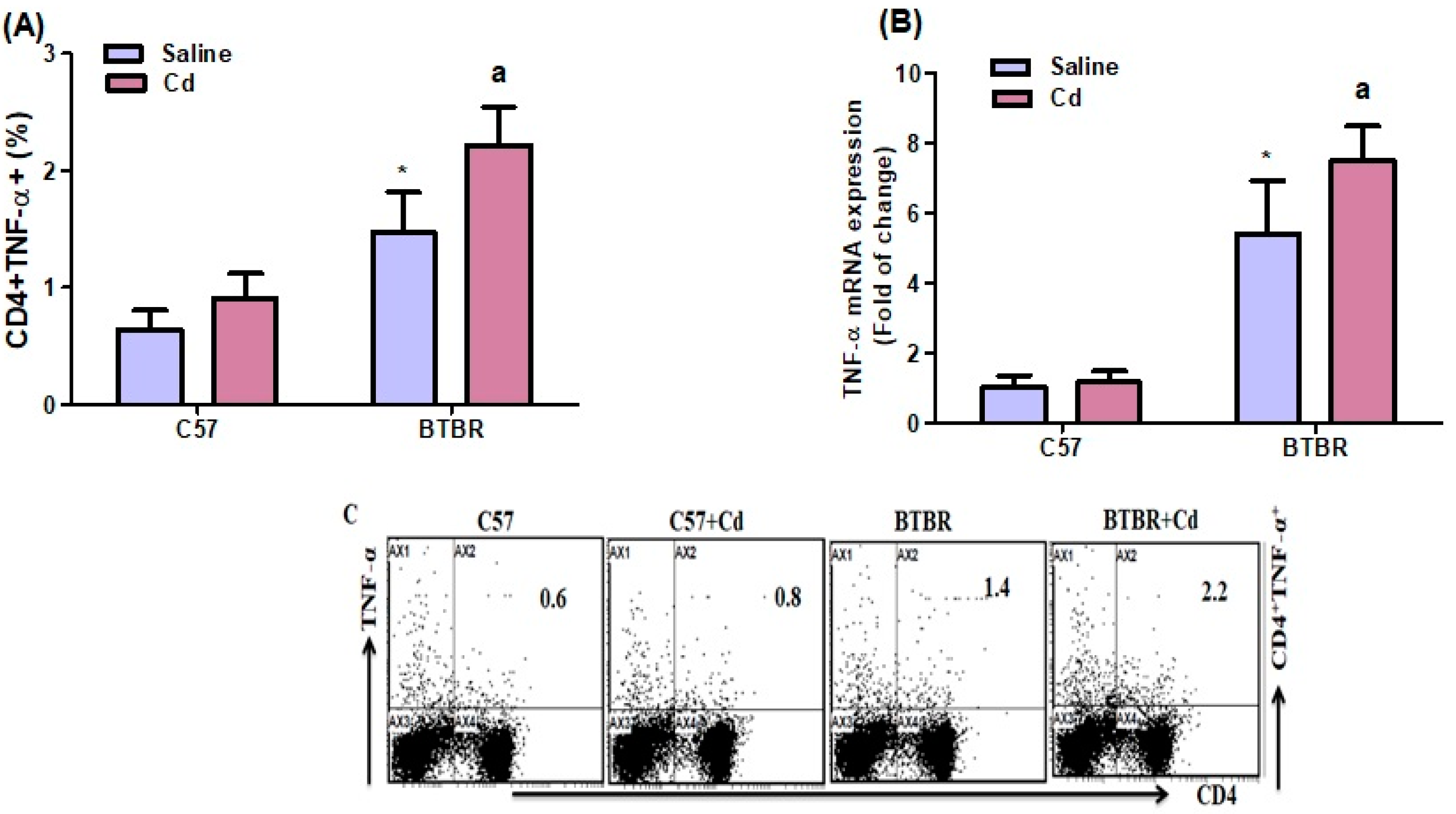
2.4. Cd Exposure Increases TNF-α Expression
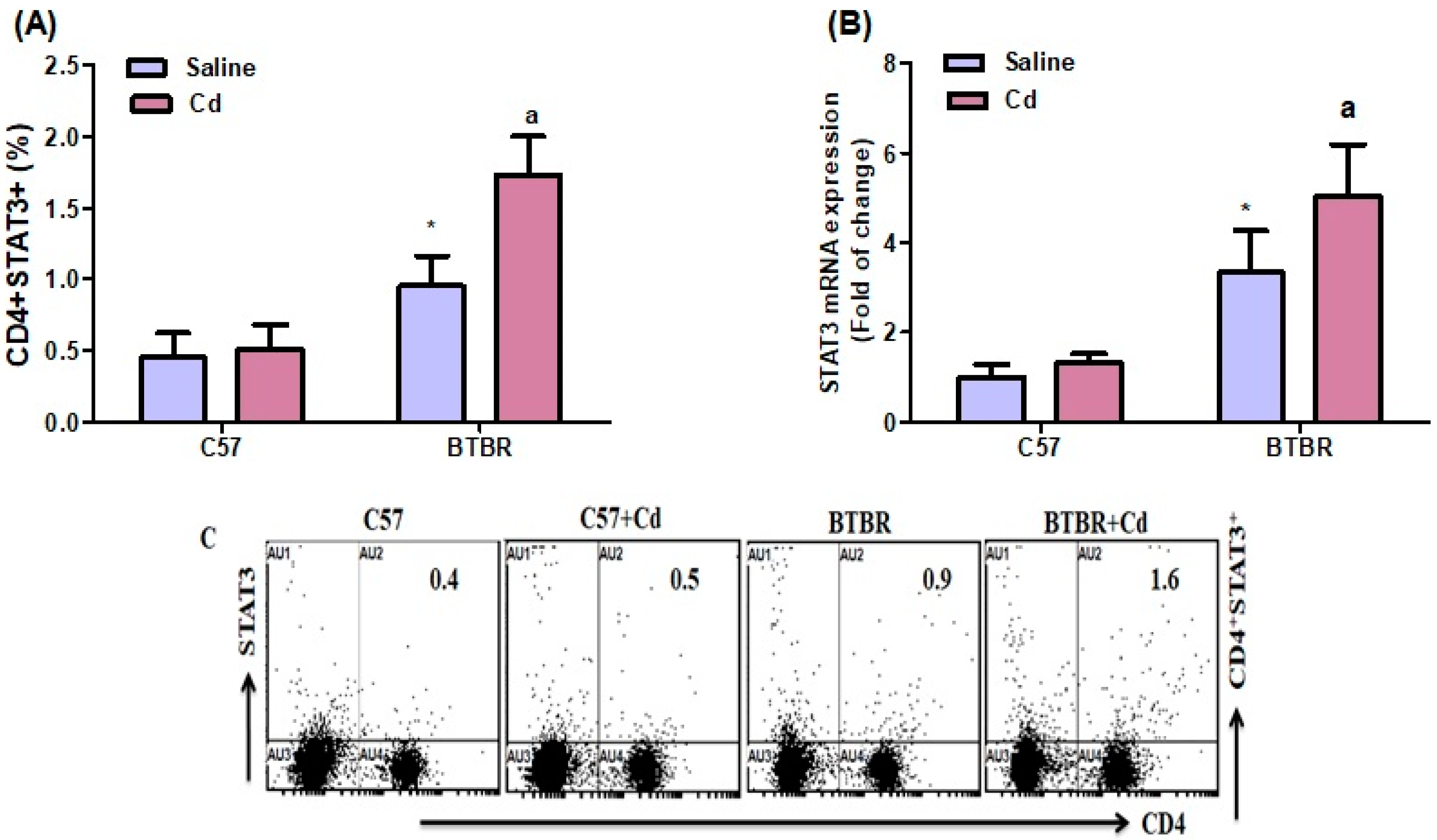
2.5. Cd Exposure Increases STAT-3 Expression
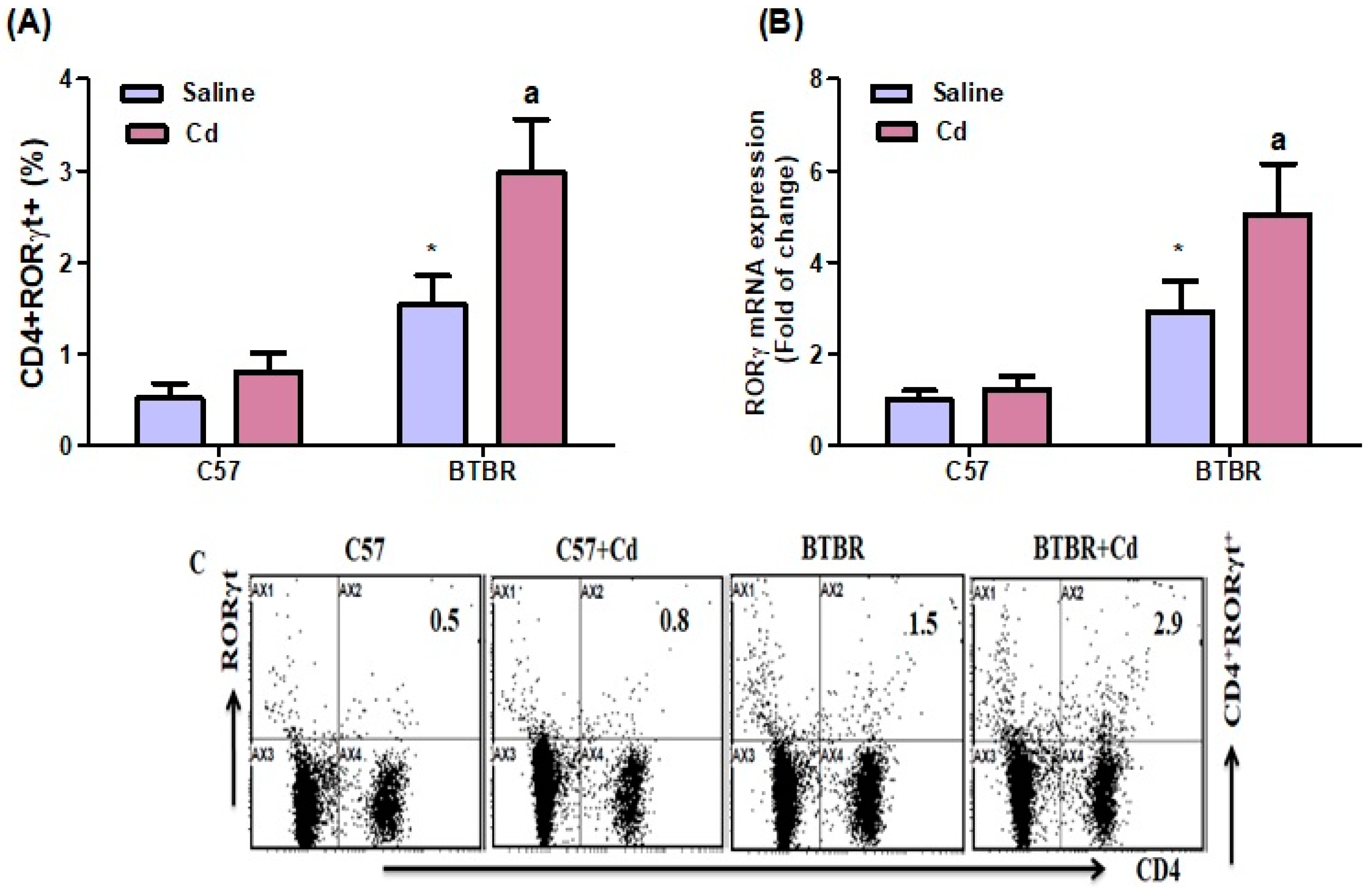
2.6. Cd Exposure Upregulates RORγT Transcription-Factor Signaling
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Animals and Cd Administration
4.3. Self-Grooming
4.4. Marble-Burying Test
4.5. Three-Chamber Test
4.6. Preparation of Mouse Spleen Cells
4.7. Flow Cytometry
4.8. Gene Expression
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Autism Spectrum Disorder; American Psychiatric Publishing: Washington, DC, USA, 2015. [Google Scholar]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef]
- Freeman, K. Nutrient protection against arsenic toxicity: Folate, cysteine support methylation in children. Environ. Health Perspect. 2009, 117, A211. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ahn, R.; Van den Bossche, J.; Thompson, D.H.; O’Halloran, T.V. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol. Cancer Ther. 2009, 8, 195563. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Liu, X.; Slavkovich, V.; Ilievski, V.; Pilsner, J.R.; Alam, S.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Folate, Cobalamin, Cysteine, Homocysteine, and Arsenic Metabolism among Children in Bangladesh. Environ. Health Perspect. 2009, 117, 825–831. [Google Scholar] [CrossRef]
- Hughes, H.K.; Mills Ko, E.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Bakheet, S.A.; Ibrahim, K.E.; Alqahtani, F.; Alqinyah, M. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T+ tf/J mice. Behav. Brain Res. 2019, 364, 213–224. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van de Water, J. Altered T cell responses in children with autism. Brain Behav. Immun. 2011, 25, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Zoheir, K.M.A.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; AL-Ayadhi, L.Y.; Alzahrani, M.Z.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Attia, M. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol. Neurobiol. 2017, 54, 4390–4400. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Alzahrani, M.Z.; Ansari, M.A.; Nadeem, A.; Zoheir, K.M.A.; Attia, S.M.; AL-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol Ameliorates Dysregulation of Th1, Th2, Th17, and T Regulatory Cell-Related Transcription Factor Signaling in a BTBR T+ tf/J Mouse Model of Autism. Mol. Neurobiol. 2017, 54, 5201–5212. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Bonefeld-Jørgensen, E.C.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, E.L. Neonatal chemokine levels and risk of autism spectrum disorders: Findings from a Danish historic birth cohort follow-up study. Cytokine 2013, 61, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayadhi, L.Y.; Mostafa, G.A. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflamm. 2012, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Hoeffer, C. Maternal IL-17A in autism. Exp. Neurol. 2018, 299 Pt A, 228–240. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Haim, L.B.; Ceyzériat, K.; Carrillo-de Sauvage, M.A.; Aubry, F.; Auregan, G.; Guillermier, M.; Ruiz, M.; Ruiz, F.; Houitte, D.; Houitte, E.; et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 2015, 35, 2817–2829. [Google Scholar] [CrossRef]
- Khan, D.; Fernando, P.; Cicvaric, A.; Berger, A.; Pollak, A.; Monje, F.J.; Pollak, D.D. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl. Psychiatry 2014, 4, e363. [Google Scholar] [CrossRef]
- Parker-Athill, E.; Luo, D.; Bailey, A.; Giunta, B.; Tian, J.; Shytle, R.D.; Murphy, T.; Legradi, G.; Tan, J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J. Neuroimmunol. 2009, 217, 20–27. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Craner, M.J.; Friese, M.A.; Jakobsen, K.B.; Newcombe, J.; Esiri, M.M.; Fugger, L. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am. J. Pathol. 2011, 178, 794–802. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Ling, C.; Shi, Y.; Harris, M.G.; Rayasam, A.; Sun, D.; Salamat, M.S.; Kuchroo, V.; Lambris, J.D.; Sandor, M.; et al. T cell-derived interleukin (IL)-21 promotes brain injury following stroke in mice. J. Exp. Med. 2014, 211, 595–604. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Attia, S.M.; Zoheir, K.M.; Al-Ayadhi, L.Y.; Alzahrani, M.Z.; Alsaad, A.M.; Alotaibi, M.R.; et al. Imbalance between the anti- and pro-inflammatory milieu in blood leukocytes of autistic children. Mol. Immunol. 