Oral Exposure to Titanium Dioxide E171 and Zinc Oxide Nanoparticles Induces Multi-Organ Damage in Rats: Role of Ceramide
Abstract
:1. Introduction
2. Results
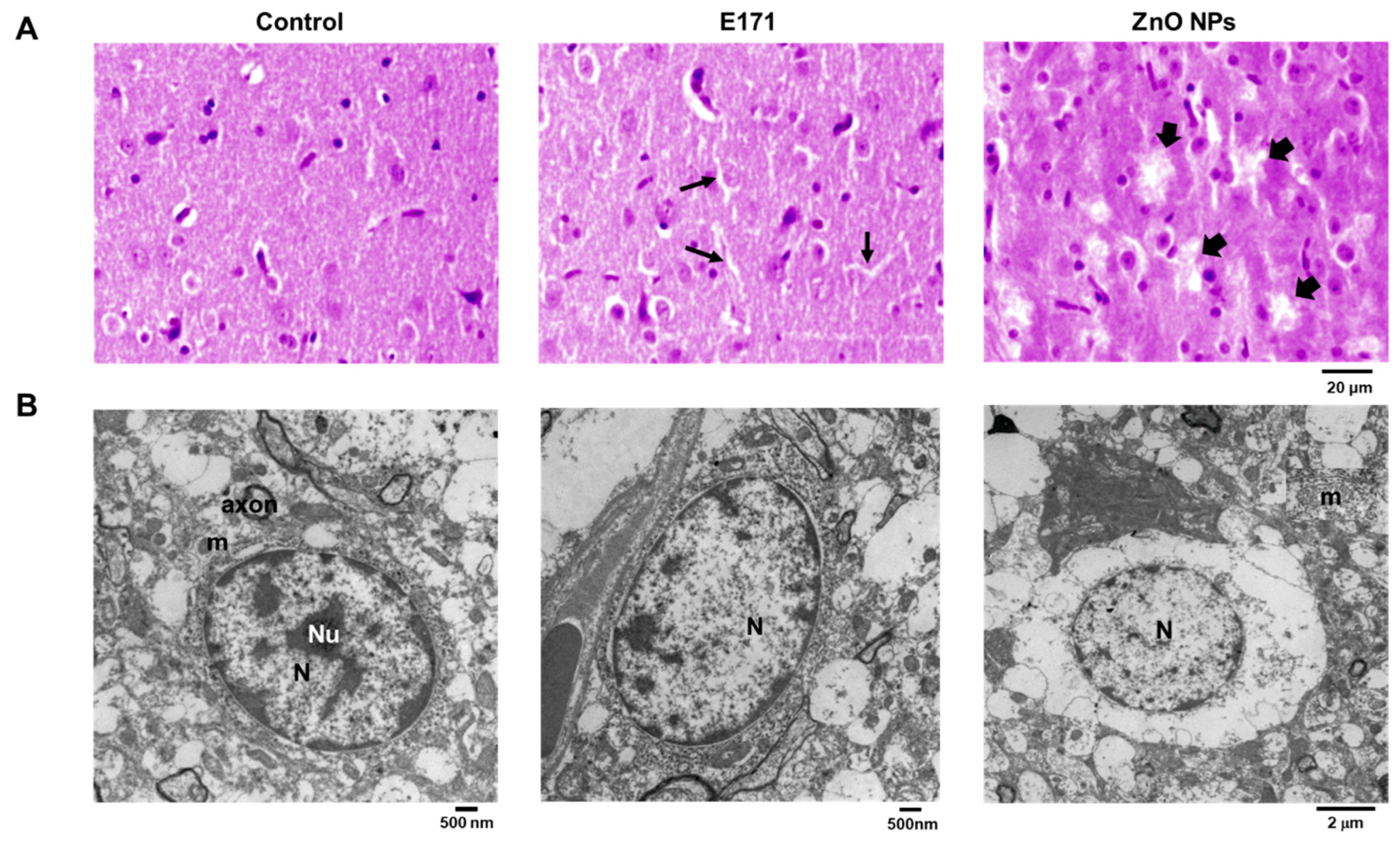
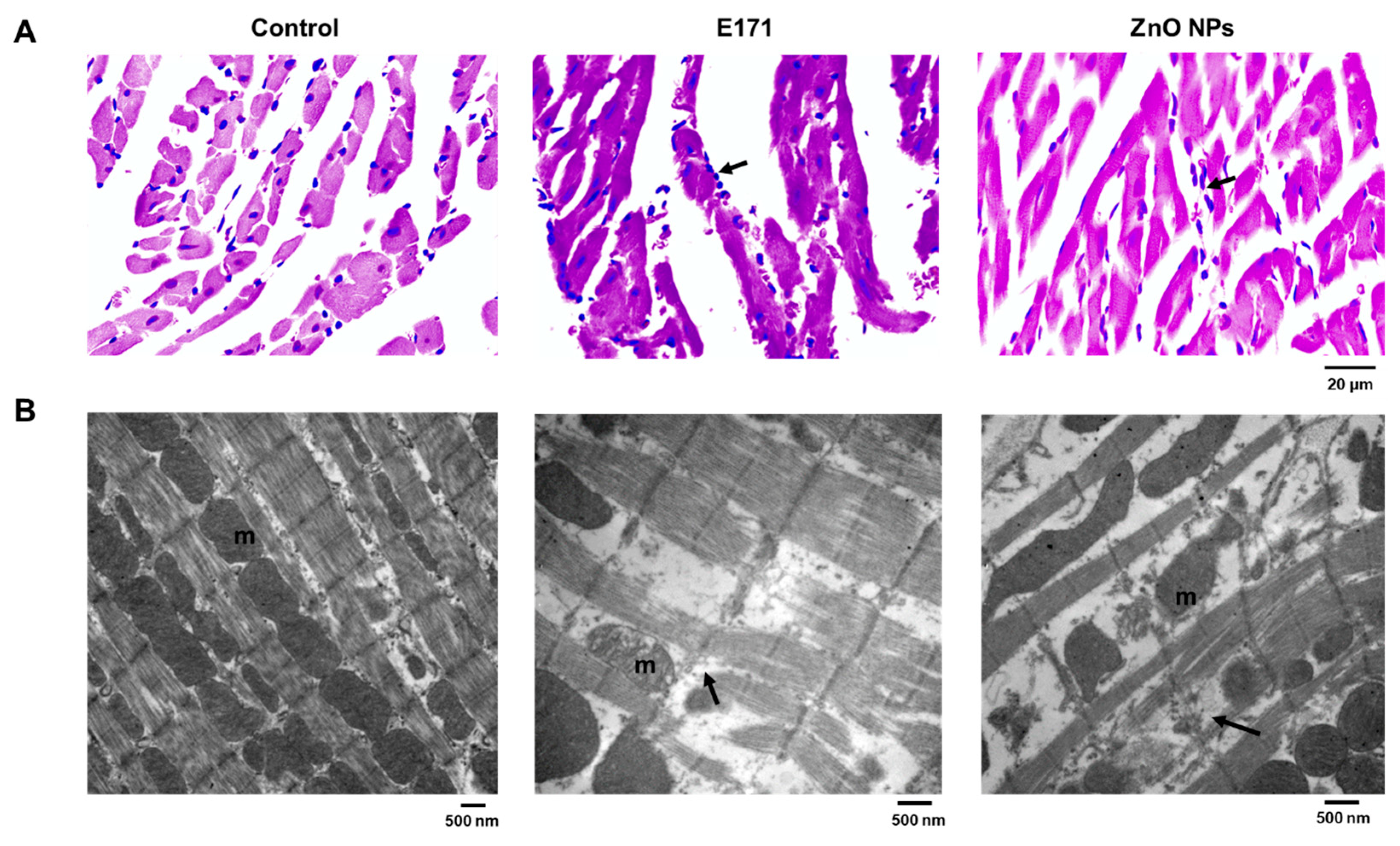
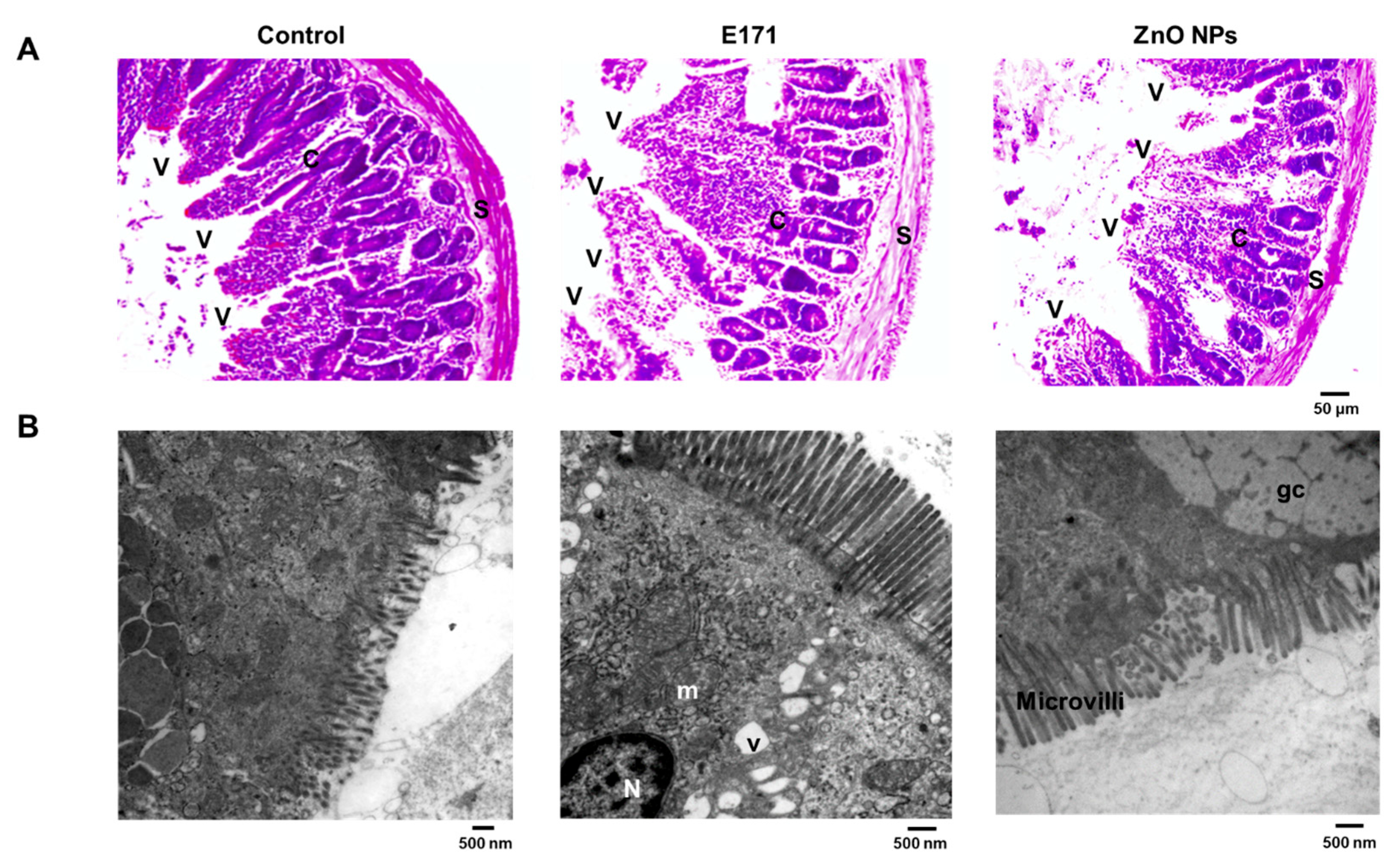
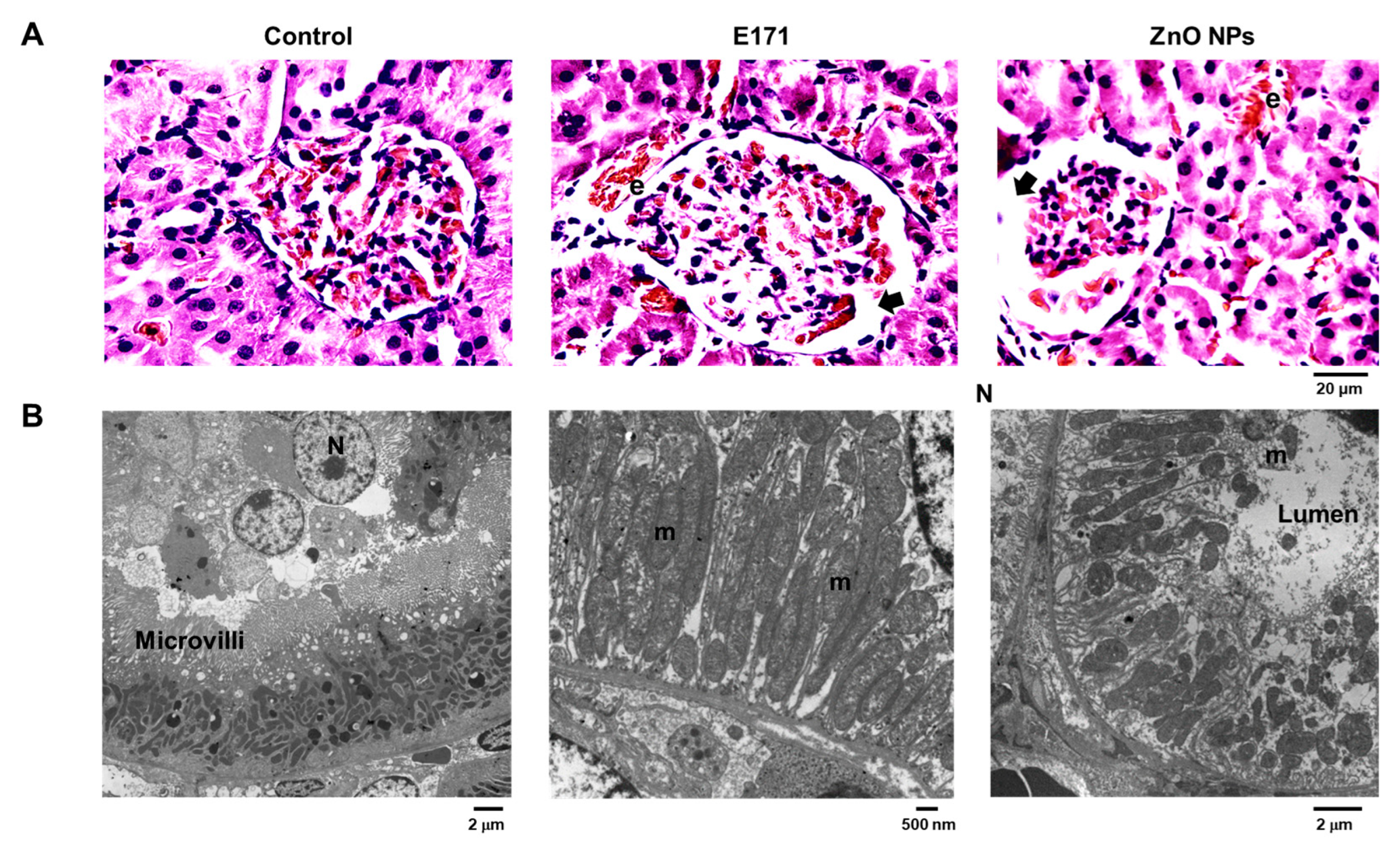
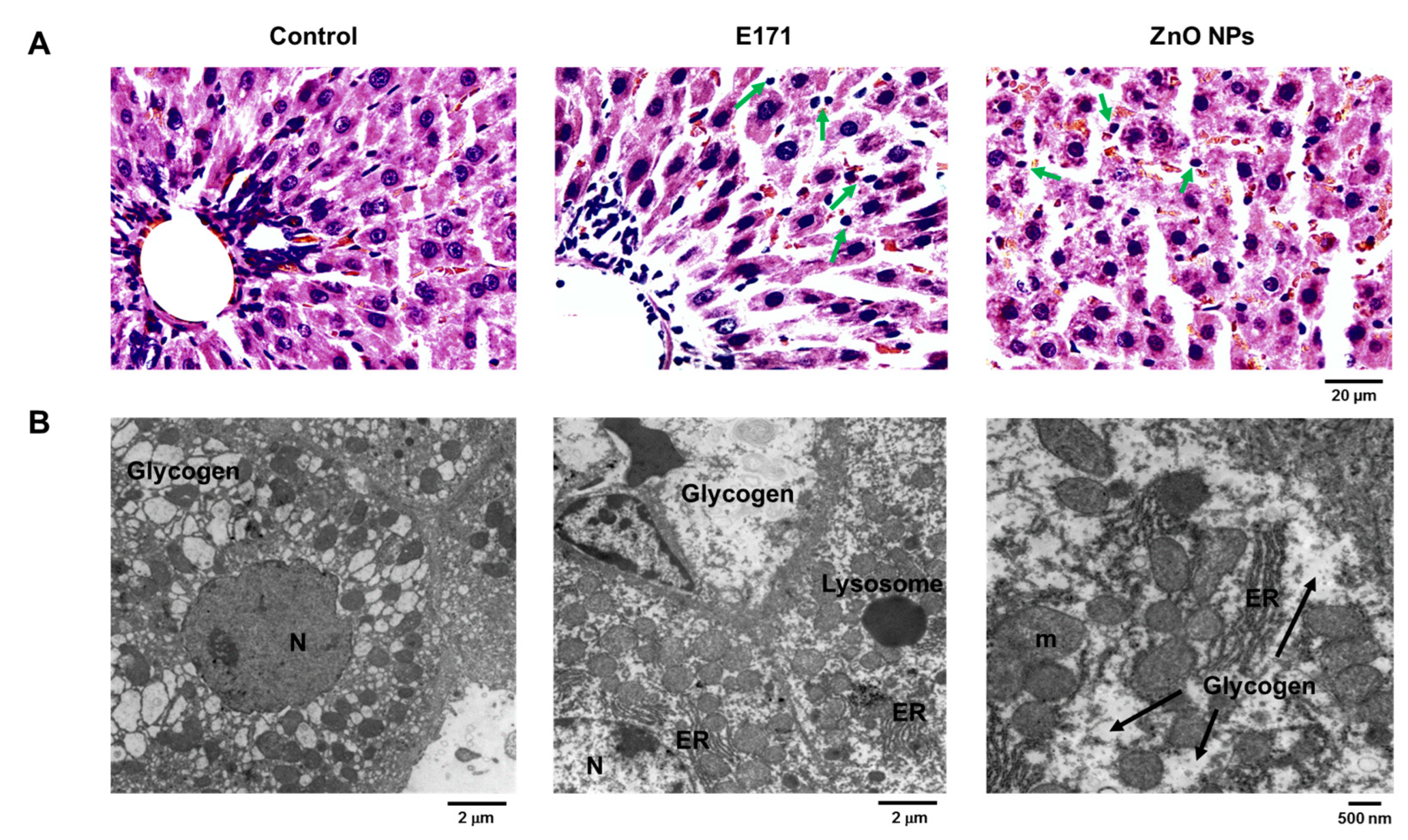
2.1. Organ Histology and Cell Morphology
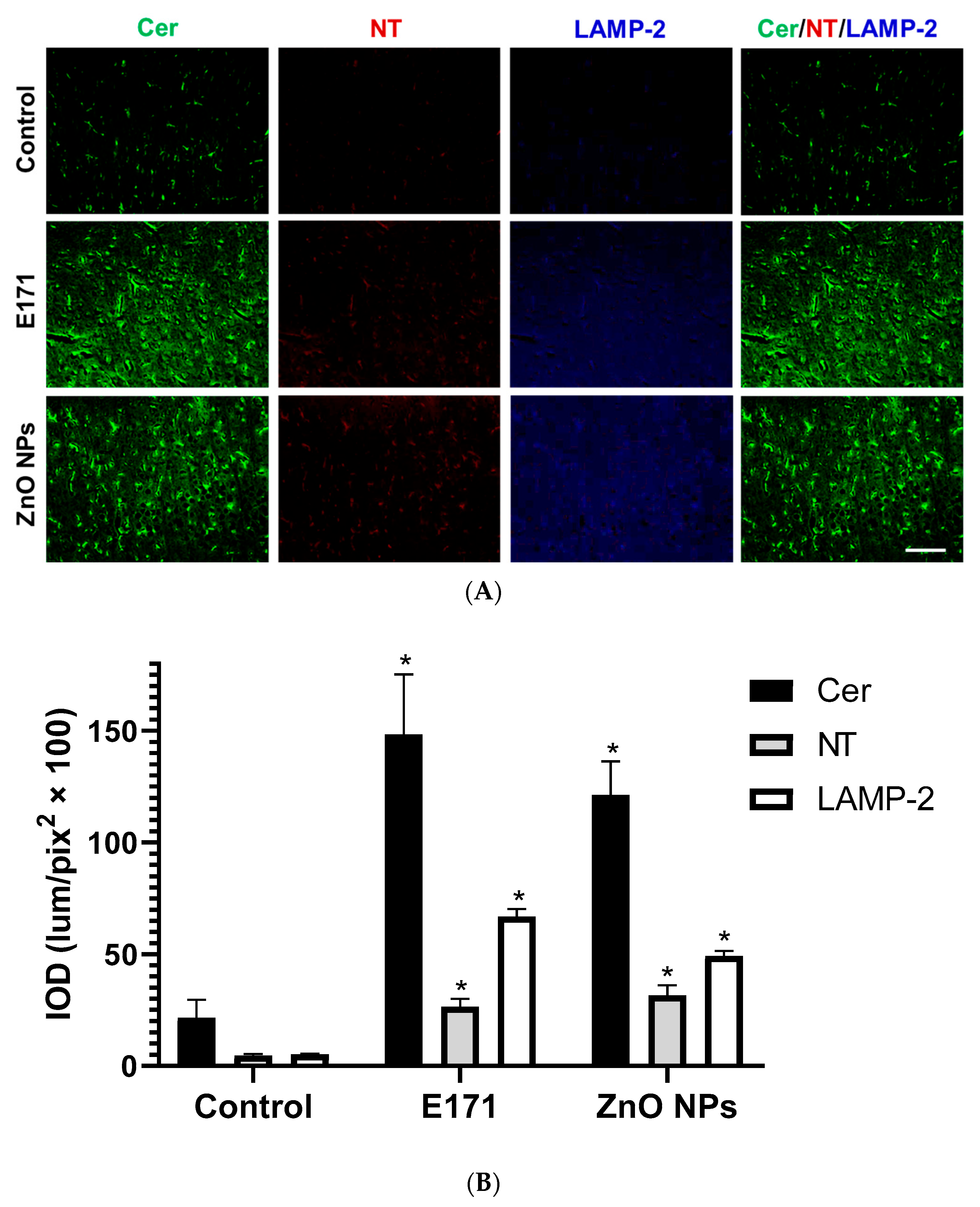
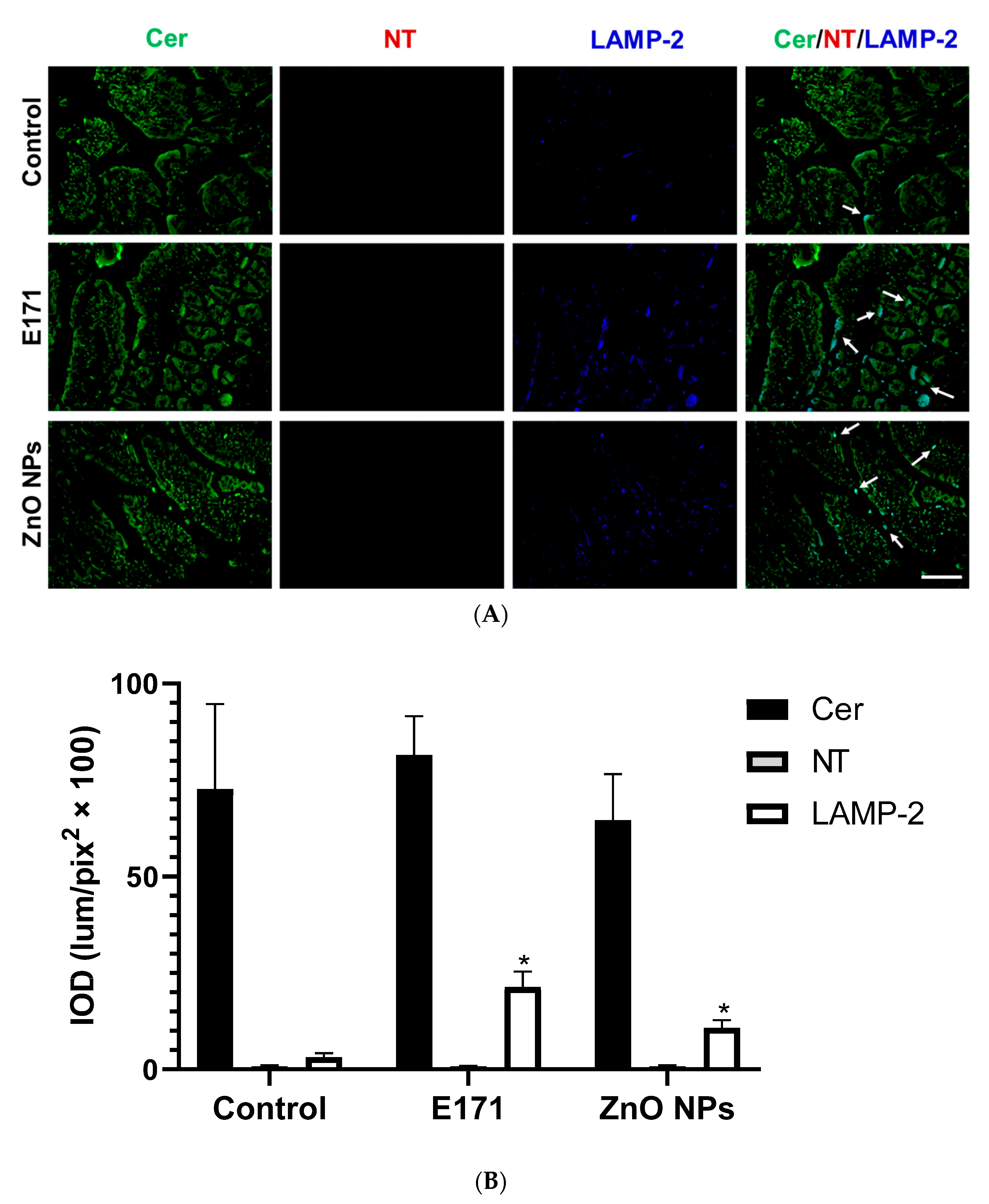
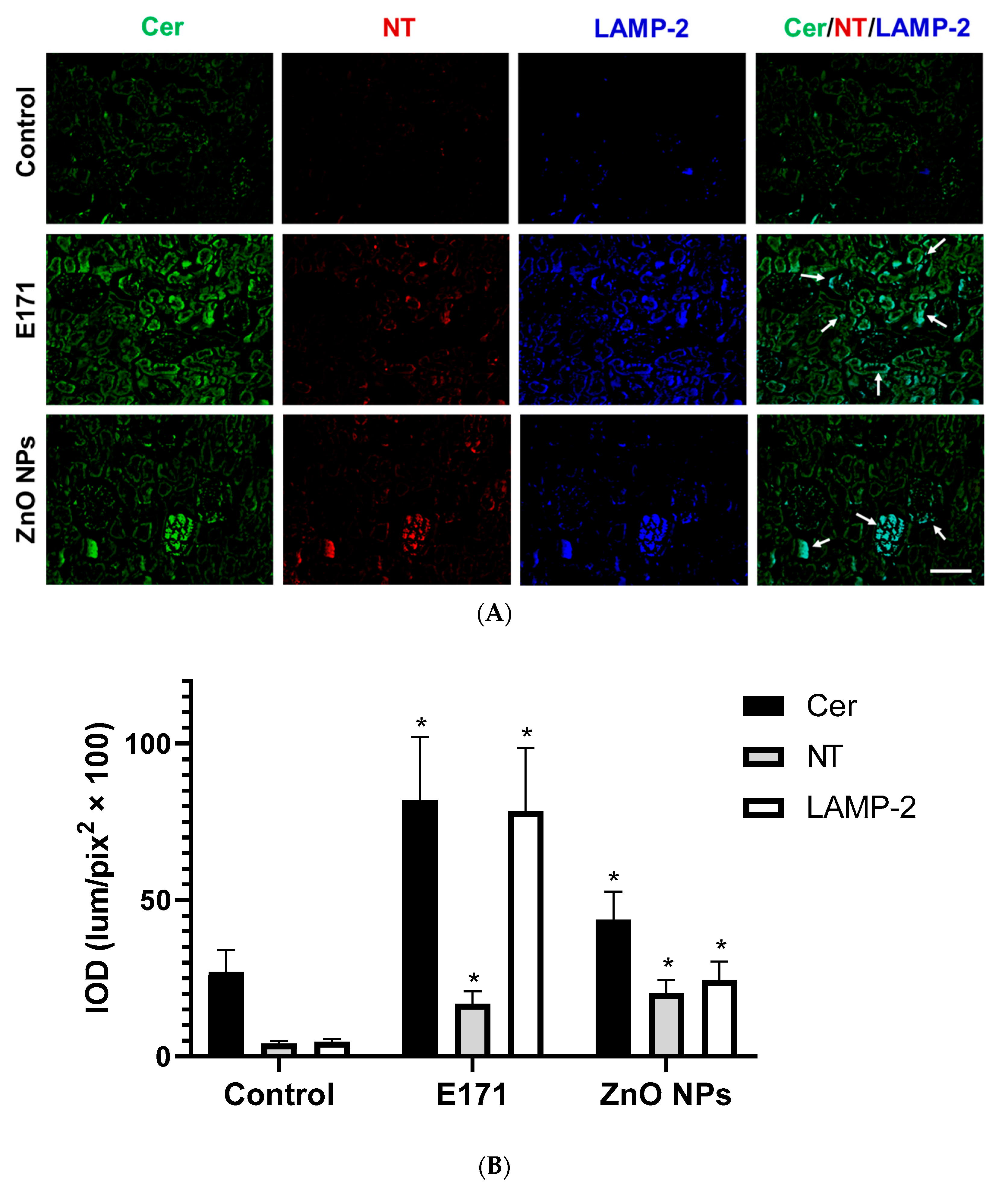
2.2. Ceramide, Nitrotyrosine, and LAMP-2 Expression
3. Discussion
4. Materials and Methods
4.1. E171 and ZnO NP Characterization
4.2. Rat Protocol
4.3. Histology
4.4. TEM Analysis
4.5. Immunofluorescence
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Dos Santos, C.A.; Ingle, A.P.; Rai, M. The emerging role of metallic nanoparticles in food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food. 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Jeong, H.J.; Kim, D.W.; Sohn, M.H.; Lim, S.T. The effect of fluorination of zinc oxide nanoparticles on evaluation of their biodistribution after oral administration. Nanotechnology 2012, 23, 205102. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Wu, D.; Saber, A.T.; Decan, N.; Jacobsen, N.R.; Williams, A.; Yauk, C.L.; Wallin, H.; Vogel, U.; Halappanavar, S. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology 2015, 9, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Jeong, U.; Yoon, C.; Kim, Y. Comparison of distribution and toxicity of different types of zinc-based nanoparticles. Environ. Toxicol. 2017, 32, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Gaté, L.; Disdier, C.; Cosnier, F.; Gagnaire, F.; Devoy, J.; Saba, W.; Brun, E.; Chalansonnet, M.; Mabondzo, A. Biopersistence and translocation to extrapulmonary organs of titanium dioxide nanoparticles after subacute inhalation exposure to aerosol in adult and elderly rats. Toxicol. Lett. 2017, 265, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Talamini, L.; Gimondi, S.; Violatto, M.B.; Fiordaliso, F.; Pedica, F.; Tran, N.L.; Sitia, G.; Aureli, F.; Raggi, A.; Nelissen, I.; et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology 2019, 13, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Yang, M.J.; Yoon, C.; Lee, G.H.; Kim, D.W.; Kim, T.W.; Kwak, M.; Heo, M.B.; Lee, T.G.; Kim, S.; et al. Toxicity of orally administered food-grade titanium dioxide nanoparticles. J. Appl. Toxicol. 2021, 41, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Skovsted, G.F.; Lykkesfeldt, J.; Dreier, R.; Berg, J.O.; Jeppesen, J.L.; Sheykhzade, M.; Loft, S.; Møller, P. Vasomotor dysfunction in human subcutaneous arteries exposed ex vivo to food-grade titanium dioxide. Food Chem. Toxicol. 2018, 120, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Q.; Long, J.; Ding, Y.; Zou, X.; Liao, G.; Cao, Y. A comparative study of toxicity of TiO2, ZnO, and Ag nanoparticles to human aortic smooth-muscle cells. Int. J. Nanomed. 2018, 13, 8037–8049. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Li, F.; Li, L.; Feng, Y.; Min, L.; Ma, D.; Yu, S.; Liu, J.; Zhang, H.; et al. Zinc Oxide Nanoparticle Caused Plasma Metabolomic Perturbations Correlate with Hepatic Steatosis. Front. Pharmacol. 2018, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Bian, Q.; Ning, J.; Yong, L.; Ou, T.; Song, Y.; Wei, S. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol. Lett. 2014, 226, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Goyary, D.; Karmakar, S.; Chattopadhyay, P. Exploration of cytotoxic and genotoxic end points following sub-chronic oral exposure to titanium dioxide nanoparticles. Toxicol. Ind. Health 2019, 35, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, Y.; Liu, S.; Lv, S.; Chen, L.; Wang, W.; Feng, Y.; Fu, F.; Xu, H. Zinc Oxide Particles Can Cause Ovarian Toxicity by Oxidative Stress in Female Mice Model. Int. J. Nanomed. 2022, 17, 4947–4960. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, H.; Zhou, M.; Duan, Y.; Li, N.; Gong, X.; Hu, R.; Hong, M.; Hong, F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. A 2011, 96, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: In vivo and in vitro study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, B.; Hong, W.; Chen, L.; Yao, L.; Zhao, Y.; Aguilar, Z.P.; Xu, H. ZnO Nanoparticles Induced Male Reproductive Toxicity Based on the Effects on the Endoplasmic Reticulum Stress Signaling Pathway. Int. J. Nanomed. 2019, 14, 9563–9576. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, J.; Li, B.; Zhang, J.; He, K.; Duan, X.; Huang, R.; Wu, Z.; Xiang, G. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated apoptotic cell death in liver cancer cells. J. Int. Med. Res. 2020, 48, 300060520903652. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, X.; Xu, L.; Liu, X.; Zhu, X.; Song, E.; Song, Y. Zinc oxide nanoparticles effectively regulate autophagic cell death by activating autophagosome formation and interfering with their maturation. Part. Fibre Toxicol. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Lee, T.G.; Reipa, V.; Heo, M.B. Titanium Dioxide Induces Apoptosis under UVA Irradiation via the Generation of Lysosomal Membrane Permeabilization-Dependent Reactive Oxygen Species in HaCat Cells. Nanomaterials 2021, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Eno, C.; Zhao, G.; Venkatanarayan, A.; Wang, B.; Flores, E.R.; Li, C. Noxa couples lysosomal membrane permeabilization and apoptosis during oxidative stress. Free Rad. Biol. Med. 2013, 65, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ziglari, T.; Wang, Z.; Holian, A. Contribution of Particle-Induced Lysosomal Membrane Hyperpolarization to Lysosomal Membrane Permeabilization. Inter. J. Mol. Sci. 2021, 22, 2277. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Alswady-Hoff, M.; Erdem, J.S.; Phuyal, S.; Knittelfelder, O.; Sharma, A.; Fonseca, D.M.; Skare, Ø.; Slupphaug, G.; Zienolddiny, S. Long-Term Exposure to Nanosized TiO2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5349. [Google Scholar] [CrossRef] [PubMed]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998, 356, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Shen, C.C.; Cheng, Y.W.; Huang, S.H.; Wu, C.C.; Kao, C.C.; Liao, J.W.; Kang, J.J. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology 2012, 6, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gong, Y.; Liao, W.; Luo, Y.; Wu, C.; Wang, M.; Yang, Q. A review of cardiovascular toxicity of TiO2, ZnO and Ag nanoparticles (NPs). Biometals 2018, 31, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, M.; Tan, Z.; Dai, M.; Xie, M.; Lin, J.; Hua, H.; Ma, Q.; Zhao, J.; Liu, A. Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ. Toxicol. Pharmacol. 2017, 49, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Zhang, Z.; Zheng, L.; Cui, Y.; Liu, X.; Li, N.; Sang, X.; Sun, Q.; Gao, G.; Cheng, Z.; et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 195, 365–370. [Google Scholar] [CrossRef]
- Albi, E.; Alessenko, A.; Grösch, S. Sphingolipids in Inflammation. Mediators Inflamm. 2018, 2018, 7464702. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Brown, R.P.; Shaw, M.; Vaidya, V.S.; Zhou, Y.; Espandiari, P.; Sadrieh, N.; Stratmeyer, M.; Keenan, J.; Kilty, C.G.; et al. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury, or chromium-treated rats: Relationship to renal distributions of iNOS and nitrotyrosine. Toxicol. Pathol. 2008, 36, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Albuck, A.L.; Sakamuri, S.S.V.P.; Sperling, J.A.; Evans, W.R.; Kolli, L.; Sure, V.N.; Mostany, R.; Katakam, P.V.G. Peroxynitrite decomposition catalyst enhances respiratory function in isolated brain mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H630–H641. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Tani, M.; Hannun, Y.A. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. J. Biol. Chem. 2007, 282, 10047–10056. [Google Scholar] [PubMed]
- Tirodkar, T.S.; Voelkel-Johnson, C. Sphingolipids in apoptosis. Exp. Oncol. 2012, 34, 231–242. [Google Scholar] [PubMed]
- Novgorodov, S.A.; Gudz, T.I. Ceramide and mitochondria in ischemic brain injury. Int. J. Biochem. Mol. Biol. 2011, 2, 347–361. [Google Scholar] [PubMed]
