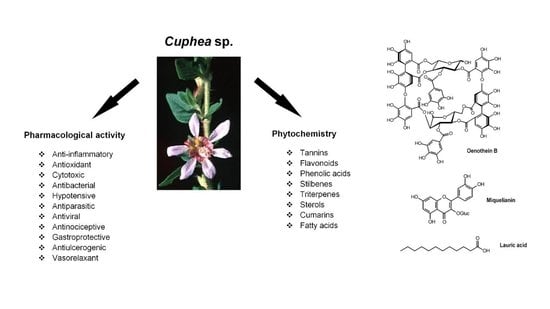
The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review
Abstract
:1. Introduction
- -
- subgenus Cuphea Koehne (Lythrocuphea Koehne); sections: Archocuphea Koehne, Cuphea;
- -
2. Botanical Characteristics
3. Phytochemistry
3.1. Cuphea Seed Oil and Fatty Acids
3.2. Polyphenols
3.3. Other Phytochemicals
4. Cuphea Plants in Traditional Medicine
5. Pharmacological Activity of Cuphea Plants and Phytochemicals
5.1. Hypotensive Activity of Cupheas
5.2. Anti-Inflammatory Activity of Cupheas
5.3. Antiparasitic, Antibacterial, and Antiviral Effects
5.4. Antioxidant Activity
5.5. Cytotoxic Activity of Cuphea Plants
| Cuphea Species/Positive Control | Cell Line * | Cytotoxic Activity | Assay | References |
|---|---|---|---|---|
| C. aequipetala (acetone-aqueous extract of the whole plant) Colchicine | HEp-2 HCT-15 DU145 HEp-2 HCT-15 DU145 | ED50 [μg/mL] >50 18.70 8.1 <0.006 0.006 0.099 | Sulforhodamine B assay | [129] |
| C. aequipetala (chloroform extract of aerial parts) | % inhibition at the conc. of 6.25 μg/mL | Not mentioned | [130] | |
| HeLa | 36.47 ± 4.04 | |||
| DU145 | 23.16 ± 9.21 | |||
| C. aequipetala (methanol extract from leaves, flowers and stems) | ED50 [μg/mL] | Oyama and Eagle method | [93] | |
| UISO-SQC1 | 17.4 | |||
| C. aequipetala (aerial parts) (a) methanol extract (b) aqueous extract | CC50 [mg/mL] | MTT assay | [128] | |
| B16F10 | 0.269 | |||
| HepG2 | 0.145 | |||
| MCF-7 | 0.096 | |||
| B16F10 | 0.364 | |||
| HepG2 | 0.212 | |||
| MCF-7 | 0.173 | |||
| C. hyssopifolia (aqueous-methanol extract of aerial parts) Doxorubicin (positive control) | IC50 [μg/mL] | Sulforhodamine B assay | [48] | |
| MCF-7 | 92.5 | |||
| HEp-2 | 84.9 | |||
| HCT-116 | 81.0 | |||
| HepG2 | 73.4 | |||
| MCF-7 | ||||
| HEp-2 | 3.7–5 | |||
| HCT-116 | ||||
| HepG2 | ||||
| C. hyssopifolia (methanol extract) (a) aerial parts (b) roots | EC50 [μg/mL] | MTT assay | [126] | |
| MK-1 | 50–100 | |||
| HeLa | 25–50 | |||
| B16F10 | 50–100 | |||
| MK-1 | 25–50 | |||
| HeLa | 50–100 | |||
| B16F10 | 50–100 | |||
| Compounds isolated from C. hyssopifolia Cuphiin D1 Cuphiin D2 Oenothein B Woodfordin C Adriamycin (positive control) | IC50 [μg/mL] | MTT assay | [51] | |
| KB | 20.0 | |||
| DU145 | 51.4 | |||
| HeLa | 36.5 | |||
| Hep3B | 54.2 | |||
| S-180 | 39.2 | |||
| WISH | 100.0 | |||
| KB | 20.7 | |||
| DU145 | 74.0 | |||
| HeLa | 28.5 | |||
| Hep3B | 55.0 | |||
| S-180 | 24.5 | |||
| WISH | 69.0 | |||
| KB | 26.8 | |||
| DU145 | 54.5 | |||
| HeLa | 29.0 | |||
| Hep 3B | 19.0 | |||
| S-180 | 11.4 | |||
| WISH | 67.2 | |||
| KB | 28.9 | |||
| DU145 | 70.5 | |||
| HeLa | 34.1 | |||
| Hep 3B | 34.0 | |||
| S-180 | 24.7 | |||
| WISH | 102.5 | |||
| KB | <0.15 | |||
| DU145 | <0.15 | |||
| HeLa | <0.15 | |||
| Hep3B | <0.15 | |||
| S-180 | <0.15 | |||
| WISH | <0.15 | |||
| Compound isolated from C. hyssopifolia Cuphiin D1 | IC50 [μM] | MTT assay | [131] | |
| HL-60 | 16 | |||
| C. ignea (aqueous–ethanol extract of aerial parts) | IC50 [μg/mL] | MTT assay NRU assay | [105] | |
| A549 | 376 | |||
| A549 | 369.6 | |||
| C. ignea (aqueous–ethanol extract of whole plant) 7-hydroxy 3-methoxy coumarin 5-O-β-glucopyranoside | IC50 [μg/mL] | NRU assay | [57] | |
| HaCaT | 397.34 ± 19.83 | |||
| HCT-116 | 70.88 ± 0.62 | |||
| HuH-7 | 98 ± 2.91 | |||
| MRC-9 | 83.65 ± 13.43 | |||
| NCI-H460 | 37.76 ± 3.41 | |||
| NCI-H23 | 32.44 ± 5.23 | |||
| HaCaT | 220.52 ± 28.83 | |||
| HCT-116 | 59.29 ± 6.21 | |||
| HuH-7 | 66.39 ± 2.39 | |||
| MRC-9 | 340.67 ± 22.21 | |||
| NCI-H460 | 45.56 ± 1.61 | |||
| NCI-H23 | 40.38 ± 2.75 | |||
| C. ingrata (methanol extract of the aerial parts) (ethyl acetate fraction) (n-butanol fraction) Doxorubicin (positive control) | IC50 [μg/mL] | LDH assay | [127] | |
| A375 | 36.07 | |||
| HTB-140 | >100 | |||
| WM793 | 43.37 | |||
| HaCaT | 9.18 | |||
| DU145 | >100 | |||
| PC3 | >100 | |||
| PNT2 | >100 | |||
| A375 | 15.90 | |||
| HTB-140 | 3.40 | |||
| WM793 | 18.75 | |||
| HaCaT | 6.12 | |||
| DU145 | >100 | |||
| PC3 | >100 | |||
| PNT2 | >100 | |||
| A375 | 22.60 | |||
| HTB-140 | 5.65 | |||
| WM793 | 29.39 | |||
| HaCaT | 7.23 | |||
| DU145 | >100 | |||
| PC3 | >100 | |||
| PNT2 | >100 | |||
| A375 | 0.59 | |||
| HTB-140 | 5.71 | |||
| WM793 | >40 | |||
| HaCaT | 4.68 | |||
| DU145 | 3.18 | |||
| PC3 | >50 | |||
| PNT2 | 1.38 | |||
| C. procumbens (aqueous extract of leaves) | IC50 [μg/mL] | MTT assay | [123] | |
| MCF-7 | >100 | |||
| MDA-MB-468 | >100 | |||
| A375 | >100 | |||
| HCT-116 | >100 |
6. Cuphea for Commercial Use
7. Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, S.A. Cuphea: A new plant source of medium-chain fatty acids. Crit. Rev. Food Sci. Nutr. 1989, 28, 139–173. [Google Scholar] [CrossRef]
- Barber, J.C.; Ghebretinsae, A.; Graham, S.A. An expanded phylogeny of Cuphea (Lythraceae) and a North American monopoly. Plant Syst. Evol. 2010, 289, 35–44. [Google Scholar] [CrossRef]
- Graham, S.A.; Freudenstein, J.V.; Luker, M. A phylogenetic study of Cuphea (Lythraceae) based on morphology and nuclear rDNA ITS sequences. Sys. Bot. 2006, 31, 764–778. [Google Scholar] [CrossRef]
- Brauner, L.M.; Cavalcanti, T.B. A new species, a new synonym and lectotypification in Cuphea (Lythraceae) from Brazil. Phytotaxa 2018, 350, 155–160. [Google Scholar] [CrossRef]
- Graham, S.A. A revision of Cuphea section Amazoniana s.s. (Lythraceae). Sys. Bot. 2019, 44, 146–183. [Google Scholar] [CrossRef]
- Graham, S.A.; Cavalcanti, T.B. Taxonomic revision of Cuphea sect. Euandra subsect. Oidemation (Lythraceae). Phytotaxa 2013, 113, 1–86. [Google Scholar]
- Cavalcanti, T.B.; Graham, S.A.T.; Facco, M.G.; Brauner, L.M. Cuphea. Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB8735 (accessed on 30 June 2022).
- Cavalcanti, T.B. Flora da Serra do Cipo, Minas Gerais: Lythraceae. Bolm. Botânica 1990, 12, 67–93. [Google Scholar] [CrossRef] [Green Version]
- El Bassam, N. (Ed.) Energy plant species. Cuphea. In Energy Plant Species: Their Use and Impact on Environment and Development, 1st ed.; James and James (Science Publishers) Ltd.: London, UK, 1998; pp. 137–140. [Google Scholar]
- African Plant Database (version 4.0.0). Conservatoire et Jardin Botaniques De La Ville De Genève and South African National Biodiversity Institute, Pretoria. Available online: http://africanplantdatabase.ch (accessed on 28 November 2022).
- de Wilde, W.J.J.O. Lythraceae. In Flora Malesiana. Series I—Seed Plants; De Wilde, W.J.J.O., Duyfjes, B.E.E., Eds.; Naturalis Biodiversity Center/Foundation Flora Malesiana: Leiden, The Netherlands, 2016; Volume 22, pp. 1–64. [Google Scholar]
- PIER: US Forest Service, Pacific Island Ecosystems at Risk. Available online: http://www.hear.org.pier/ (accessed on 6 June 2022).