2017, 82, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chez, M.G.; Dowling, T.; Patel, P.B.; Khanna, P.; Kominsky, M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007, 36, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Henn, B.C.; Schnaas, L.; Ettinger, A.S.; Schwartz, J.; Lamadrid-Figueroa, H.; Hernández-Avila, M.; Amarasiriwardena, C.; Hu, H.; Bellinger, D.C.; Wright, B.R.; et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 2012, 120, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kwon, H.J.; Lim, M.H.; Jee, Y.K.; Hong, Y.C.; Leem, J.H.; Sakong, J.; Bae, J.M.; Hong, S.J.; Roh, Y.M.; et al. Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: A report of the children’s health and environment research (CHEER). Neurotoxicology 2009, 30, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ran, G.; Lu, G.; Ni, X.; Liu, D.; Sun, J.; Xie, C.; Yao, D.; Bai, W. Highly sensitive label-free electrochemical aptasensor based on screen-printed electrode for detection of cadmium (II) ions. J. Electrochem. Soc. 2019, 166, B449–B455. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Long, M.; Luo, T.; Bian, J.; Liu, X.; Gu, J.; Zou, H.; Song, R.; Wang, Y.; et al. Beclin-1-mediated Autophagy Protects Against Cadmium-activated Apoptosis via the Fas/FasL Pathway in Primary Rat Proximal Tubular Cell Culture. Sci. Rep. 2017, 7, 977. [Google Scholar] [CrossRef]
- Thevenod, F.; Lee, W.K. Toxicology of cadmium and its damage to mammalian organs. Met. Ions Life Sci. 2013, 11, 415–490. [Google Scholar]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine Modulates Cadmium-Induced Oxidative Stress, Neuroinflammation, and Cognitive Impairments by Regulating Nrf-2/HO-1 In Vivo and In Vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef]
- Olszowski, T.; Gutowska, I.; Baranowska-Bosiacka, I.; Łukomska, A.; Drozd, A.; Chlubek, D. Cadmium Alters the Concentration of Fatty Acids in THP-1 Macrophages. Biol. Trace Elem. Res. 2018, 182, 29–36. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; Amin, O.R.; Dessoki, H.H.; Rabah, T. Toxic Metals and Essential Elements in Hair and Severity of Symptoms among Children with Autism. Maedica 2012, 7, 38–48. [Google Scholar] [PubMed]