- Zager, R.A.; Conrad, S.; Lochhead, K.; Sweeney, E.A.; Igarashi, Y.; Burkhart, K.M. Altered sphingomyelinase and ceramide expression in the setting of ischemic and nephrotoxic acute renal failure. Kidney Int. 1998, 53, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kalhorn, T.; Zager, R.A. Renal cortical ceramide patterns during ischemic and toxic injury: Assessments by HPLC-mass spectrometry. Am. J. Physiol. 1999, 277, F723–F733. [Google Scholar] [CrossRef]
- de Wit, N.M.; den Hoedt, S.; Martinez-Martinez, P.; Rozemuller, A.J.; Mulder, M.T.; de Vries, H.E. Astrocytic ceramide as possible indicator of neuroinflammation. J. Neuroinflamm. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.J.; Holland, W.L.; Summers, S.A. Ceramides and Acute Kidney Injury. Semin. Nephrol. 2022, 42, 151281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Zou, A.P.; Li, P.L. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H605–H612. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P.; Faraci, F.M. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Zhang, L.; Xu, G.; Palmero, L.C.; Albini, P.T.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2013, 95, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Doppler, C.; Arnhard, K.; Dumfarth, J.; Heinz, K.; Messner, B.; Stern, C.; Koal, T.; Klavins, K.; Danzl, K.; Pitterl, F.; et al. Metabolomic profiling of ascending thoracic aortic aneurysms and dissections—Implications for pathophysiology and biomarker discovery. PLoS ONE 2017, 12, e0176727. [Google Scholar] [CrossRef] [PubMed]
- Porto, H.K.P.; Grando, M.D.; Ramalho, L.N.Z.; Valadares, M.C.; Bendhack, L.M.; Batista, A.C.; Rocha, M.L. Exposure to acetaminophen impairs vasodilation, increases oxidative stress and changes arterial morphology of rats. Arch. Toxicol. 2019, 93, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Theus, M.H.; Brickler, T.; Meza, A.L.; Coutermarsh-Ott, S.; Hazy, A.; Gris, D.; Allen, I.C. Loss of NLRX1 Exacerbates Neural Tissue Damage and NF-κB Signaling following Brain Injury. J. Immunol. 