- Baret, S.; Rouget, M.; Richardson, D.M.; Lavergne, C.; Egoh, B.; Dupont, J.; Strasberg, D. Current distribution and potential extent of the most invasive alien plant species on La Réunion (Indian Ocean, Mascarene islands). Austral. Ecology 2006, 31, 747–758. [Google Scholar] [CrossRef]
- Jaworski, C.A.; Phatak, S.C. Cuphea glutinosa selections for flowering ornamental ground cover in southeast United States. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 467–469. [Google Scholar]
- Jaworski, C.A. Flowering ornamental Cuphea glutinosa ‘Purple Passion’ and ‘Lavender Lei’. Hortscience 1992, 27, 940. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Bonfil, B.P.; Pineda-Montero, M.; López-Laredo, A.R.; Salecado-Morales, G.; Evangelista-Lozano, S.; Trejo-Tapia, G. A propagation procedure for Cuphea aequipetala Cav. (Lythraceae) and antioxidant properties of wild and greenhouse-grown plants. Bol. Latinoam. Caribe. Plant. Med. Aromát. 2013, 12, 1–14. [Google Scholar]
- Rather, M.A.; Gupta, K.; Mandal, M. Inhibition of biofilm and quorum sensing-regulated virulence factors in Pseudomonas aeruginosa by Cuphea carthagenensis (Jacq.) J.F.Macbr. leaf extract: An in vitro study. J. Ethnopharmacol. 2021, 269, 113699. [Google Scholar] [CrossRef]
- Garibay-Castro, L.R.; Gutiérrez-Yurrita, P.J.; López-Laredo, A.R.; Hernández-Ruíz, J.; Trejo-Espino, J.L. Potential distribution and medicinal uses of the Mexican plant Cuphea aequipetala Cav. (Lythraceae). Diversity 2022, 14, 403. [Google Scholar] [CrossRef]
- Olejniczak, J. Cuphea. In Wild Crop Relatives: Genomic and Breeding Resources: Oilseeds; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 117–136. [Google Scholar]
- Ghebretinsae, A.G.; Graham, S.A.; Camilo, G.R.; Barber, J.C. Natural infraspecific variation in fatty acid composition of Cuphea (Lythraceae) seed oil. Ind. Crops Prod. 2008, 27, 279–287. [Google Scholar] [CrossRef]
- Floh, E.l.S.; Handro, W.; Rita, I. Cuphea species: Tissue culture, micropropagation and production of medium-chain fatty acids. In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer GmbH: Berlin/Heidelberg, Germany, 1999; Volume 43, pp. 78–84. [Google Scholar]
- Phippen, W.B. Cuphea. In Handbook of Plant Breeding; Vollmann, J., Rajcan, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, pp. 517–533. [Google Scholar]
- Abel, C.A.; Roekel, J.W.V.; Wilson, R.L. Cuphea lanceolata and Cuphea ignia seed increase using three pollinators in insect-proof cages in the field. Southwest. Entomol. 2019, 44, 95–98. [Google Scholar]
- Hirsinger, F.; Knowles, P.F. Morphological and agronomic description of selected Cuphea germplasm. Econom. Bot. 1984, 38, 439–451. [Google Scholar] [CrossRef]
- Knapp, S.J.; Crane, J.M. Registration of reduced seed shattering Cuphea germplasm PSR23. Crop Sci. 2000, 40, 299–300. [Google Scholar] [CrossRef]
- Knothe, G.; Cermak, S.C.; Evangelista, R.L. Cuphea oil as source of biodiesel with improved fuel properties caused by high content of methyl decanoate. Energy Fuels 2009, 23, 1743–1747. [Google Scholar] [CrossRef]
- Bergmeier, D.; Berres, P.H.D.; Filippi, D.; Bilibio, D.; Bettiol, V.R.; Priamo, W.L. Extraction of total polyphenols from hibiscus (Hibiscus sabdariffa L.) and waxweed/’sete-sangrias’ (Cuphea carthagenensis) and evaluation of their antioxidant potential. Acta Sci. Technol. 2014, 36, 545–551. [Google Scholar] [CrossRef]
- Paxton, J. Cuphea melvilla. In Paxton’s Magazine of Botany and Register of Flowering Plants; Orr, W.S. & Co.: London, UK, 1841; Volume 8, pp. 197–198. [Google Scholar]
- Graham, S.A.; Cavalcanti, T.B. The yellow-flowered species of Cuphea (Lythraceae), including three new taxa. Brittonia 1999, 51, 24–30. [Google Scholar] [CrossRef]
- Elgindi, M.R.; Ayoub, N.; Milad, R. A comprehensive review of Cuphea (Lythraceae). Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 847–855. [Google Scholar]
- Gesch, R.W.; Barbour, N.W.; Forcella, F.; Voorhees, W.B. Cuphea growth and development. Responses to temperature. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 213–215. [Google Scholar]
- Gesch, R.W.; Forcella, F. Differential sensitivity to temperature of Cuphea vegetative and reproductive growth. Ind. Crops Prod. 2007, 25, 305–309. [Google Scholar] [CrossRef]
- Crane, J.; Miller, A.L.; Van Roekel, J.W.; Walters, C. Triacylglycerols determine the unusual storage physiology of Cuphea seed. Planta 2003, 217, 699–708. [Google Scholar] [CrossRef]
- Graham, S.A.; Coelho-José, G.P.; Murad, A.M.; Rech, E.L.; Cavalcanti, T.B.; Inglis, P.W. Patterns of fatty acid composition in seed oils of Cuphea, with new records from Brazil and Mexico. Ind. Crops Prod. 2016, 87, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.E.; Kleiman, R. Effect of seed maturity on seed oil and protein content of Cuphea species. JAOCS 1988, 65, 139–146. [Google Scholar] [CrossRef]
- Forcella, F.; Gesch, R.W.; Isbell, T.A. Seed yield, oil, and fatty acids of Cuphea in the Northwestern Corn Belt. Crop Ecol. Manag. Qual. 2005, 45, 2195–2202. [Google Scholar] [CrossRef] [Green Version]
- Jaradat, A.A. Evolution of Cuphea PSR23 under cultivation. Euphytica 2016, 210, 41–55. [Google Scholar] [CrossRef]
- Eller, F.J.; Cermak, S.C.; Taylor, S.L. Supercritical carbon dioxide extraction of Cuphea seed oil. Ind. Crops Prod. 2011, 33, 554–557. [Google Scholar] [CrossRef]
- Evangelista, R.L.; Cermak, S.C. Full-press oil extraction of Cuphea (PSR23) seeds. J. Am. Oil Chem. Soc. 2007, 84, 1169–1175. [Google Scholar] [CrossRef]
- Evangelista, R.L.; Cermak, S.C.; Isbell, T.A. Dehulling of Cuphea PSR23 seeds to reduce color of the extracted oil. Ind. Crops Prod. 2010, 31, 437–443. [Google Scholar] [CrossRef]
- Phippen, W.B.; Isbell, T.A.; Phippen, M.E. Total seed oil and fatty acid methyl ester contents of Cuphea accessions. Ind. Crops Prod. 2006, 24, 52–59. [Google Scholar] [CrossRef]
- Wolf, R.B.; Graham, S.A.; Kleiman, R. Fatty acid composition of Cuphea seed oils. JACOS 1983, 60, 103–104. [Google Scholar]
- Hovoraková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against Gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Henriques, A.T.; Mendez, A.S.L. Analytical methods of phytochemicals from the Cuphea genus—A review. Drug Anal. Res. 2021, 5, 4–10. [Google Scholar] [CrossRef]
- Krepsky, P.B.; Isidório, R.G.; de Souza Filho, J.D.; Côrtes, S.F.; Castro Braga, F. Chemical composition and vasodilatation induced by Cuphea carthagenensis preparations. Phytomedicine 2012, 19, 953–957. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Farias, L.S.; Merlugo, L.; de Oliveira, T.V.; Barbosa, F.S.; Fuentefria, A.M.; Henriques, A.T.; Garcia, C.V.; Mendez, A.S.L. UPLC-MS for identification of quercetin derivatives in Cuphea glutinosa Cham. & Schltdl (Lythraceae) and evaluation of antifungal potential. Curr. Pharm. Anal. 2018, 14, 586–594. [Google Scholar]
- Sobolewska, D.; Owczarek, A.; Olszewska, M.; Paulino, N.; Podolak, I.; Paśko, P.; Wróbel-Biedrawa, D.; Michalska, K. UHPLC-PDA-ESI-MS profile of phenolic compounds in the aerial parts of Cuphea ingrata Cham. & Schltdl. Nat. Prod. Res. 2022, 36, 3721–3725. [Google Scholar] [PubMed]
- Elgindi, M.R.; Ayoub, N.; Milad, R.; Mekky, R. Antioxidant and cytotoxic activities of Cuphea hyssopifolia Kunth (Lythraceae) cultivated in Egypt. J. Pharmacogn. Phytochem. 2012, 1, 67–77. [Google Scholar]
- Santos, D.Y.A.; Salatino, M.L.F.; Salatino, A. Flavonoids of species of Cuphea (Lythraceae) from Brazil. Biochem. Sys. Ecol. 1995, 23, 99–103. [Google Scholar] [CrossRef]
- Krepsky, P.B.; Farias, M.R.; Côrtes, S.F.; Castro Braga, F. Quercetin-3-sulfate: A chemical marker for Cuphea carthagenensis. Biochem. Sys. Ecol. 2010, 38, 125–127. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, L.-G.; Yang, L.-L. Antitumor activity of four macrocyclic ellagitannins from Cuphea hyssopifolia. Cancer Lett. 1999, 140, 195–200. [Google Scholar] [CrossRef]
- Ramírez-Atehortúa, A.M.; Morales-Agudelo, L.; Osorio, E.; Lara-Guzmán, O.J. The traditional medicinal plants Cuphea calophylla, Tibouchina kingii, and Pseudoelephantopus spiralis attenuate inflammatory and oxidative mediators. Evid. Based Complement. Alternat. Med. 2018, 1953726. [Google Scholar] [CrossRef] [Green Version]
- Cardenas-Sandoval, B.A.; López-Laredo, A.R.; Martínez-Bonfil, B.P.; Bermúdez-Torres, K.; Trejo-Tapia, G. Advances in the phytochemistry of Cuphea aequipetala, C. aequipetala var. hispida and C. lanceolata: Extraction and quantification of phenolic compounds and antioxidant activity. Rev. Mex. Ing. Quím. 2012, 11, 401–413. [Google Scholar]
- Ismail, W.M.; Ezzat, S.M.; Michel, H.E.; El Deeb, K.S.; El-Fishawy, A.M. Angiotensin converting enzyme and renin inhibition activities, antioxidant properties, phenolic and flavonoid contents of Cuphea ignea A. DC. J. Rep. Pharm. Sci. 2020, 9, 92–96. [Google Scholar] [CrossRef]
- Scio, E.; Mendes, R.F.; Motta, E.V.S.; Bellozi, P.M.Q.; Aragāo, D.M.O.; Mello, J.; Fabri, R.L.; Moreira, J.R.; de Assis, I.V.L.; Bouzada, M.L.M. Antimicrobial and antioxidant activities of some plant extracts. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; InTech: London, UK, 2012; pp. 21–42. [Google Scholar]
- Gonzalez, A.G.; Valencia, E.; Exposito, T.S.; Barrera, J.B.; Gupta, M.P. Chemical components of Cuphea species. Carthagenol: A new triterpene from C. carthagenensis. Planta Med. 1994, 60, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.S.; Swilam, N.F.; Ghanem, O.B.; Hashim, A.N.; Nawwar, M.A.; Lindequist, U.; Linscheid, M.W. A coumarin with an unusual structure from Cuphea ignea, its cytotoxicity and antioxidant activities. Pharmazie 2018, 73, 241–243. [Google Scholar] [PubMed]