- Ciesielski, T.; Weuve, J.; Bellinger, D.C.; Schwartz, J.; Lanphear, B.; Wright, R.O. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 2012, 120, 758–763. [Google Scholar] [CrossRef]
- Yorbik, O.; Kurt, I.; Haşimi, A.; Oztürk, O. Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol. Trace Elem. Res. 2010, 135, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Chiu, S.J.; Lin, K.A.; Lin, L.Y. Effect of cadmium on cell cycle progression in Chinese hamster ovary cells. Chem. Biol. Interact. 2004, 149, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Mar, W.; Cho, M.H. Cadmium affects genes involved in growth regulation during twostage transformation of Balb/3T3 cells. Toxicology 2002, 177, 253–265. [Google Scholar] [CrossRef]
- Dong, S.; Shen, H.M.; Ong, C.N. Cadmium-induced apoptosis and phenotypic changes in mouse thymocytes. Mol. Cell. Biochem. 2001, 222, 11–20. [Google Scholar] [CrossRef]
- Kern, J.K.; Grannemann, B.D.; Trivedi, M.H.; Adams, J.B. Sulfhydryl-reactive metals in autism. J. Toxicol. Environ. Health A 2007, 70, 715–721. [Google Scholar] [CrossRef]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, D.; Kluetzman, K.; Mendoza, A.; Bolivar, V.J.; Reilly, A.; Jolly, J.K.; Lawrence, D.A. The maternal autoimmune environment affects the social behavior of offspring. J. Neuroimmunol. 2013, 258, 51–60. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alsanea, S.; Al-Hosaini, K.A.; Mahmood, H.M.; Alzahrani, M.Z.; Attia, S.M. Inhibition of tyrosine kinase signaling by tyrphostin AG126 downregulates the IL-21/IL-21R and JAK/STAT pathway in the BTBR mouse model of autism. Neurotoxicology 2020, 77, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Gene Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Attia, S.M.; Albekairi, T.H.; Alhosaini, K.; Algahtani, M.; Alsaad, A.M.S.; Al-Mazroua, H.A.; et al. Lead (Pb) exposure exacerbates behavioral and immune abnormalities by upregulating Th17 and NF-κB-related signaling in BTBR T+ Itpr3tf/J autistic mouse model. Neurotoxicology 2022, 91, 340–348. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Alzahrani, M.Z.; Nadeem, A.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; Al-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol treatment attenuates chemokine receptor expression in the BTBR T+tf/J mouse model of autism. Mol. Cell. Neurosci. 