Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media
Abstract
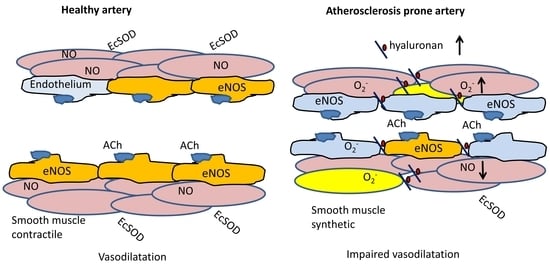
:1. Introduction
2. Results
2.1. Functional Studies and NO Measurements
2.2. eNOS Expression and Phosphorylation
2.3. Assessment of Vascular O2- and O2- Scavenging
2.4. EC–EC Distance and Expression of Occludin, Vascular Endothelial Cadherin, and the LDL Receptor
3. Discussion
3.1. Sex-Dependent Differences in Endothelial Dysfunction
3.2. Effect of Hyaluronan Overexpression on Endothelial Cell Function
3.3. Pro-Atherogenic Effects of Hyaluronan Overexpression
3.4. Limitations
4. Materials and Methods
4.1. Animals
4.2. Contractility
4.3. Diaminofluorescence
4.4. Detection of Vascular O2−
4.5. Immunoblotting
4.6. EC–EC Distance
4.7. Data and Statistical Analyses
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Wasty, F.; Alavi, M.Z.; Moore, S. Distribution of Glycosaminoglycans in the Intima of Human Aortas: Changes in Atherosclerosis and Diabetes Mellitus. Diabetologia 1993, 36, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Prenner, S.B.; Chirinos, J.A. Arterial Stiffness in Diabetes Mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Yamashita, T.; Takaya, T.; Shinohara, M.; Shiraki, R.; Takeda, M.; Emoto, N.; Fukatsu, A.; Hayashi, T.; Ikemoto, K.; et al. Augmentation of Vascular Remodeling by Uncoupled Endothelial Nitric Oxide Synthase in a Mouse Model of Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Ladeia, A.M. Prognostic Value of Endothelial Dysfunction in Type 1 Diabetes Mellitus. World J. Diabetes 2014, 5, 601. [Google Scholar] [CrossRef]
- Seike, M.; Ikeda, M.; Matsumoto, M.; Hamada, R.; Takeya, M.; Kodama, H. Hyaluronan Forms Complexes with Low Density Lipoprotein While Also Inducing Foam Cell Infiltration in the Dermis. J. Dermatol. Sci. 2006, 41, 197–204. [Google Scholar] [CrossRef]
- Heickendorff, L.; Ledet, T.; Rasmussen, L.M. Glycosaminoglycans in the Human Aorta in Diabetes Mellitus: A Study of Tunica Media from Areas with and without Atherosclerotic Plaque. Diabetologia 1994, 37, 286–292. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 Interactions as Potential Targets for Cancer Therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Williams, K.J.; Tabas, I. The Response-to-Retention Hypothesis of Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–562. [Google Scholar] [CrossRef]
- Nakashima, Y.; Wight, T.N.; Sueishi, K. Early Atherosclerosis in Humans: Role of Diffuse Intimal Thickening and Extracellular Matrix Proteoglycans. Cardiovasc. Res. 2008, 79, 14–23. [Google Scholar] [CrossRef]
- Chai, S.; Chai, Q.; Danielsen, C.C.; Hjorth, P.; Nyengaard, J.R.; Ledet, T.; Yamaguchi, Y.; Rasmussen, L.M.; Wogensen, L. Overexpression of Hyaluronan in the Tunica Media Promotes the Development of Atherosclerosis. Circ. Res. 2005, 96, 583–591. [Google Scholar] [CrossRef]
- Lorentzen, K.A.; Chai, S.; Chen, H.; Danielsen, C.C.; Simonsen, U.; Wogensen, L. Mechanisms Involved in Extracellular Matrix Remodeling and Arterial Stiffness Induced by Hyaluronan Accumulation. Atherosclerosis 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A. Long-Term Follow-up of Patients with Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Nitenberg, A.; Chemla, D.; Antony, I.; Verdier, C.J. Long-Term Follow-up of Hypertensive Patients with Angiographically Normal Coronary Arteries: Prognostic Value of Epicardial Coronary Endothelial Dysfunction. J. Am. Coll. Cardiol. 2003, 41, 282. [Google Scholar] [CrossRef]
- Nitenberg, A.; Pham, I.; Antony, I.; Valensi, P.; Attali, J.R.; Chemla, D. Cardiovascular Outcome of Patients with Abnormal Coronary Vasomotion and Normal Coronary Arteriography Is Worse in Type 2 Diabetes Mellitus than in Arterial Hypertension: A 10 Year Follow-up Study. Atherosclerosis 2005, 183, 113–120. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.; Warnholtz, A.; et al. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Lund, D.D.; Chu, Y.; Miller, J.D.; Heistad, D.D. Protective Effect of Extracellular Superoxide Dismutase on Endothelial Function during Aging. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H1920–H1925. [Google Scholar] [CrossRef]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular Endothelium in Atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef]