- Mousa, A.M.; El-Sammad, N.M.; Hassan, S.K.; Madboli, A.E.N.A.; Hashim, A.N.; Moustafa, E.S.; Bakry, S.M.; Elsayed, E.A. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement. Altern. Med. 2019, 19, 345. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Toson, N.S.B.; Pimentel, M.C.B.; Bordignon, S.A.; Mendez, A.S.; Henriques, A.T. Polyphenols composition from leaves of Cuphea spp. and inhibitor potential, in vitro, of angiotensin I-converting enzyme (ACE). J. Ethnopharmacol. 2020, 255, 112781. [Google Scholar] [CrossRef]
- Aguilar-Rodríguez, S.; Echeveste- Ramírez, N.L.; López-Villafranco, E.; Aguilar-Contreras, A.; Vega-Ávilla, E.; Reyes-Chilpa, R. Etnobotánica, micrografía analítica de hojas y tallos y fitoquimica de Cuphea aequipetala Cav. (Lythraceae): Una contribución a la Farmacopea Herbolaria de los Estados Unidos Mexicanos (FHEUM). Bol. Latinoam. Caribe. Plantas Med. Aromát. 2012, 11, 316–330. [Google Scholar]
- Martins, D.; Roque, N.F. Constituents of Cuphea aperta. Fitoterapia 1995, 66, 187. [Google Scholar]
- Klider, L.M.; Machado, C.D.; Almeida, V.P.; Tirloni, C.A.S.; Marques, A.A.M.; Palozi, R.A.C.; Lorençone, B.R.; Romão, P.V.M.; Guarnier, L.P.; Casserimo, N.S.; et al. Cuphea calophylla var. mesostemon (Koehne) S.A. Graham: A whole-ethnopharmacological investigation. J. Med. Food 2021, 24, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Zago, A.M.; Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Fernandes, M.D.C.; Morandini, L.M.; Coelho, H.S.; Fogaça, A.O.; Andrade, C.M.; Mostardeiro, M.A.; et al. A phytochemical study of the Cuphea glutinosa from Southern Brazil: Na+,K+- ATPase activity inhibition and antioxidant properties. Nat. Prod. Res. 2019, 33, 3426–3431. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Ismail, W.M.; Moatasim, Y.; Kutkat, O.; ElMeshad, A.N.; Ezzat, S.M.; El Deeb, S.M.; El Deeb, K.S.; El-Fishawy, A.M.; Gomaa, M.R.; et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: In silico and in vitro studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102845. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; Godoy, H.T. Methylxanthines in 100 Brazilian herbs and infusions: Determination and consumption. Emir. J. Food Agric. 2019, 31, 125–133. [Google Scholar] [CrossRef]
- Santos, M.C.; Soares, K.D.; Beltrame, B.M.; Toson, N.S.; do Carmo, B.; Pimentel, M.; Bordignon, S.A.; Apel, M.A.; Mendez, A.S.L.; Henriques, A.T. Polyphenolic composition and in vitro anti-hypertensive and anti-inflammatory effects of Cuphea lindmaniana and Cuphea urbaniana. Chem. Biodivers. 2021, 18, e2100041. [Google Scholar] [CrossRef]
- Lechner, M.; Reiter, B.; Lorbeer, E. 1999. Determination of free and esterified sterols in potential new oil seed crops by coupled on-line liquid chromatography-gas-chromatography. Lipid/Fett 1999, 101, 171–177. [Google Scholar] [CrossRef]
- Calzada, F.; Alanís, A.D.; Meckes, M.; Tapia-Contreras, A.; Cedillo-Rivera, R. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother. Res. 1998, 12, 70–72. [Google Scholar] [CrossRef]
- Calzada, F. Additional antiprotozoal constituents from Cuphea pinetorum, a plant used in Mayan traditional medicine to treat diarrhoea. Phytother. Res. 2005, 19, 725–727. [Google Scholar] [CrossRef]
- Perez-Castorena, A.L.; Maldonado, E. Triterpenes and flavonoid glycosides from Cuphea wrightii. Biochem. Syst. Ecol. 2003, 31, 331–334. [Google Scholar] [CrossRef]
- Elisabetsky, E.; Addison Posey, D. Use of contraceptive and related plants by the Kayapo Indians (Brazil). J. Ethnopharmacol. 1989, 26, 299–316. [Google Scholar] [CrossRef]
- Alvarado, T.D.; Mariezcurrena Berasain, M.D.; Salem, A.Z.M.; Pinzón Martínez, D.L. Antimicrobial and antioxidant activities of two medicinal plants Cuphea aequipetala var. hispida (Cav.) Koehne and Eryngium comosum Delaroche F against bacteria related to equine infections. J. Equine Vet. Sci. 2020, 94, 103269. [Google Scholar]
- Garcia, D.; Domingues, M.V.; Rodrigues, E. Ethnopharmacological survey among migrants living in the Southeast Atlantic Forest of Diadema, São Paulo, Brazil. J. Ethnobiol. Ethnomed. 2010, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Juárez, I.; González, V.; Jaime-Aguilar, H.; Martinez, G.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J. Ethnopharmacol. 2009, 122, 402–405. [Google Scholar] [CrossRef]
- Amat, A.G.; Yajia, M.E.; Gonzalez, C.F.; Lorca, G.L.; Sanchez Gonzalez, F.; Riglos, A.G.; Veron, J.R. Evaluation of cytological parameters induced by aqueous extracts of seven plants used as anti-hypertensive agents in Argentine folk medicine. Acta Farm. Bonaerense 2002, 21, 37–42. [Google Scholar]
- Menetrier, J.V.; Bonkoski, V.R.; Medeiros, K.A.; Estevan, D.A.; Palozi, R.A.C.; Livero, F.A.D.R.; Velasquez, L.G.; Lourenço, E.L.B.; Gasparotto Junior, A. Ethnomedicinal plants used for the treatment of cardiovascular diseases by healers in the southwestren state of Paraná, Brazil, and their validation based on scientific pharmacological data. J. Relig. Health. 2020, 59, 3004–3036. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Sauini, T.; Stern da Fonseca-Kruel, V.; Baptistela Yazbek, P.; Matta, P.; Cassas, F.; da Cruz, C.; Hortal Pereira Barretto, E.; Santos, M.A.; Silva Gomez, M.A.; Francischetti Garcia, R.J.; et al. Participatory methods on the recording of traditional knowledge about medicinal plants in Atlantic forest, Ubatuba, São Paulo, Brazil. PLoS ONE 2020, 15, e0232288. [Google Scholar] [CrossRef]
- Castro Braga, F.; Wagner, H.; Lombardi, J.A.; de Oliveira, A.B. Screening the Brazilian flora for anti-hypertensive plant species for in vitro angiotensin-I-converting enzyme inhibiting activity. Phytomedicine 2000, 7, 245–250. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Crepin, J.; Maina, S.L. Medicinal plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004; p. 165. [Google Scholar]
- Vendruscolo, G.S.; Mentz, L.A. Estudo da concordância das citações de uso e importância das espécies e famílias utilizadas como medicinais pela comunidade do bairro Ponta Grossa, Porto Alegre, RS, Brasil. Acta Bot. Bras. 2006, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.P.; Solís, P.N.; Calderón, A.I.; Guinneau-Sinclair, F.; Correa, M.; Galdames, C.; Guerra, C.; Espinosa, A.; Alvenda, G.I.; Robles, G.; et al. Medical ethnobotany of the Teribes of Bocas del Toro, Panama. J. Ethnopharmacol. 2005, 96, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Schmeda-Hirschmann, G. The use of medicinal plants by Paraguayan migrants in the Atlantic Forest of Misiones, Argentina, is based on Guaraní tradition, colonial and current plant knowledge. J. Ethnopharmacol. 2022, 283, 114702. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Ferdausi, D.; Mollik, M.A.H.; Jahan, R.; Chowdhury, M.H.; Haque, W.M. A survey of medicinal plants used by Kavirajes of Chalna Area, Khulna District, Bangladesh. Afr. J. Trad. Complement. Altern. Med. 2010, 7, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolson, M.; Hefler, S.R.; Dall’Oglio Chavez, E.I.; Gasparotto Junior, A.; Cardozo Junior, E.L. Ethno-medicinal study of plants used for treatment of human ailments, with residents of the surrounding region of forest fragments of Paraná, Brazil. J. Ethnopharmacol. 2015, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, I.; Zardini, E.; Ortiz, M. Medicinal plants of Paraguay: Underground organs. Econ. Bot. 1991, 45, 86–96. [Google Scholar] [CrossRef]
- Pavetti, C.; Basualdo, I.; Ortiz, M.; Soria, N. Plantas nativas de uso en medicina popular en el Paraguay. Parte III. Acta Amazon. 1988, 18, 39–48. [Google Scholar] [CrossRef]
- Lentz, D. Medicinal and other economic plants of the Paya of Honduras. Econ. Bot. 1993, 47, 358–370. [Google Scholar] [CrossRef]
- Aquino, A.M. “Living with joy”: History, sociability, and alterity in Kaingang ritual life. Vibrant 2021, 18, e18505. [Google Scholar] [CrossRef]
- Queiroz, I.B.; Lino, J.T. Kaingang’s Kiki ritual: Material culture of an indigenous religious ritual in Southern Brazil. R. Museu. Arq. Etn. 2021, 36, 46–58. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Arana-Argáez, V.; Yáñez-Barrientos, E.; Ramirez-Camacho, M.A.; Wrobel, K.; Torres-Romero, J.C.; León-Callejas, C.; Wrobel, K. Antinociceptive and anti-inflammatory effects of Cuphea aequipetala Cav (Lythraceae). Inflammopharmacology 2021, 29, 295–306. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Zurita-Vásquez, G.G.; Pacheco-Hernández, Y.; Betancourt-Jiménez, M.G.; Cruz-Durán, R.; Duque-Bautista, H. Anti-lipase and antioxidant properties of 30 medicinal plants used in Oaxaca, México. Biol. Res. 2013, 46, 153–160. [Google Scholar] [CrossRef]
- Waizel-Bucay, J.; Martinez-Porcayo, G.; Villareal-Ortega, M.L.; Alonso-Cortés, D.; Pliego-Castañeda, A. Estudio preliminar etnobotánico, fitoquímico, de la actividad citotóxica y antimicrobiana de Cuphea aequipetala Cav. (Lythraceae). Polibotánica 2003, 15, 99–108. [Google Scholar]
- Palacios-Espinosa, J.F.; Arroyo-García, O.; García-Valencia, G.; Linares, E.; Bye, R.; Romero, I. Evidence of the anti-Helicobacter pylori, gastroprotective and anti-inflammatory activities of Cuphea aequipetala infusion. J. Ethnopharmacol. 2014, 151, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Baracho, N.C.V.; Brügger, P.G.; Camanducaia, D.S.M.; Sanches, A.I.F.; Sanches, R.S. Effects of chronic treatment with aqueous extract of Cuphea balsamona L. on the lipid profile of rats submitted to a high-cholesterol diet. In Proceedings of the Congresso Brasileiro de Farmacologia e Terapêutica Experimental, Ribeirão Preto, Brasil, 18–21 October 2010; p. 42. [Google Scholar]