2016, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cimmino, F.; Cavaliere, G.; Catapano, A.; Fogliano, C.; Lama, A.; Pirozzi, C.; Cristiano, C.; Russo, R.; Petrella, L.; et al. The Hepatic Mitochondrial Alterations Exacerbate Meta-Inflammation in Autism Spectrum Disorders. Antioxidants 2022, 11, 1990. [Google Scholar] [CrossRef]
- Fido, A.; Al-Saad, S. Toxic trace elements in the hair of children with autism. Autism 2005, 9, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.B.; Allshire, A.; van Pelt, F.N. In the acute reporter antigen-popliteal lymph node assay, immune modulation by cadmium and lead. Toxicol. Sci. 2006, 91, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, N.Y.; Emmrich, F.; Sack, U.; Wichmann, G.; Lehmann, J.; Adham, K.; Lehmann, I. The in vitro immune modulation by cadmium depends on the way of cell activation. Toxicology 2006, 222, 37–45. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Morucci, G.; Pacini, A. Cadmium-induced neurotoxicity: Still much ado. Neural Regen. Res. 2018, 13, 1879–1882. [Google Scholar] [PubMed]
- Richter, P.; Faroon, O.; Pappas, R.S. Cadmium and Cadmium/Zinc Ratios and Tobacco-Related Morbidities. Int. J. Environ. Res. Public Health 2017, 14, 1154. [Google Scholar] [CrossRef] [PubMed]
- Demenesku, J.; Mirkov, I.; Ninkov, M.; Popov Aleksandrov, A.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Acute cadmium administration to rats exerts both immunosuppressive and proinflammatory effects in spleen. Toxicology 2014, 326, 96–108. [Google Scholar] [CrossRef]
- Djokic, J.; Popov Aleksandrov, A.; Ninkov, M.; Mirkov, I.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Cadmium administration affects circulatory mononuclear cells in rats. J. Immunotoxicol. 2015, 12, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Cai, D.; Li, P.; Jin, J.; Jiang, X.; Li, Z.; Tian, L.; Chen, G.; Sun, J.; et al. Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem. Toxicol. 2021, 148, 111944. [Google Scholar] [CrossRef] [PubMed]
- Phuagkhaopong, S.; Ospondpant, D.; Kasemsuk, T.; Sibmooh, N.; Soodvilai, S.; Power, C.; Vivithanaporn, P. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology 2017, 60, 82–91. [Google Scholar] [CrossRef]