- Jung, O.; Marklund, S.L.; Geiger, H.; Pedrazzini, T.; Busse, R.; Brandes, R.P. Extracellular Superoxide Dismutase Is a Major Determinant of Nitric Oxide Bioavailability: In Vivo and Ex Vivo Evidence from EcSOD-Deficient Mice. Circ. Res. 2003, 93, 622–629. [Google Scholar] [CrossRef]
- Gao, F.; Koenitzer, J.R.; Tobolewski, J.M.; Jiang, D.; Liang, J.; Noble, P.W.; Oury, T.D. Extracellular Superoxide Dismutase Inhibits Inflammation by Preventing Oxidative Fragmentation of Hyaluronan. J. Biol. Chem. 2008, 283, 6058–6066. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Siegfried, M.R.; Ushio-Fukai, M.; Cheng, Y.; Kojda, G.; Harrison, D.G. Regulation of the Vascular Extracellular Superoxide Dismutase by Nitric Oxide and Exercise Training. J. Clin. Investig. 2000, 105, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell. Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Califano, J.P.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-Related Intimal Stiffening Enhances Endothelial Permeability and Leukocyte Transmigration. Sci. Transl. Med. 2011, 3, 12ra122. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. CardioRenal Med. 2014, 4, 60–71. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M.D.; Østergaard, L.; Andersen, M.R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic Acid Induces Relaxation and Calcium-Independent Release of Endothelium-Derived Nitric Oxide. Br. J. Pharmacol. 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Matlung, H.L.; Neele, A.E.; Groen, H.C.; van Gaalen, K.; Tuna, B.G.; van Weert, A.; de Vos, J.; Wentzel, J.J.; Hoogenboezem, M.; van Buul, J.D.; et al. Transglutaminase Activity Regulates Atherosclerotic Plaque Composition at Locations Exposed to Oscillatory Shear Stress. Atherosclerosis 2012, 224, 355–362. [Google Scholar] [CrossRef]
- Van Herck, J.L.; Schrijvers, D.M.; De Meyer, G.R.Y.; Martinet, W.; Van Hove, C.E.; Bult, H.; Vrints, C.J.; Herman, A.G. Transglutaminase 2 Deficiency Decreases Plaque Fibrosis and Increases Plaque Inflammation in Apolipoprotein-E-Deficient Mice. J. Vasc. Res. 2010, 47, 231–240. [Google Scholar] [CrossRef]
- Wellman, G.C.; Bonev, A.D.; Nelson, M.T.; Brayden, J.E. Gender Differences in Coronary Artery Diameter Involve Estrogen, Nitric Oxide, and Ca2+-Dependent K+ Channels. Circ. Res. 1996, 79, 1024–1030. [Google Scholar] [CrossRef]
- Takenouchi, Y.; Kobayashi, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Gender Differences in Vascular Reactivity of Aortas from Streptozotocin-Induced Diabetic Mice. Biol. Pharm. Bull. 2010, 33, 1692–1697. [Google Scholar] [CrossRef]
- Taguchi, K.; Matsumoto, T.; Kamata, K.; Kobayashi, T. Akt/ENOS Pathway Activation in Endothelium-Dependent Relaxation Is Preserved in Aortas from Female, but Not from Male, Type 2 Diabetic Mice. Pharmacol. Res. 2012, 65, 56–65. [Google Scholar] [CrossRef]
- Chen, H.; Simonsen, U.; Aalkjaer, C. A Sex-Specific, COX-Derived/Thromboxane Receptor Activator Causes Depolarization and Vasoconstriction in Male Mice Mesenteric Resistance Arteries. Basic Clin. Pharmacol. Toxicol. 2020, 127, 152–159. [Google Scholar] [CrossRef]
- Kauser, K.; Rubanyi, G.M. Gender Difference in Endothelial Dysfunction in the Aorta of Spontaneously Hypertensive Rats. Hypertension 1995, 25, 517–523. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Biggs, M.L.; Barzilay, J.; Kuller, L.H.; Mozaffarian, D.; Mukamal, K.; Smith, N.L.; Siscovick, D. Diabetes and Coronary Heart Disease as Risk Factors for Mortality in Older Adults. Am. J. Med. 2010, 123, 556.e1. [Google Scholar] [CrossRef]
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex Differences in the Vascular Function and Related Mechanisms: Role of 17β-Estradiol. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1499–H1518. [Google Scholar] [CrossRef]
- Beck, L.; Su, J.; Comerma-Steffensen, S.; Pinilla, E.; Carlsson, R.; Hernanz, R.; Sheykhzade, M.; Danielsen, C.C.; Simonsen, U. Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic Db/Db Mice. Front. Physiol. 2020, 11, 667. [Google Scholar] [CrossRef]