- Santos, M.C.; Soares, K.D.; Beltrame, B.M.; Bordignon, S.A.; Apel, M.A.; Mendez, A.S.; Henriques, A.T. Cuphea ssp.: Antichemotactic study for a potential anti-inflammatory drug. Nat. Prod. Res. 2021, 35, 6058–6061. [Google Scholar] [CrossRef] [PubMed]
- Lima Prando, T.B.; Barboza, L.N.; Gasparotto, F.M.; Araújo Vde, O.; Slgnor Tirloni, C.A.; de Souza, L.M.; Lourenço, E.L.; Gasparotto Junior, A. Ethnopharmacological investigation of the diuretic and hemodynamic properties of native species of the Brazilian biodiversity. J. Ethnopharmacol. 2015, 174, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, F.R.; Santos, A.L.; Arruda, A.M.S.; Vasques-Pinto, L.M.C.; Godinho, R.O.; Torres, L.M.B.; Lapa, A.J.; Souccar, C. Antinociceptive and anti-inflammatory activities of the aqueous extract and isolated Cuphea carthagenensis (Jacq.)J.F.Macbr. Rev. Bras. Farmacogn. 2002, 12, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Barboza, L.N.; Livero, F.A.; Prando, T.B.; Ribeiro, R.C.; Lourenço, E.L.; Budel, J.M.; de Souza, L.M.; Acco, A.; Dalsenter, P.R.; Gasparotto, A. Junior. Atheroprotective effects of Cuphea carthagenensis (Jacq.)J.F.Macbr. in New Zealand rabbits fed with cholesterol-rich diet. J. Ethnopharmacol. 2016, 187, 134–145. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Farias, C.; Curtius, F.; Brasil, L.M.; Hort, S.; Leite, S.N.; Prado, S.R.T. Preliminary studies on Campomanesia xanthocarpa (Berg.) and Cuphea carthagenensis (Jacq.)J.F.Macbr. aqueous extract: Weight control and biochemical parameters. J. Ethnopharmacol. 2004, 93, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, E.Z.; Ckless, K.; Simas, M.E.; Farias, M.R.; Ribeiro-Do-Valle, R.M. Butanolic fraction from Cuphea carthagenensis Jacq McBride relaxes rat thoracic aorta through endothelium-dependent and endothelium-independent mechanisms. J. Cardiovasc. Pharmacol. 2000, 35, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Schaedler Schuldt, M.I.; Palozi, R.A.C.; Tirloni, C.A.S.; Silva, A.O.; de Oliveira Araújo, V.; Lourenço, E.L.B.; Souza, L.M.; Lívero, F.A.R.; Junior, A.G. Redox regulation and NO/cGMP plus K+ channel activation contributes to cardiorenal protection induced by Cuphea carthagenensis (Jacq.) J.F.Macbr. in ovariectomized hypertensive rats. Phytomedicine 2018, 51, 7–19. [Google Scholar] [CrossRef]
- Schuldt, E.Z.; Farias, M.R.; Ribeiro-do-Valle, R.M.; Ckless, K. Comparative study of radical scavenger activities of crude extract and fractions from Cuphea carthagenesis leaves. Phytomedicine 2004, 11, 523–529. [Google Scholar] [CrossRef]
- Moke, E.G.; Mordi, J.C.; Umukoro, E.K. Effects of methanol leaf extract of Cuphea hyssopifolia Kunth on liver enzymes activity and antioxidant indices of paracetamol-induced hepatotoxicity in Wistar rats. Afr. J. Biomed. Res. 2020, 23, 123–126. [Google Scholar]
- Hassan, S.K.; Mousa, A.M.; El-Sammad, N.M.; Abdel-Halim, A.H.; Khalil, W.K.B.; Elsayed, E.A.; Anwar, N.; Linscheid, M.W.; Moustafa, E.S.; Hashim, A.N.; et al. Antitumor activity of Cuphea ignea extract against benzo(a)pyrene-induced lung tumorigenesis in Swiss Albino mice. Toxicol. Rep. 2019, 6, 1071–1085. [Google Scholar] [CrossRef]
- Ismail, W.M. A pharmacognostical Study of Cuphea Ignea A.DC. Family: Lythraceae, Cultivated in Egypt. Ph.D. Dissertation, Cairo University, Giza, Egypt, 2020. [Google Scholar]
- Carvalho Siqueira, M.; Stutz, E.T.G.; Silva, T.C.; Carvalho Alves, J.N. Evaluation of the hypocholesterolemic effect of Cuphea ingrata Cham.& Schltdl. in mice induced to hypercholesterolemia. Braz. J. Develop. 2020, 6, 60518–60531. [Google Scholar]
- Sülsen, V.; Güida, C.; Coussio, J.; Paveto, C.; Muschetti, L.; Martino, V. In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol. Res. 2006, 98, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; Calzada, F.; Campos, R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J. Ethnopharmacol. 2007, 109, 552–554. [Google Scholar] [CrossRef]
- Meckes, M.; Villarreal, M.L.; Tortoriello, J. A microbiological evaluation of medicinal plants used by the Maya people of Southern Mexico. Phytother. Res. 1995, 9, 244–250. [Google Scholar] [CrossRef]
- Otenio, J.K.; Baisch, R.G.; Poplawski Carneiro, V.P.; Botelho Lourenço, E.L.; Alberton, O.; Soares, A.A.; Boleta Ceranto, D.C.F.; Jacomassi, E. Etnofarmacologia da Cuphea carthagenensis (Jacq.) J. F. Macbr: Uma revisão. Braz. J. Dev. 2020, 6, 10206–10219. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Chaudary, S.K.; Bhat, H.R.; Shakya, A. Cuphea carthagenensis: A review of its ethnobotany, pharmacology and phytochemistry. Bull Arunachal Res. 2018, 33, 1–14. [Google Scholar]
- Ataabadi, E.A.; Golshiri, K.; Jüttner, A.; Krenning, G.; Danser, A.H.J.; Roks, A.J.M. Nitric oxide-cGMP signaling in hypertension.Current and future options for pharmacotherapy. Hypertension 2020, 76, 1055–1068. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pejuadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.A. Revision of Cuphea sect. Leptocalyx (Lythraceae). Sys. Bot. 1989, 14, 43–76. [Google Scholar] [CrossRef]
- Toson, N.; Santos, M.C.; Fasolo, J.A.; Rico, E.P.; Vendruscolo, M.H.; Mendez, A.; Henriques, A. Evaluation of neuroinflammatory cytokine production in miquelianin isolated from Cuphea glutinosa in adult zebrafish. In Proceedings of the 7th Brazilian Conference on Natural Product/XXXIII RESEM Proceedings, Rio de Janeiro, Brasil, 10–13 November 2019; Volume 1, p. 117742. [Google Scholar]
- Campana, P.R.V.; Mansur, D.S.; Gusman, G.S.; Ferreira, D.; Teixeira, M.M.; Castro Braga, F. Anti TNF-α activity of Brazilian medicinal plants and compounds from Ouratea semiserrata. Phytother. Res. 2015, 29, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Gusman, G.S.; Campana, P.R.V.; Castro, L.C.; Castilho, R.O.; Teixeira, M.M.; Castro Braga, F. Evaluation of the effects of some Brazilian medicinal plants on the production of TNF-α and CCL2 by THP-1 cells. Evid. Based Complement. Alternat. Med. 2015, 497123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madboli, A.-N.A.; Mousa, A.M.; El-Sammad, N.M.; Hassan, S.K.; Nawwar, M.; Seif, M. Cuphea ignea extract relieved the histological changes and activated the NF-κB protein of female reproductive organs and stomach in Et-OH-treated rats. Egypt. J. Chem. 2023, ahead of print. [CrossRef]
- Botsaris, A.S. Plants used traditionally to treat malaria in Brazil: The archives of Flora Medicinal. J. Ethnobiol. Ethnomed. 2007, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Bussmann, R.W.; Paniagua Zambrana, N.Y.; Romero, C.; Hart, R.E. Astonishing diversity—The medicinal plant markets of Bogotá, Colombia. J. Ethnobiol. Ethnomed. 2018, 14, 43. [Google Scholar] [CrossRef]
- Rather, M.A.; Deori, P.J.; Gupta, K.; Daimary, N.; Deka, D.; Quershi, A.; Dutta, T.K.; Joardar, S.N.; Mandal, M. Ecofriendly phytofabrication of silver nanoparticles using aqueous extract of Cuphea carthagenensis and their antioxidant potential and antibacterial activity against clinically important human pathogens. Chemosphere 2022, 300, 134497. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Benítez, A.R.T.; Navarro-Marchal, S.A.; Martínez-Martínez, E.; Marchal, J.A.; Boulaiz, H.; Morales-Luckie, R. Biogeneration of silver nanoparticles from Cuphea procumbens for biomedical and environmental applications. Sci. Rep. 2023, 13, 790. [Google Scholar] [CrossRef]
- Andrighetti-Fröhner, C.R.; Sincero, T.C.M.; Da Silva, A.C.; Savi, L.A.; Gaido, C.M.; Bettega, J.M.R.; Mancini, M.; De Almeida, M.T.R.; Barbosa, R.A.; Farais, M.R.; et al. Antiviral evaluation of plants from Brazilian Atlantic Tropical Forest. Fitoterapia 2005, 76, 374–378. [Google Scholar] [CrossRef]
- Chen, L.-G.; Yen, K.-Y.; Yang, L.-L.; Hatano, T.; Okuda, T.; Yoshida, T. Macrocyclic ellagitannin dimers, cuphiins D1 and D2, and accompanying tannins from Cuphea hyssopifolia. Phytochemistry 1999, 50, 307–312. [Google Scholar] [CrossRef]
- Kinjo, J.; Nakano, D.; Fujioka, T.; Okabe, H. Screening of promising chemotherapeutic candidates from plants extracts. J. Nat. Med. 2016, 70, 335–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolewska, D.; Galanty, A.; Granica, S.; Podolak, I.; Olszewska, M.A.; Owczarek, A.; Paulino, N.; Michalska, K. In vitro cytotoxic activity of Cuphea ingrata Cham. & Schltdl. extracts related to the oenothein B content. Nat. Prod. Res. 2022, 1–5, ahead of print. [CrossRef]
- Uscanga-Palomeque, A.C.; Zapata-Benavides, P.; Saavedra-Alonso, S.; Zamora-Ávila, D.E.; Franco-Molina, M.A.; Arellano-Rodríguez, M.; Manilla-Muñoz, E.; Martínez-Torres, A.C.; Trejo-Ávila, L.M.; Rodríguez-Padilla, C. Inhibitory effect of Cuphea aequipetala extracts on murine B16F10 melanoma in vitro and in vivo. BioMed. Res. Int. 2019, 8560527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, E.V.; Aguilar, R.T.; Estrada, M.J.; Ortega, M.L.; Ramos, R.R. Cytotoxic activity of Cuphea aequipetala. Proc. West. Pharmacol. Soc. 2004, 47, 129–133. [Google Scholar]
- Calleros, F.G.; Gomez, H.M.; Gonzales, L.A.; Zavala, S.M.A. Evaluation of the cytotoxic effect of extracts of Cuphea aequipetala Cav. and Verbena carolina L. on cancer cell lines. Nat. Prod. Chem. Res. 2018, 6, 79. [Google Scholar]
- Wang, C.-C.; Chen, L.-G.; Yang, L.-L. Cuphiin D1, the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell line. Cancer Lett. 2000, 149, 77–83. [Google Scholar] [CrossRef]
- Husain, I.; Dale, O.R.; Martin, K.; Gurley, B.J.; Adams, S.J.; Avula, B.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Screening of medicinal plants for possible herb-drug interactions through modulating nuclear receptors, drug-metabolizing enzymes and transporters. J. Etnopharmacol. 2023, 301, 115822. [Google Scholar] [CrossRef]