- Seok, S.M.; Park, D.H.; Kim, Y.C.; Moon, C.H.; Jung, Y.S.; Baik, E.J.; Moon, C.K.; Lee, S.H. COX-2 is associated with cadmium-induced ICAM-1 expression in cerebrovascular endothelial cells. Toxicol. Lett. 2006, 165, 212–220. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Gu, C.; Zhang, H.; Zhang, R.; Dong, X.; Liu, C.; Hu, X.; Ji, X.; Huang, S.; et al. Celastrol ameliorates Cd-induced neuronal apoptosis by targeting NOX2-derived ROS-dependent PP5-JNK signaling pathway. J. Neurochem. 2017, 141, 48–62. [Google Scholar] [CrossRef]
- Careaga, M.; Schwartzer, J.; Ashwood, P. Inflammatory profiles in the BTBR mouse: How relevant are they to autism spectrum disorders? Brain Behav. Immun. 2015, 43, 11–16. [Google Scholar] [CrossRef]
- Akintunde, M.E.; Rose, M.; Krakowiak, P.; Heuer, L.; Ashwood, P.; Hansen, R.; Hertz-Picciotto, I.; Van de Water, J. Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. J. Neuroimmunol. 2015, 286, 33–41. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Chow, J.; Mazmanian, S.K.; Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 2012, 109, 12776–12781. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tome, S.; Takei, Y. Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Mol. Brain 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jäger, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Xu, X.; Xiong, G.-L.; Xu, Q.; Zhou, B.-R.; Li, C.-Y.; Qin, Q.; Liu, C.-X.; Li, H.-P.; Sun, Y.-J.; et al. Alterations in plasma cytokine levels in chinese children with autism spectrum disorder. Autism Res. 2018, 11, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Weissenbock, H.; Horscroft, N.; Lipkin, W.I. An infection-based model of neurodevelopmental damage. Proc. Natl. Acad. Sci. USA 1999, 96, 12102–12107. [Google Scholar] [CrossRef]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef]
- Packer, A. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci. Biobehav. Rev. 2016, 64, 185–195. [Google Scholar] [CrossRef]
- Chez, M.; Low, R.; Parise, C.; Donnel, T. Safety and observations in a pilot study of lenalidomide for treatment in autism. Autism Res. Treat. 2012, 2012, 291601. [Google Scholar] [CrossRef]