2017, 199, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Hashimoto, N.; Naritomi, H.; Nagata, I.; Nozaki, K.; Kondo, S.; Kurino, M.; Kikuchi, H. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 2000, 101, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Kleinloog, R.; Verweij, B.H.; van der Vlies, P.; Deelen, P.; Swertz, M.A.; de Muynck, L.; Van Damme, P.; Giuliani, F.; Regli, L.; van der Zwan, A.; et al. RNA Sequencing Analysis of Intracranial Aneurysm Walls Reveals Involvement of Lysosomes and Immunoglobulins in Rupture. Stroke 2016, 47, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.A.; Hou, Y.; Yi, D.; Qiu, Y.; Wu, G.; Kong, X.; Yin, Y. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim. Nutr. 2015, 1, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Li, L.; Luo, M.; He, X.; Zou, Z.; Gu, Z.; Su, L. Heat stress induces intestinal injury through lysosome- and mitochondria-dependent pathway in vivo and in vitro. Oncotarget 2017, 8, 40741–40755. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed]
- Soon, G.S.T.; Torbenson, M. The Liver and Glycogen: In Sickness and in Health. Int. J. Mol. Sci. 2023, 24, 6133. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Sayed, H.A. Hazards of nanotechnology: Effect of titanium dioxide nanoparticles on the liver and renal cortex of albino rats. An electron microscopic study. Egypt. J. Histol. 2013, 36, 389–399. [Google Scholar] [CrossRef]
- Hassanein, K.M.A.; El-Amir, Y.O. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. Med. 2018, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Beertsen, W.; Eskelinen, E.L. LAMP-2: A control step for phagosome and autophagosome maturation. Autophagy 2008, 4, 510–512. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, L.P.; Ye, J.Y.; Yang, L.; Huang, Y.; Jiang, X.P.; Zhang, Q.; Jia, J.Z.; Zhang, D.X.; Huang, Y. The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes. Front. Cell. Dev. Biol. 2020, 8, 31. [Google Scholar] [CrossRef]