- Golonko, A.; Olichwier, A.J.; Swislocka, R.; Szczerbinski, L.; Lewandowski, W. Why do dietary flavonoids have a promising effect as enhancer of anthracyclines? Hydroxyl substituents, bioavailability and biological activity. Int. J. Mol. Sci. 2023, 24, 391. [Google Scholar] [CrossRef]
- Berti, M.T.; Gesch, R.W. Cuphea production and management. In Industrial Crops, Handbook of Plant Breeding; Cruz, V.M.V., Dierig, D.A., Eds.; Springer Science + Business Media: New York, NY, USA, 2015; pp. 291–313. [Google Scholar]
- Jackson, M.A.; Evans, K.O.; Price, N.P.J.; Blackburn, J.A.; Ward, C.J.; Ray, K.J.; Vermillion, K.E. New family of surfactants from biobased materials. ACS Sustain. Chem. Eng. 2021, 9, 13842–13850. [Google Scholar] [CrossRef]
- Cermak, S.C.; Evangelista, R.L.; Jackson, M.A.; Compton, D.L.; Knothe, G.; Laszlo, J.A.; Evans, K.O. Biobased lubricants and functional products from Cuphea oil. In Surfactants in Tribology; Biresaw, G., Mittal, K.L., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 443–482. [Google Scholar]
- Cermak, S.C.; Isbell, T.A. Synthesis and physical properties of Cuphea-oleic estolides and esters. JAOCS 2004, 81, 297–303. [Google Scholar] [CrossRef]
- Isbell, T.A. US effort in the development of new crops (Lesquerella, Pennycress Coriander and Cuphea). OCL 2009, 4, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Tisserat, B.; O’kuru, R.H.; Cermak, S.C.; Evangelista, R.L.; Doll, K.M. Potential uses for Cuphea oil processing byproducts and processed oils. Ind. Crops Prod. 2012, 35, 111–120. [Google Scholar] [CrossRef]
- Tao, L.; Milbrandt, A.; Zhang, Y.; Wang, W.-C. Techno-economic and resources analysis of hydroprocessed renewable jet fuel. Biotechnol. Biofuels 2017, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Dominant Fatty Acid | Cuphea Species | Total Fatty Acid Content in Oil (%) | Dominant Fatty Acid | Cuphea Species | Total Fatty Acid Content in Oil (%) |
|---|---|---|---|---|---|
| Caprylic (C8:0) | C. avigera var. pulcherrima (R.C.Foster) S.A.Graham | 75–94 | Lauric (C12:0) | C. laminuligera Koehne | 63; 52–60 *** |
| C. cordata Ruiz & Pav. | 50 | C. lobophora Koehne | 66 | ||
| C. cyanea Moc. & Sessé | 68 | C. lutea Rose ex Koehne | 38; 34–42 *** | ||
| C. hookeriana Walp. | 50 | C. lutescens Pohl ex Koehne | 66; 76; 66 * | ||
| C. painteri Rose ex Koehne | 65 | C. melanium (L.) R.Br. ex Steud. | 77; 86 | ||
| C. pinetorum Benth. | 48 | C. melvilla Lindl. | 46; 52 | ||
| Capric (C10:0) | C. angustifolia Jacq. ex Koehne | 67–80 | C. micrantha Kunth | 43; 53 | |
| C. avigera B.L.Rob. & Seaton | 43 | C. parsonsia (L.) R.Br. ex Steud. | 74; 63 *** | ||
| C. bustamanta Lex. | 63 | C. pohlii Lourteig | 44 | ||
| C. caesariata S.A.Graham | 86 | C. polymorphoides Koehne | 80 | ||
| C. calaminthifolia Schltdl. | 44; 44 * | C. pseudovaccinium A.St.-Hil. | 69; 83 | ||
| C. calcarata Benth. | 64 | C. pulchra Moric. | 56 | ||
| C. cordata Ruiz & Pav. | 50 | C. retroscabra S.Watson | 55 | ||
| C. crassiflora S.A.Graham | 87 | C. rupestris T.B.Cavalc. & S.A.Graham | 54 | ||
| C. ferrisiae Bacig. | 82; 82 * | C. sclerophylla Koehne | 60; 67 | ||
| C. hookeriana Walp. | 50 | C. sessiliflora A.St.-Hil. | 64; 37 * | ||
| C. humifusa S.A.Graham | 82 | C. setosa Koehne | 62 | ||
| C. ignea A.DC. | 87; 54 **** | C. sincorana T.B.Cavalc. | 39 | ||
| C. inflata S.A.Graham | 86 | C. spermacoce A.St.-Hil. | 49 | ||
| C. koehneana Rose | 92: 92 * | C. splendida Lourteig | 51 | ||
| C. lanceolata W.T.Aiton | 83; 78–91 *** | C. strigulosa Kunth | 53 ** | ||
| C leptopoda Hemsl. | 87 | C. teleandra Lourteig | 71 | ||
| C. llavea Lex. | 86; 88; 83 ***; 92 *** | C. tolucana Peyr. | 53; 46–64 *** | ||
| C. lophostoma Koehne | 62; 81 | C. trochilus S.A.Graham | 62; 62 * | ||
| C. micropetala Kunth | 26 | C. thymoides Cham. & Schltdl. | 56; 65 | ||
| C. nitidula Kunth | 74 | C. tuberosa Cham. & Schltdl. | 56 | ||
| C. paucipetala S.A.Graham | 87 | C. urbaniana Koehne | 48 | ||
| C. procumbens Ortega | 80; 82; 81–89 *** | C. urens Koehne | 76 | ||
| C. quaternata Bacig. | 63; 63 * | C. vesiculigera R.C.Foster | 71; 71 * | ||
| C. schumannii Koehne | 94 | C. viscosa Rose ex Koehne | 60; 60 * | ||
| C. viscosissima Jacq. | 76; 76 * | C. wrightii A.Gray | 54; 54 ** | ||
| Lauric (C12:0) | C. acinifolia A.St.-Hil. | 65 | C. wrightii var. wrightii | 58–73 *** | |
| C. acinos A.St.-Hil. | 64 | Myristic (C14:0) | C. aequipetala Cav. | 56 | |
| C. adenophylla T.B.Cavalc. | 73 | C. epilobiifolia Koehne | 55; 55 * | ||
| C. appendiculata Benth. | 73; 83; 83 * | C. palustris Koehne | 64; 71 | ||
| C. bahiensis (Lourteig) S.A.Graham & T.B.Cavalc. | 47 | C. rasilis S.A.Graham | 49 | ||
| C. brachiata Mart. ex Koehne | 47 | C. salvadorensis (Standl.) Standl. | 65 | ||
| C. brachypoda T.B.Cavalc. | 47 | C. sessiifolia Mart. | 37 | ||
| C. calophylla Cham. & Schltdl. | 62–85; 85 *; 56–65 *** | C. strigulosa subsp. nitens Koehne | 37 | ||
| C. calophylla subsp. calophylla | 58–72 *** | C. strigulosa subsp. opaca Koehne | 45; 45 * | ||
| C. calophylla subsp. mesostemon (Koehne) Lourteig | 59–70 *** | C. tetrapetala Koehne | 51 | ||
| C. carthagenensis (Jacq.) J.F.Macbr. | 61; 81; 59 **; 59–67 *** | Oleic (C18:1) | C. circaeoides Sm. ex Sims | 48 | |
| C. confertiflora A.St.-Hil. | 73 | C. denticulata Koehne | 53 | ||
| C. diosmifolia A.St.-Hil. | 64 | Linoleic (C18:2) | C. decandra Dryand. | 45 | |
| C. egleri Lourteig | 57 | C. flavovirens S.A.Graham | 23; 23 * | ||
| C. ericoides Cham. & Schltdl. | 43 | C. fruticosa Spreng. | 67 | ||
| C. ferrisiae Bacig. | 35 | C. linarioides Cham. & Schltdl. | 34–62 | ||
| C. ferruginea Pohl ex Koehne | 55 | C. lindmaniana Koehne ex Bacig. | 55; 55 * | ||
| C. flava Spreng. | 43 | C. linifolia Koehne | 49; 63 | ||
| C. gardneri Koehne | 68 | C. mimuloides Schltdl. & Cham. | 30 | ||
| C. glareosa T.B.Cavalc. | 49 | C. pascuorum Mart. ex Koehne | 53 | ||
| C. glossostoma Koehne | 58 *** | C. purpurascens Bacig. | 36; 36 * | ||
| C. glutinosa Cham. & Schltdl. | 50; 82; 54 *** | C. subuligera Koehne | 29 | ||
| C. grandiflora Pohl ex Koehne | 62 | C. utriculosa Koehne | 31 | ||
| C. heterophylla Benth. | 48; 42 *** | Linolenic (C18:3) | C. spectabilis | 31; 31 * | |
| C. hyssopifolia Kunth | 79 | ||||
| C. ingrata Cham. & Schltdl. | 65; 69 | ||||
| C. jorullensis Kunth | 53; 53 * |
| Cuphea Species | Compound | Reference | Cuphea Species | Compound | Reference |
|---|---|---|---|---|---|
| C. acinos A.St.-Hil. (a) leaves | Apigenin-C-glycoside Isorhamnetin-3-O-galactoside | [49] | C. appendiculata Benth. (a) aerial part | β-Amyrin Betulinic acid Epifriedelanol β-Sitosterol Stigmasterol Mannitol | [56] |
| C. adenophylla T.B.Cavalc. (a) leaves | Quercetin-3-O-arabinoside Quercetin-3-O-glucoside Quercetin-3-O-rhamnosylglucoside Quercetin-3-O-galactosylgalactosid | [49] | C. calophylla Cham. & Schltdl. (a) leaves | Quercetin Quercetin-3-(2-galloylglucoside) Quercetin-3-O-(6′′-O-α-L-rhamnose)-β-D-glucoside Quercetin-3-arabinoside Quercetin-3-O-α-L-rhamnoside Quercetin-3-O-β-glucoside Kaempferol Kaempferol-3-glucoside Kaempferol-galloyl-glucoside Kaempferol-3-xyloside Kaempferol-7-rhamnoside Myricetin-3-(2-galloyl-glucoside) Myricetin-3-glucoside Myricetin-3-xyloside Myricetin-3-O-α-L-rhamnoside | [59] |
| C. aequipetala Cav. (a) aerial parts (b) leaves | Mannitol Quercetin-3-β-D-glucoside | [60] [53] | |||
| C. aequipetala var. hispida Koehne (a) leaves | Quercetin-3-β-D-glucoside Sitotenone Stigmastenone | [53] | |||
| C. aperta Koehne (a) whole plant | Quercetin Kaempferol Gallic acid Methyl gallate Protocatechuic acid α-Amyrin, β-Amyrin Lupeol Stigmasterol β-Sitosterol Campestenone, Sitostenone, Stigmastenone | [61] | C. calophylla subsp. mesostemon (Koehne) Lourteig (a) fresh aerial parts | Kaempferol Gallic acid O-Galloylquinic acid Di-O-galloylquinic acid Brevifolincarboxylic acid Epigallocatechin Ellagic acid 3-O-Methyl ellagic acid 4′-sulfate 3-O-Methyl ellagic acid | [62] |
| C. carthagenensis (Jacq.) J.F.Macbr. (a) aerial parts (b) fresh aerial parts (c) aerial parts (d) leaves | β-Sitosterol, Stigmasterol Epifriedelanol Ergosterol, Carthagenol β-Amyrin Lauric acid, Myristic acid Betulinic acid, Ursolic acid Mannitol Quercetin-3-sulfate Quercetin-5-O-β -glucoside Quercetin-3-O-β-arabinofuranoside Quercetin-3-sulfate Quercetin Quercetin-5-O-β-glucoside Quercetin-3-O-(6′′-O-α-L-rhamnosyl)-β-D-glucoside Quercetin-3-O-β-D-glucuronide Quercetin-3-O-β-glucoside Quercetin-3-sulfate Quecertin-3-O-arabinofuranoside Kaempferol Kaempferol-rutinoside Kaempferol-3-glucoside Kaempferol 3,7-dirhamnoside Myricetin-glucoside Chlorogenic acid | [56] [50] [45] [60,62] | C. crulsiana Koehne (a) leaves | Quercetin Quercetin-3-O-arabinoside Quercetin-3-O-(glucose-rhamnose) Rhamnetin-3-O-glucoside Isorhamnetin-3-O-arabinoside | [49] |
| C. diosmifolia A.St.-Hil. (a) leaves | Quercetin Quercetin-3-O-galactoside Quercetin-3-O-(glucose-glucuronic acid) Rhamnetin-3-O-galactoside Myricetin-3-O-galactoside Myricetin-3-O-glucoside | [49] | |||
| C. disperma A.St.-Hil. (a) leaves | Apigenin-C-glycoside Quercetin-3-O-arabinoside Quercetin-3-O-galactoside Quercetin-3-O-glucosyl-glucosyl-glucoside | [49] | |||
| C. epilobiifolia Koehne (a) aerial part | β-Sitosterol, β-Amyrin Epifriedelanol Betulinic acid Mannitol | [56] | |||
| C. cipoensis T.B.Cavalc. (a) leaves | Isorhamnetin-3-O-galactoside Myricetin-3-O-galactoside | [49] | C. ericoides Cham. & Schltdl. (a) leaves | Quercetin-3-O-galactoside Kaempferol-3-O-galactoside Myricetin-3-O-arabinosyl-arabinoside Myricetin-3-O-galactosyl-galactosyl-galactoside | [49] |