- Yamauchi, T.; Makinodan, M.; Toritsuka, M.; Okumura, K.; Kayashima, Y.; Ishida, R.; Kishimoto, N.; Takahashi, M.; Komori, T.; Yamaguchi, Y.; et al. Tumor necrosis factor-α expression aberration of M1/M2 macrophages in adult high-functioning autism spectrum disorder. Autism Res. 2021, 14, 2330–2341. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Cheng, M.; Zhao, Y.; Wang, M.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J. Neuroinflamm. 2017, 14, 187. [Google Scholar] [CrossRef]
- Nelson, T.E.; Olde Engberink, A.; Hernandez, R.; Puro, A.; Huitron-Resendiz, S.; Hao, C.; De Graan, P.N.; Gruol, D.L. Altered synaptic transmission in the hippocampus of transgenic mice with enhanced central nervous systems expression of interleukin-6. Brain Behav. Immun. 2012, 26, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Raish, M.; Ahmad, S.F. Adenosine A2A receptor modulates neuroimmune function through Th17/retinoid-related orphan receptor gamma t (RORγt) signaling in a BTBR T+ Itpr3tf/J mouse model of autism. Cell. Signal. 2017, 36, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Pantelyushin, S.; Haak, S.; Ingold, B.; Kulig, P.; Heppner, F.L.; Navarini, A.A.; Becher, B. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Investig. 2012, 122, 2252–2256. [Google Scholar]
- Mickael, M.E.; Bhaumik, S.; Chakraborti, A.; Umfress, A.A.; van Groen, T.; Macaluso, M.; Totenhagen, J.; Sorace, A.G.; Bibb, J.A.; Standaert, D.G.; et al. RORγt-Expressing Pathogenic CD4+ T Cells Cause Brain Inflammation during Chronic Colitis. J. Immunol. 2022, 208, 2054–2066. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Winger, R.C.; Lee, P.W.; Nuro-Gyina, P.K.; Minc, A.; Larson, M.; Liu, Y.; Pei, W.; Rieser, E.; Racke, M.K.; et al. Impact of suppressing retinoic acid-related orphan receptor gamma t (ROR)γt in ameliorating central nervous system autoimmunity. Clin. Exp. Immunol. 2015, 179, 108–118. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; El-Sherbeeny, A.M.; Al-Harbi, N.O.; Bakheet, S.A.; Attia, S.M. Systemic inflammation in asocial BTBR T+ tf/J mice predisposes them to increased psoriatic inflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 83, 8–17. [Google Scholar] [CrossRef]
- Alam, S.I.; Kim, M.W.; Shah, F.A.; Saeed, K.; Ullah, R.; Kim, M.O. Alpha-Linolenic Acid Impedes Cadmium-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration in Mouse Brain. Cells 2021, 10, 2274. [Google Scholar] [PubMed]