- Comerma-Steffensen, S.; Prat-Duran, J.; Mogensen, S.; Fais, R.; Pinilla, E.; Simonsen, U. Erectile Dysfunction and Altered Contribution of KCa1.1 and KCa2.3 Channels in the Penile Tissue of Type-2 Diabetic Db/Db Mice. J. Sex. Med. 2022, 19, 697–710. [Google Scholar] [CrossRef]
- Chen, H.; Kold-Petersen, H.; Laher, I.; Simonsen, U.; Aalkjaer, C. Impaired Endothelial Calcium Signaling Is Responsible for the Defective Dilation of Mesenteric Resistance Arteries from Db/Db Mice to Acetylcholine. Eur. J. Pharmacol. 2015, 767, 17–23. [Google Scholar] [CrossRef]
- Boittin, F.X.; Alonso, F.; Le Gal, L.; Allagnat, F.; Bény, J.L.; Haefliger, J.A. Connexins and M3 Muscarinic Receptors Contribute to Heterogeneous Ca Signaling in Mouse Aortic Endothelium. Cell. Physiol. Biochem. 2013, 31, 166–178. [Google Scholar] [CrossRef]
- Tangirala, R.K.; Rubin, E.M.; Palinski, W. Quantitation of Atherosclerosis in Murine Models: Correlation between Lesions in the Aortic Origin and in the Entire Aorta, and Differences in the Extent of Lesions between Sexes in LDL Receptor-Deficient and Apolipoprotein E-Deficient Mice. J. Lipid Res. 1995, 36, 2320–2328. [Google Scholar] [CrossRef]
- Russell, R. Mechanisms of Disease: Atherosclerosis An Inflammatory Disease. N. Engl. J. Med. 1999, 1, 115–126. [Google Scholar]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement Fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, E.; Comerma-Steffensen, S.; Prat-Duran, J.; Rivera, L.; Matchkov, V.V.; Buus, N.H.; Simonsen, U. Transglutaminase 2 Inhibitor LDN 27219 Age-Dependently Lowers Blood Pressure and Improves Endothelium-Dependent Vasodilation in Resistance Arteries. Hypertension 2020, 77, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Swoap, S.J.; Gutilla, M.J. Cardiovascular Changes during Daily Torpor in the Laboratory Mouse. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R769–R774. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard Operating Procedures for Describing and Performing Metabolic Tests of Glucose Homeostasis in Mice. DMM Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Buus, N.H.; Hansson, N.C.; Rodriguez-Rodriguez, R.; Stankevicius, E.; Andersen, M.R.; Simonsen, U. Antiatherogenic Effects of Oleanolic Acid in Apolipoprotein e Knockout Mice. Eur. J. Pharmacol. 2011, 670, 519–526. [Google Scholar] [CrossRef]
- Bangshaab, M.; Gutierrez, A.; Huynh, K.D.; Knudsen, J.S.; Arcanjo, D.D.R.; Petersen, A.G.; Rungby, J.; Gejl, M.; Simonsen, U. Different Mechanisms Involved in Liraglutide and Glucagon-like Peptide-1 Vasodilatation in Rat Mesenteric Small Arteries. Br. J. Pharmacol. 2019, 176, 386–399. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Stain-Free Total Protein Staining Is a Superior Loading Control to b-Actin for Western Blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef]









| Male WT | Male HAS-2 | Female WT | Female HAS-2 | |
|---|---|---|---|---|
| PhE −log EC50 | 6.49 ± 0.11 # (13) | 6.35 ± 0.11 # (13) | 7.19 ± 0.24 (5) | 6.92 ± 0.08 (5) |
| ACh −log EC50 | 7.45 ± 0.20 # (11) | 7.26 ± 0.23 # (11) | 8.13 ± 0.25 (5) | 7.92 ± 0.11 (5) |
| SNP −log EC50 | 8.05 ± 0.05 # (11) | 7.70 ± 0.07 #* (119) | 8.62 ± 0.05 # (5) | 8.31 ± 0.05 #* (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorentzen, K.A.; Hernanz, R.; Pinilla, E.; Nyengaard, J.R.; Wogensen, L.; Simonsen, U. Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media. Int. J. Mol. Sci. 2023, 24, 8436. https://doi.org/10.3390/ijms24098436
Lorentzen KA, Hernanz R, Pinilla E, Nyengaard JR, Wogensen L, Simonsen U. Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media. International Journal of Molecular Sciences. 2023; 24(9):8436. https://doi.org/10.3390/ijms24098436
Chicago/Turabian StyleLorentzen, Karen Axelgaard, Raquel Hernanz, Estéfano Pinilla, Jens Randel Nyengaard, Lise Wogensen, and Ulf Simonsen. 2023. "Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media" International Journal of Molecular Sciences 24, no. 9: 8436. https://doi.org/10.3390/ijms24098436