| C. glutinosa Cham. & Schltdl. (a) whole plant (b) leaves | Quercetin Quercetin-3-O-β-glucoside Kaempferol β-Sitosterol-3-O-β-glucoside Methyl gallate Gallic acid Quercetin Quercetin-3-O-β-D-glucuronide Quercetin-3-arabinoside Quercetin-3-O-α-L-rhamnoside Quercetin-acetyl-glucuronide Quercetin-3-O-β-glucoside Kaempferol Kaempferol-3-glucoside Kaempferol-3-glucuronide 6"-O-Galloylquercimeritrin Isorhamnetin Myricetin-3-O-glucuronide 3-Feruloylquinic acid | [63] [45,60] | C. hyssopifolia (a) aerial part (cont.) | Methyl gallate Epifriedelanol Ursolic acid Mannitol 1,3-O-Digalloyl-4-,6-hexahydroxydiphenoyl-β-D−4C1-glucoside Genistein-7-O-β-D-4C1-glucoside Myricetin-3-O-β-D-4C1-glucoside Valoneic acid dilactone Gallic acid 3,4,5-Trimethoxy benzoic acid Vanillic acid | |
| C. hyssopifolia Kunth (a) aerial part | 1,2,3,6-Tetra-O-galloyl-β-D-glucose 1,2,3,4,6-Penta-O-galloyl-β-D-glucose Myricetin 3-O-α-L-rhamnoside Tellimagrandin II Woodfordin C Oenothein B Cuphiin D1 Cuphiin D2 Quercetin Quercetin-3-O-α-rhamnoside | [47,64,65] | C. ignea A.DC. (a) fresh plant (b) leaves | 7-Hydroxy-3-methoxycoumarin 5-O-β-glucoside Quercetin Quercetin-3-O-(6″-O-α-L-rhamnose)-β-D-glucoside Naringenin Myricetin-3-O-rhamnoside Catechin p-Coumaric acid o-Coumaric acid Gallic acid Caffeic acid Syringic acid Vanillic acid Cinnamic acid Rosmaric acid Chlorogenic acid Resveratrol | [57] [64] |
| C. ingrata Cham. & Schltdl. (a) leaves and thalli (b) aerial parts | Caffeine Quercetin Quercetin-3-O-(6′′-O-α-L-rhamnose)-β-D-glucoside Quercetin-3-O-β-D-glucoside Quercetin-3-O-β-D-glucuronide Quercetin-3-O-α-L-arabinoside Quercetin-3-O-α-L-arabinofuranoside Quercetin sulfate Quercetin-3-O-β-D-glucuronide butyl ester Kaempferol Kaempferol-3-O-(6′′-O-α-L-rhamnose)-β-D-glucoside Kaempferol-3-O-β-D-glucoside Methyl gallate, Gallic acid Protocatechuic acid p-Hydroxybenzoic acid Caffeic acid, Syringic acid Vanillic acid, p-Coumaric acid 1,3-Dicaffeoylquinic acid Ferulic acid Ellagic acid 1,5-Dicaffeoylquinic acid Oenothein B Cuphiin D2/Woodfordin C | [65] [47] | C. linarioides Cham. & Schltdl. (a) leaves | Myricetin-3-O-glucoside Myricetin-3-O-rhamnoside Myricetin-3-O-(glucose-rhamnose) | [49] |
| C. lindmaniana Koehne ex Bacig. (a) leaves | Quercetin Quercetin 3-O-β-D-glucuronide Quercetin-3-arabinoside Quercetin-acetyl-glucuronide Quercetin-3-(4″-malonylrhamnoside) Quercetin-3-O-β-glucoside Kaempferol Kaempferol-3-xyloside Kaempferol-3-glucuronide 3-Methylellagic acid 8-rhamnoside Chlorogenic acid Chicoric acid | [66] | |||
| C. lutea Rose ex Koehne (a) seed oil | Campesterol Stigmasterol β-Sitosterol Δ5-Avenasterol Δ7-Stigmastenol | [67] | |||
| C. lanceolata W.T.Aiton (a) seed oil (b) leaves | Campesterol Stigmasterol β-Sitosterol Δ5-Avenasterol Δ7-Stigmastenol Quercetin-3-β-D-glucoside | [67] [53] | C. lutescens Pohl ex Koehne (a) leaves | Quercetin-3-O-galactoside Quercetin-3-O-glucoside Quercetin-3-O-(arabinose-glucose) Isorhamnetin-3-O-(glucose-rhamnose) Myricetin-3-O-arabinoside Myricetin-3-O-galactoside Myricetin-3-O-(arabinose-galactose) | [49] |
| C. paucipetala S.A.Graham (a) seed oil | Campesterol Stigmasterol β-Sitosterol Δ5-Avenasterol Δ7-Stigmastenol | [67] | C. racemosa (L.f.) Spreng. (a) leaves | Quercetin Quercetin-3,7-diglucoside Quercetin-3-O-(6′′-O-α-L-rhamnose)-β-D-glucoside Quercetin-3-O-β-D-glucuronide Quercetin-3-arabinoside Quercetin-3-O-β-glucoside Kaempferol Kaempferol-3-O-rutinoside Kaempferol-3-glucuronide Myricetin-3-O-glucuronide Myricetin-3-O-glucoside Myricetin-3-O-α-L-rhamnoside Chlorogenic acid, 3-Feruloylquinic acid | [59] |
| C. pinetorum Benth. (a) roots (b) aerial part | Quercetin Kaempferol Quercetin Quercetin-3-O-α-rhamnoside Kaempferol Luteolin-7-O-β-D-glucoside Apigenin-7-O-α-rhamnoside Apigenin-7-O-β-D-glucoside Squalene, β-Sitosterol | [68] [69] | C. rubrovirens T.B.Cavalc. (a) leaves | Quercetin-3-O-galactoside Quercetin-3-O-(galactose-glucose) Rhamnetin-3-O-galactoside | [49] |
| C. pseudovaccinium A.St.-Hil. (a) leaves | Quercetin Quercetin-3-O-galactoside Quercetin-3-O-(galactose-rhamnose) Kaempferol-3-O-(galactose-glucose) Kaempferol-3-O-(glucose-rhamnose) Myricetin | [49] | C. sclerophylla Koehne (a) leaves | Quercetin Quercetin-3-O-galactoside Luteolin-7-O-galactoside Luteolin-7-O-(glucose-glucuronic acid) Myricetin-3-O-glucoside | [49] |
| C. pulchra Moric. (a) leaves | Quercetin-3-O-arabinoside Quercetin-3-O-galactosyl-galactoside Quercetin-3-O-rhamnosyl-glucoside Rhamnetin-3-O-glucoside Isorhamnetin-3-O-xyloside Myricetin | [49] | C. sessilifolia Mart. (a) leaves | Quercetin-3-O-arabinoside Quercetin-3-O-galactoside Quercetin-3-O-(galactose-glucose) Quercetin-3-O-(galactose-glucuronic acid) Quercetin-3-O-glucosyl-glucoside Quercetin-3-O-(glucose-glucuronic acid) Quercetin-3-O-rhamnosyl-glucoside Myricetin-3-O-galactoside | [49] |
| C. sperguloides A.St.-Hil (a) leaves | Myricetin-3-O-galactoside | [49] | C. viscosissima Jacq. (a) seed oil | Campesterol Stigmasterol β-Sitosterol Δ5-Avenasterol Δ7-Stigmastenol | [67] |
| C. teleandra Lourteig (a) leaves | Quercetin-3-O-arabinoside Quercetin-3-O-glucoside Quercetin-3-O-(glucose-rhamnose) Isorhamnetin-3-O-galactoside | [49] | |||
| C. urbaniana Koehne (a) leaves | Quercetin Quercetin-4′-galactoside Quercetin-3-O-(6′′-O-α-L-rhamnose)-β-D-glucoside Quercetin-3-O-β-D-glucuronide Quercetin-3-O-malonylglucoside Quercetin-galloyl rhamnoside Quercetin-3-O-α-L-rhamnoside Quercetin-3-O-β-glucoside Kaempferol Kaempferol-3-glucoside Apigenin-7-O-glucoside | [66] | C. wrightii A.Gray (a) seed oil (b) whole plant | Campesterol Stigmasterol β-Sitosterol Δ5-Avenasterol Δ7-Stigmastenol Quercetin-3-O-β-D-galactoside Luteolin-7-O-β-D-glucoside β-Sitosterol-3-O-β-D-glucoside Epifriedelanol Fernenol Germanicol Ursolic acid Mannitol | [67] [70] |
| Species | Part of the Plant | Form (Route of Administration) | Traditional Use | Reference |
|---|---|---|---|---|
| C. aequipetala | aerial parts | decoction (topically; wound washing) | wound healing bumps bruises throat pain | [18] |
| infusion | cough gastrointestinal disorders | |||
| not mentioned | diarrhea stomachache | [74] | ||
| C. calophylla var. macrostemon | aerial parts | decoction | anti-hypertensive | [75] |
| C. carthagenensis | leaves, aerial parts | decoction (orally) | anti-hypertensive lipid-lowering | [76] |
| whole plant leaves roots | maceration infusion | not mentioned | [77] | |
| roots | decoction (orally) | anti-hypertensive | [78] | |
| aerial parts | infusion (orally) | intestinal and heart problems | [79] | |
| stems and leaves | maceration in rum (topically) | sprains | [80] | |
| infusion (orally) | colds, chills | |||
| not mentioned | not mentioned | digestive problems diarrhea stomachache bowel infections leg pain varicose veins | [81] | |
| C. epilobiifolia | stems | decoction (orally) | rheumatism | [82] |
| leaves | decoction (baths) | rheumatism | ||
| C. glutinosa | aerial parts | infusion (orally) | hypercholesteremia | [83] |
| C. hyssopifolia | leaves and flowers | cough fever as insecticide and tonic | [84] | |
| C. ingrata | whole plant leaves stems | maceration infusion | cardiovascular system diseases musculoskeletal and joint diseases | [85] |
| C. lysimachioides | xylopodium | infusion | diarrhea as astringent | [86] |
| decoction | throatache | |||
| C. pinetorum | aerial parts | decoction (orally) | diarrhea dysentery | [69] |
| C. racemosa | not mentioned | infusion decoction (orally) | anti-hypertensive | [87] |
| C. urticulosa | leaves | ground up leaves (topically) | rashes lice | [88] |
| Cuphea Species | Biological Activity Tested | Results | Assay/Model | References |
|---|---|---|---|---|
| C. aequipetala (ethanol extract from leaves and stems) | antinociceptive anti-inflammatory | - antinociception in the acetic acid test (dose-dependent ↓ in the number of abdominal constrictions, ED50 = 90 mg/kg) and in the second phase of the formalin test (ED50 = 158 mg/kg), probably due to the involvement of nitric oxide and ATP-sensitive K+ channels - no effect in hot-plate test (doses: 50–200 mg/kg) - inhib. of production of NO (IC50 = 420 μM/mL) and H2O2 (IC50 = 416 μM/mL) in LPS-treated macrophages in a concentration-dependent manner - significant ↑ in the production of IL-10 (EC50 = 10 pg/mL) - ↓ of ear oedema by 25.7% after topical application of 2 mg of the extract - ↓ of the levels of IL-1β, IL-6, TNF-α, and PGE2 induced by the extract at the concentration of 100 mg/kg and 200 mg/kg | male Balb/c mice in vivo acetic acid-induced writhing test in vivo formalin test in vivo hot plate test in vitro LPS-stimulated primary murine macrophages male Balb/c mice in vivo TPA-induced ear oedema male Balb/c mice in vivo carrageenan-induced mouse paw oedema | [91] |
| C. aequipetala (ethanol extract from shoots and leaves) | anti-lipase antioxidant | - non-competitive inhib. of porcine pancreatic lipase (PPL) up to 60% - effect on the kinetic parameters of PPL: Km (mM) 0.365 ± 0.014 at the concentration of 50 μg/mL 0.362 ± 0.019 at the concentration of 100 μg/mL - high antioxidant activity against the DPPH radical with IC50 = 6.5 μg/mL | in vitro inhib. of PPL in vitro DPPH assay | [92] |
| C. aequipetala (methanol extracts from leaves, stems and roots of wild-grown and greenhouse grown plants) | antioxidant | - free-radical scavenging activity of extracts [μM trolox/g DW] - from wild-grown plants: leaves 169.33 ± 2.10 stems 19.19 ± 0.10 roots 85.62 ± 0.48 leaves 494.37 ± 8.6 stems 106.71 ± 0.3 roots 209.38 ± 1.2 - from greenhouse grown plants: leaves 87.83 ± 0.8 stems 21.86 ± 0.3 roots 43.26 ± 0.2 leaves 119.50 ± 0.3 stems 117.74 ± 0.2 roots 43.38 ± 0.1 | - in vitro DPPH assay - in vitro ABTS assay - in vitro DPPH assay - in vitro ABTS assay | [16] |