- Leo, A.; De Caro, C.; Mainardi, P.; Tallarico, M.; Nesci, V.; Marascio, N.; Striano, P.; Russo, E.; Constanti, A.; De Sarro, G.; et al. Increased efficacy of combining prebiotic and postbiotic in mouse models relevant to autism and depression. Neuropharmacology 2021, 198, 108782. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, D.A.; Pahua, A.E.; Zarate, M.; Taylor, J.A.; Peterson, S.; Posadas, R.; Oliver, B.L.; Amodeo, L.R. Differences in the expression of restricted repetitive behaviors in female and male BTBR T+ tf/J mice. Behav. Brain Res. 2019, 372, 112028. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, M.M.; Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Al-Mazroua, H.A.; Aldossari, A.A.; Almutairi, M.M.; Albekairi, T.H.; Hussein, M.H.; et al. Cadmium Exposure Is Associated with Behavioral Deficits and Neuroimmune Dysfunction in BTBR T+ Itpr3tf/J Mice. Int. J. Mol. Sci. 2023, 24, 6575. https://doi.org/10.3390/ijms24076575
Alanazi MM, Ansari MA, Nadeem A, Attia SM, Bakheet SA, Al-Mazroua HA, Aldossari AA, Almutairi MM, Albekairi TH, Hussein MH, et al. Cadmium Exposure Is Associated with Behavioral Deficits and Neuroimmune Dysfunction in BTBR T+ Itpr3tf/J Mice. International Journal of Molecular Sciences. 2023; 24(7):6575. https://doi.org/10.3390/ijms24076575
Chicago/Turabian StyleAlanazi, Mohammed M., Mushtaq A. Ansari, Ahmed Nadeem, Sabry M. Attia, Saleh A. Bakheet, Haneen A. Al-Mazroua, Abdullah A. Aldossari, Mohammed M. Almutairi, Thamer H. Albekairi, Marwa H. Hussein, and et al. 2023. "Cadmium Exposure Is Associated with Behavioral Deficits and Neuroimmune Dysfunction in BTBR T+ Itpr3tf/J Mice" International Journal of Molecular Sciences 24, no. 7: 6575. https://doi.org/10.3390/ijms24076575
APA StyleAlanazi, M. M., Ansari, M. A., Nadeem, A., Attia, S. M., Bakheet, S. A., Al-Mazroua, H. A., Aldossari, A. A., Almutairi, M. M., Albekairi, T. H., Hussein, M. H., Al-Hamamah, M. A., & Ahmad, S. F. (2023). Cadmium Exposure Is Associated with Behavioral Deficits and Neuroimmune Dysfunction in BTBR T+ Itpr3tf/J Mice. International Journal of Molecular Sciences, 24(7), 6575. https://doi.org/10.3390/ijms24076575