| C. aequipetala (extracts from leaves, flowers and stems) | antimicrobial | - no significant inhib. of bacteria and yeast cultures growth compared to common antibiotics: amoxicillin, ampicillin, carbenicillin, cephalotaxin, cephalothin, chloramphenicol, fosfomycin, gentamicin, penicillin, sulfamethoxazole, trimethopim | in vitro disc-diffusion method Staphyllococcus aureus, Staphyllococcus sp. coagulase-negative, Enterococcus faecalis, Escherichia coli, Candida albicans | [93] |
| C. aequipetala (methanol and aqueous extracts from aerial parts) | anti-Helicobacter pylori | - inhib. of the growth of H. pylori - aqueous extract: MIC 125 μg/mL - methanol extract: MIC >500 μg/mL | in vitro agar dilution method in vitro broth dilution method Helicobacter pylori | [74] |
| C. aequipetala (aqueous extracts from aerial parts prepared by infusion) | anti-Helicobacter pylori gastroprotective anti-inflammatory | - inhib. of the growth of H. pylori in a concentration dependent manner - promotion of bacterial lysis - MIC 125 μg/mL - ↓ of the ethanol-induced gastric lesions in a dose-dependent manner - 88% protective effect of the extract at the dose of 300 mg/kg, comparable to the effect (87%) of the reference drug carbenoxolone at the dose of 100 mg/kg - xylene-induced ear edema inhib. [%] after topical application of the extract 2.4 ± 2.7 at the dose of 0.1 mg of the extract 14.6 ± 2.5 at the dose of 0.25 mg 22.0 ± 4.0 at the dose of 0.5 mg - xylene-induced ear edema inhib. [%] after oral application of the extract 16.9 ± 4.4 at the dose of 10 mg/kg of the extract 36.4 ± 7.7 at the dose of 30 mg/kg 35.0 ± 3.0 at the dose of 100 mg/kg - TPA-induced ear edema inhib. [%] after topical application of the extract 10.4 ± 2.0 at the dose of 0.1 mg of the extract 14.3 ± 3.0 at the dose of 0.25 mg 23.7 ± 4.9 at the dose of 0.5 mg - TPA-induced ear edema inhib. [%] after oral application of the extract 12.2 ± 1.4 at the dose of 10 mg/kg of the extract 15.6 ± 2.2 at the dose of 30 mg/kg 27.3 ± 1.0 at the dose of 100 mg/kg | in vitro broth dilution method Helicobacter pylori male CD-1 mice in vivo ethanol-induced gastric ulcer model male CD-1 mice in vivo xylene and TPA-induced ear edema | [94] |
| C. aequipetala var. hispida (aqueous–ethanol extract) | antimicrobial antioxidant | - inhib. halo sizes [mm] (the preparation of 50% ethanolic extracts carried out with a 125 mg/mL dried matter plant concentration) L. monocytogenes 7.0 ± 0.0 Staphylococcus sp. 10 ± 1.0 E. coli 8 ± 0.03 S. enterica 8.0 ± 1.0 - free-radical scavenging activity [uM TEAC/g]—1756.59 ± 1.9 | in vitro agar diffusion susceptibility test disc method Listeria monocytogenes (ATCC 19115), Staphylococcus sp., Escherichia coli (ATCC 25922), Salmonella enterica serotype Enteritidis (ATCC 13076) in vitro ABTS assay | [72] |
| C balsamona Cham. & Schltdl. (aqueous extract) | hypocholesteremic | - significant ↓ in cholesterol and triglycerides blood levels (vs. control) during chronic treatment with different concentrations of aqueous extract - 50 mg/L total cholesterol 500.0 ± 108.25 (vs. 857.81 ± 56.22) triglycerides 80.95 ± 27 (vs. 173.80 ± 63.35) HDL 38.65 ± 1.03 (vs. 69.32 ± 3.34) VLDL 16.31 ± 5.36 (vs. 34.75 ± 12.67) LDL 445.16 ± 101.71 (vs. 753.73 ± 55.17) - 100 mg/L total cholesterol 684.37 ± 98.22 (vs. 857.81 ± 56.22) triglycerides 61.90 ± 22.67 (vs. 173.80 ± 63.35) HDL 48.28 ± 7.33 (vs. 69.32 ± 3.34) VLDL 12.37 ± 4.53 (vs. 34.75 ± 12.67) LDL 623.72 ± 92 (vs. 753.73 ± 55.17) | young adult male Wistar rats submitted to a high cholesterol diet in vivo dyslipidemia model | [95] |
| C. calophylla (aqueous–ethanol extract of aerial parts) | antioxidant | - free-radical scavenging activity [μM ET/g] - 1761.92 ± 3.05 - 3756.65 ± 2.48 | in vitro FRAP assay in vitro ORAC assay | [52] |
| C. calophylla (aqueous–ethanol extract of leaves) | anti-inflammatory | - significant ↓ in the ROS levels - no significant cytoprotective effect on the cell death induced by LPS and no effect on NO production in macrophages - inhib. activity against COX and LOX - 100% inhib. of PMNs migration at the concentration 10 μg/mL | in vitro inhib. of rat PMNs chemotaxis, employing a modified Boyden chamber | [96] |
| C. carthagenensis (ethanol–aqueous extract of leaves) | antihypertensive | - ACE-inhib. activity: 26.12% at the concentration of 100 ng/mL | in vitro ACE-inhib. assay | [59] |
| C. carthagenensis (dichloromethane– methanol extract of leaves) | antihypertensive | - ACE-inhib. activity: 50% at the concentration of 100 μg/mL | in vitro ACE-inhib. assay | [79] |
| C. carthagenensis (infusion of aerial parts and ethanol-soluble fraction) | diuretic antioxidant | - no changes in renal function or cortical blood flow - DPPH free radical scavenging of ethanol-soluble fraction: - IC50 = 18 ± 4.1 ug/mL - max activity—95 ± 1.8% at the concentration of 30 ug/mL - NO radical scavenging of ethanol-soluble fraction: - IC50 = 465 ± 4.1 ug/mL - max activity—68 ± 2.5% at the concentration of 1000 ug/mL | male Wistar rats in vivo laser-Doppler flowmetry in vitro DPPH assay in vitro nitric oxide radical assay | [97] |
| C. carthagenensis (aqueous extract of aerial parts and isolated fractions) | antinociceptive anti-inflammatory | - ↓ of the acetic acid-induced writhing in mice by aqueous extract (10 to 100 mg/kg) and semi-purified fraction (0.1 to 10 mg/kg) by 40 to 50% and by 46 to 70% of control, respectively; no effect in the tail flick response - the carrageenin-induced paw edema volume ↓ by semi-purified fraction at a dose of 100 mg/kg (p.o.) by 82% in the 1st hour after carrageenin injection and by 37% in the 3rd hour | adult albino male mice in vivo acetic acid-induced writhing test in vivo tail flick test in vivo carrageenan-induced rat paw oedema | [98] |
| C. carthagenensis (ethanol-soluble fraction of infusion of leaves) | serum lipid-lowering | - ↓ in oxidative stress and significant ↓ of the CAT (17,274.7 μM min mg) and ↑ of the SOD (3571.2 μM min mg) activities in liver after 4-weeks treatment with the ethanol-soluble fraction (100 mg/kg) - no significant change in the glutathione-S-transferase activity - ↓ of the serum triglycerides (TG), total cholesterol fractions (LDL-C and VLDL-C) levels and ↑ of the level of HDL-C after 4-weeks-treatment (vs. positive control) - at dose of 10 mg/kg TG 166 ± 35 (vs. 190 ± 28) LDL-C 166 ± 33 (vs. 185 ± 20) VLDL-C 78 ± 9.2 (vs. 81 ± 10) HDL-C 7.8 ± 0.8 (vs. 7.2 ± 0.3) - at dose of 30 mg/kg TG 140 ± 31 (vs. 190 ± 28) LDL-C 122 ± 15 (vs. 185 ± 20) VLDL-C 57 ± 6.9 (vs. 81 ± 10) HDL-C 8.2 ± 0.2 (vs. 7.2 ± 0.3) - at dose of 100 mg/kg TG 147 ± 25 (vs. 190 ± 28) LDL-C 117 ± 17 (vs. 185 ± 20) VLDL-C 56 ± 7.1 (vs. 81 ± 10) HDL-C 8.6 ± 0.4 (vs. 7.2 ± 0.3) | New Zealand (NZ) rabbits undergoing cholesterol-rich diet in vivo dyslipidemia and atherosclerosis model | [99] |
| C. carthagenensis (infusion of herb) | body weight control | - significant ↓ in cholesterolemia while chronic (4-weeks; infusion administrated to the rats ad libitum) treatment (vs. control) - cholesterol [mg/dL] 57 ± 9 (vs. 96 ± 23) - no significant effect on glycemic level, body weight and triglyceride level in comparison to control group | male Wistar rats undergoing a high calorie diet | [100] |
| C. carthagenensis (ethanol and aqueous extracts of aerial parts and derived fractions) | vasorelaxant | - vasodilatation on pre-contracted rat aortic rings probably associated with polyphenolic compounds - vasodilatation [pIC50] (max vasodilatation %): - ethanol extract 4.92 ± 0.11 (81.8 ± 5.1) - aqueous extract not calculated (46.8 ± 14.4) - n-butanol fraction 4.98 ± 0.06 (86.2 ± 1.6) - methanol-insoluble water fraction 4.53 ± 0.03 (94.8 ± 4.3) - methanol-soluble water fraction 4.85 ± 0.11 (89.1 ± 4.5) - emulsion 4.93 ± 0.07 (86.0 ± 7.1) | ex vivo aortic rings with functional endothelium, pre-contracted with phenylephrine, from male Wistar rats | [45] |
| C. carthagenensis (aqueous–ethanol-extract of aerial parts) | vasorelaxant | - the n-butanol fraction induced relaxation in rat aortic rings (IC50 = 6.85 μg/mL) through two separate mechanisms - endothelium-dependent: stimulation and/or potentiation of NO release and stimulation and/or potentiation of NO release - endothelium-independent: free radical-scavenging properties | ex vivo endothelium-intact rings of thoracic aorta from male Wistar rats | [101] |
| C. carthagenensis (ethanol-soluble fraction of aqueous extract from aerial parts) | cardioprotective | - inhib. of the progression of the cardiorenal disease while a 4-weeks treatment - modulation of the antioxidant defense system - NO/cGMP activation and K+ channel opening-dependent vasodilator effect | female Wistar rats in vivo two-kidney, one-clip (2K1C) model | [102] |
| C. carthagenensis (aqueous–ethanol extract of leaves and n-butanol and ethyl acetate fractions) | antioxidant | - inhib. of uric acid formation and inhib. of NBT ↓ by O2− - concentration-dependent inhib. of deoxyribose degradation - inhib. of lipid peroxidation induced by t-butyl-peroxide | in vitro xanthine/xanthine oxidase assay in vitro deoxyribose degradation assay in vitro lipid peroxidation assay | [103] |
| C. carthagenensis (methanol extract of leaves) | antioxidant anti-biofilm and QS-related virulence factors | - dose-dependent DPPH scavenging activity - max activity at 1.0 mg/mL (64.79 ± 0.83%) - ↓ of ferricyanide complex (Fe3+) to the ferrous form (Fe2+) - inhib. of biofilm formation at the concentration of 1 mg/mL by - 81.88 ± 2.57% (TCP method) - 72.14 ± 3.25% (tube method) - inhib. of production of QS-dependent virulence factors in Pseudomonas aeruginosa at sub-lethal concentrations of extract without affecting bacterial growth: - significant ↓ in pyocyanin production - max inhib. at the concentration of 1.0 mg/mL by 84.55 ± 1.63% - at the concentration of 0.25 mg/mL by 77.50 ± 2.10% - inhib. of violacein production (83.31 ± 2.77%) in Chromobacterium violaceum | in vitro DPPH assay in vitro FRAP assay in vitro tissue culture plate method (TCP) in vitro tube method microscopic techniques Chromobacterium violaceum ATCC12472, Pseudomonas aeruginosa MTCC 2297 | [17] |
| C. glutinosa (aqueous–ethanol extract of leaves) | antihypertensive | - ACE-inhib. activity [%] of the extract of leaves collected in: - Alegrete 31.66 - Unistalda 26.32 - miquelianin 32.41 | in vitro ACE-inhib. | [59] |
| C. glutinosa (aqueous and ethanol extracts of whole plant and derived fractions) | antioxidant inhibitory activity on Na+, K+-ATPAse | - DPPH scavenging activity [EC50 μg/mL] - aqueous extract 64.75 - ethyl acetate fraction 16.77 - ethanolic extract 42.17 - lower antioxidant capacity compared with the standard quercetin 2.059 - inhib. of the enzyme activity by the ethanolic extract at the concentration above 100 μg/mL with EC50 = 84.54 (48.77 to 146.6) μg/mL | in vitro DPPH assay in vitro ATPase extracted from male Wistar rat heart muscle membranes | [63] |
| C. glutinosa (roots and leaves infusions and macerations) | antifungal | - MIC [μg/mL] values: - roots infusion Trichosporon asahii TBE 23 7.8 T. asahii TAH 09 1.9 Candida parapsilosis RL 36 15.9 C. parapsilosis RL 07 62.5 Candida glabrata CG 08 >500 C. glabrata CG 10 >500 Candida tropicalis 102 A 62.5 C. tropicalis 72 A 62.5 - leaf infusion Trichosporon asahii TBE 23 1.9 T. asahii TAH 09 1.9 Candida parapsilosis RL 36 7.8 C. parapsilosis RL 07 31.25 Candida glabrata CG 08 >500 C. glabrata CG 10 >500 Candida tropicalis 102 A 62.5 C. tropicalis 72 A 62.5 - root maceration Trichosporon asahii TBE 23 3.9 T. asahii TAH 09 15.6 Candida parapsilosis RL 36 62.5 C. parapsilosis RL 07 62.5 Candida glabrata CG 08 >500 C. glabrata CG 10 >500 Candida tropicalis 102 A 62.5 C. tropicalis 72 A 62.5 - leaf maceration Trichosporon asahii TBE 23 1.9 T. asahii TAH 09 500 Candida parapsilosis RL 36 31.25 C. parapsilosis RL 07 31.25 Candida glabrata CG 08 62.5 C. glabrata CG 10 >500 Candida tropicalis 102 A 15.6 C. tropicalis 72 A 15.6 | in vitro broth microdilution method Trichosporon asahii TBE 23, T. asahii TAH 09, Candida parapsilosis RL 36, C. parapsilosis RL 07, C. glabrata CG 08, C. glabrata CG 10, C. tropicalis 102 A, C. tropicalis 72 A | [46] |
| C. hyssopifolia (aqueous–methanol extract) | antioxidant | - inhib. of DPPH radical at 95.5% (IC50 = 12.34 μg/mL) compared to ascorbic acid—at 98.35% (IC50 = 1.82 μg/mL) | in vitro DPPH assay | [48] |
| C. hyssopifolia (methanol extract of leaves) | hepatoprotective | - changes in SOD, CAT, and MDA levels after pretreatment with the extract at the concentrations of 200 and 400 mg/kg, (vs. paracetamol-treated control) [IU/L] - 200 mg/kg SOD 0.25 ± 0.02 (vs. 0.27 ± 0.06) CAT 1.32 ± 0.06 (vs. 0.45 ± 0.09) MDA 0.45 ± 0.02 (vs. 0.72 ± 0.07) - 400 mg/kg SOD 0.32 ± 0.01 (vs. 0.27 ± 0.06) CAT 1.80 ± 0.01 (vs. 0.45 ± 0.09) MDA 0.45 ± 0.04 (vs. 0.72 ± 0.07) | adult Wistar rats in vivo paracetamol-induced hepatotoxicity rat model | [104] |
| C. ignea (aqueous–ethanol extract of aerial parts) | antitumor | - pre-treatment with C. ignea extract was more effective then post-treatment and provided chemopreventive effect probably due to its potential to attenuate benzo(α)pyrene-induced oxidative stress in the lung tissues through the amelioration of the antioxidant defense system | male Swiss albino mice in vivo benzo(α)pyrene-induced lung tumorigenesis mouse model; | [105] |
| C. ignea (aqueous–ethanol extract of aerial parts) | antiulcerogenic, gastroprotective | - doses of 250 and 500 mg/kg bw administrated orally a week before ulcer induction, decreased the volume of gastric juice and gastric ulcer index, increased gastric pH value and pepsin activity - anti-ulcer activity comparable to that of ranitidine - anti-inflammatory, antioxidant, and curing effect on the hemorrhagic shock induced by ethanol toxicity | adult female Sprague-Dawley rats in vivo ethanol-induced gastric ulcers in rats | [58] |
| C. ignea (aqueous and ethanol extracts of leaves, flowers, stems; n-butanol and ethyl acetate fractions) | antihypertensive | - ACE inhib. activity IC50 [mg/mL] - aqueous extract of leaves 0.491 - ethanolic extract of leaves 2.151 - ethanolic extract of the flowers 1.748 - aqueous extract of stems 2.036 - ethanolic extract of stems 5.707 - n-butanol fraction of ethanol extract of leaves 0.084 - ethyl acetate fraction of ethanol extract. of leaves 0.215 - inhib. of renin activity [%] at the sample concentration of 10 mg/mL - ethanolic extract of leaves 94.82 - ethanolic extracts of stems 88.98 - ethanolic extract of flowers 86.65 - methylene chloride of the stems 98.14 - ethyl acetate fractions of leaves 93.09 - attenuation of elevated systolic blood pressure by ethanolic extract of leaves (at doses of 250 and 500 mg/kg b.wt.) similarly to standard lisinopril | in vitro ACE inhib. in vitro renin inhib. male Sprague-Dawley rats in vivo L-NAME-induced hypertension model | [54,106] |
| C. ignea (hydrolyzed seed oil) | antibacterial | - MIC [mg/mL] values: Enterococcus cecorum CCM 3659 2.25 CCM 4285 1.13 Clostridium perfringens CIP 105178 0.56 CNCTC 5454 4.5 UGent 56 2.25 Listeria monocytogenes ATCC 7644 1.13 Staphylococcus aureus ATCC 25923 2.25 | in vitro broth microdilution method Enterococcus cecorum CCM 3659, CCM 4285 Clostridium perfringens CIP 105178, CNCTC 5454, UGent 56 Listeria monocytogenes ATCC 7644 Staphylococcus aureus ATCC 25923 Bifidobacterium animalis CCM 4988, MA5 B. longum TP 1, CCM 4990 Lactobacillus fermentum CCM 91 L. acidophilus CCM 4833 | [43] |
| C. ingrata (5% tincture) | hypocholesteremic | - significant cholesterol level ↓, no significant effect on cholesterol absorption and triglyceride profile | in vivo male mice diet-induced hypercholesterolemia model | [107] |
| C. ingrata (methanol extract of aerial parts) | antimicrobial | - B. cereus and C. albicans growth inhib. with MIC 39 μg/mL | in vitro serial dilution assay Bacillus cereus, Candida albicans | [55] |
| C. ingrata (dichloromethane–methanol (1:1) and ethanol extracts of aerial parts) | trypanocidal | - 29% inhib. at a concentration of 100 μg/mL of the dichloromethane–methanol (1:1) extract - no effect of the aqueous extract | in vitro epimastigote assay Trypanosoma cruzi | [108] |
| C. lindmaniana (aqueous–ethanol extract of leaves) | anti-inflammatory antihypertensive | - 100% PMNs migration inhib. at the concentrations of 0.01–10.0 μg/mL of the extract - ACE-inhib. activity 19.58% | in vitro inhib. of rat PMNs chemotaxis, employing a modified Boyden chamber in vitro ACE-inhib. | [66] |
| C. pinetorum (dichloromethane– methanol extract of aerial parts) | antiprotozoal | - inhib. of the growth of trophozoites by isolated flavonoids with kaempferol as the most active compound against E. hystolitica (IC50 = 7 μg/mL) and G. lamblia (IC50 = 8.7 μg/mL) | in vitro susceptibility test using a subculture method Entamoeba histolytica HM1-IMSS, Giardia lamblia IMSS:0989:1 | [69] |
| C. pinetorum (isolated flavonoids) | antiprotozoal | - antiprotozoal activity of isolated flavonoid compounds against Giardia lamblia with ED50 [μM/kg]: (-) epicatechin 0.072 kaempferol 2.057 tiliroside 1.429 | suckling female CD-1 mice in vivo experimental infection of Giardia lamblia | [109] |
| C. pinetorum (methanol extracts of stems and leaves) | antimicrobial | - inhib. effect of the extracts at dose of 10 mg on S. aureus and C. albicans | in vitro disc-diffusion method Staphylococcus aureus ATCC 15006, Candida albicans ATCC 10231 | [110] |
| C. subuligera (methanol extract of stems) | antimicrobial | - inhib. effect of the extract at dose of 10 mg on S. aureus (significant) and C. albicans | in vitro disc-diffusion method Staphylococcus aureus ATCC 15006, Candida albicans ATCC 10231 | [110] |
| C. urbaniana (aqueous–ethanol extract of leaves collected in Unistalda and Barros Cassal) | anti-inflammatory antihypertensive | - 100% PMNs migration inhib. at the concentrations of 0.001–10.0 μg/mL of the extract - ACE-inhib. activity [%] of the extract of leaves collected in: - Unistalda 22.82 - Barros Cassal 22.29 | in vitro inhib. of rat PMNs chemotaxis, employing a modified Boyden chamber. in vitro ACE-inhib. | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewska, D.; Michalska, K.; Wróbel-Biedrawa, D.; Grabowska, K.; Owczarek-Januszkiewicz, A.; Olszewska, M.A.; Podolak, I. The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review. Int. J. Mol. Sci. 2023, 24, 6614. https://doi.org/10.3390/ijms24076614
Sobolewska D, Michalska K, Wróbel-Biedrawa D, Grabowska K, Owczarek-Januszkiewicz A, Olszewska MA, Podolak I. The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review. International Journal of Molecular Sciences. 2023; 24(7):6614. https://doi.org/10.3390/ijms24076614
Chicago/Turabian StyleSobolewska, Danuta, Klaudia Michalska, Dagmara Wróbel-Biedrawa, Karolina Grabowska, Aleksandra Owczarek-Januszkiewicz, Monika Anna Olszewska, and Irma Podolak. 2023. "The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review" International Journal of Molecular Sciences 24, no. 7: 6614. https://doi.org/10.3390/ijms24076614
APA StyleSobolewska, D., Michalska, K., Wróbel-Biedrawa, D., Grabowska, K., Owczarek-Januszkiewicz, A., Olszewska, M. A., & Podolak, I. (2023). The Genus Cuphea P. Browne as a Source of Biologically Active Phytochemicals for Pharmaceutical Application and Beyond—A Review. International Journal of Molecular Sciences, 24(7), 6614. https://doi.org/10.3390/ijms24076614