- Song, X.B.; Liu, G.; Liu, F.; Yan, Z.G.; Wang, Z.Y.; Liu, Z.P.; Wang, L. Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis. 2017, 8, e2863. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.; Wang, B.; Xu, G.; Qin, Z.; Wang, J.; Wu, L.; Ju, X.; Bose, D.D.; Qiu, F.; et al. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis. 2017, 8, e2954. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Chen, C.; Xu, G.; Qin, X.; Hong, Y.; Bose, D.D.; Qiu, F.; Zou, Z. The size of zinc oxide nanoparticles controls its toxicity through impairing autophagic flux in A549 lung epithelial cells. Toxicol. Lett. 2018, 285, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Isaka, Y.; Takabatake, Y.; Tanaka, H.; Koike, M.; Shibata, M.; Uchiyama, Y.; Takahara, S.; Imai, E. Participation of autophagy in renal ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 368, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Snook, S.; Garrovillo, S.; Johnson, C.; La, D. An Immunohistochemical Investigation of Renal Phospholipidosis and Toxicity in Rats. Int. J. Toxicol. 2017, 36, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. Int. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Doudrick, K.; Bi, X.; Hristovski, K.; Herckes, P.; Westerhoff, P.; Westerhoff, P.; Kaegi, R. Characterization of food-grade titanium dioxide: The presence of nanosized particles. Environ. Sci. Technol. 2014, 48, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149, Erratum in: Mutagenesis 2018, 33, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Colin-Val, Z.; Vera-Márquez, C.D.; Herrera-Rodríguez, M.A.; Ramos-Godinez, M.P.; López-Saavedra, A.; Cano-Martínez, A.; Robledo-Cadena, D.X.; Rodríguez-Enríquez, S.; Correa, F.; Delgado-Buenrostro, N.L.; et al. Titanium Dioxide (E171) Induces Toxicity in H9c2 Rat Cardiomyoblasts and Ex Vivo Rat Hearts. Cardiovasc. Toxicol. 2022, 22, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Milla, C.; Macías Macías, F.I.; Velázquez Delgado, K.A.; Herrera Rodríguez, M.A.; Colín-Val, Z.; Ramos-Godinez, M.P.; Cano-Martínez, A.; Vega-Miranda, A.; Robledo-Cadena, D.X.; Delgado-Buenrostro, N.L.; et al. Zinc Oxide Nanoparticles Induce Toxicity in H9c2 Rat Cardiomyoblasts. Int. J. Mol. Sci. 2022, 23, 12940. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yiyuan, K.; Wei, Z.; Bin, S.; Limin, W.; Liangjiao, C.; Longquan, S. Ion-shedding zinc oxide nanoparticles induce microglial BV2 cell proliferation via the ERK and Akt signaling pathways. Toxicol. Sci. 2017, 156, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xin, H.; Yang, S.; Guo, M.; Malkoske, T.; Yin, D.; Xia, S. Environmental risks of ZnO nanoparticle exposure on Microcystis aeruginosa: Toxic effects and environmental feedback. Aquat. Toxicol. 2018, 204, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.; Nounou, H.; Shalaby, M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxics 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rodríguez, M.A.; Ramos-Godinez, M.P.; Cano-Martínez, A.; Segura, F.C.; Ruiz-Ramírez, A.; Pavón, N.; Lira-Silva, E.; Bautista-Pérez, R.; Thomas, R.S.; Delgado-Buenrostro, N.L.; et al. Food-grade titanium dioxide and zinc oxide nanoparticles induce toxicity and cardiac damage after oral exposure in rats. Part. Fibre Toxicol. 2023, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Fakra, S.C.; Xia, T.; Pokhrel, S.; Mädler, L.; Nel, A.E. The fate of ZnO nanoparticles administered to human bronchial epithelial cells. ACS Nano 2012, 6, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.; Chrisler, W.B.; Xie, Y.; Hu, D.; Szymanski, C.J.; Tolic, A.; Klein, J.A.; Smith, J.N.; Tarasevich, B.J.; Orr, G. Intracellular accumulation dynamics and fate of zinc ions in alveolar epithelial cells exposed to airborne ZnO nanoparticles at the air-liquid interface. Nanotoxicology 2015, 9, 9–22. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). EFSA Statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipicč, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; et al. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources. EFSA J. 2018, 16, e05294. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Pérez, R.; Del Valle-Mondragón, L.; Cano-Martínez, A.; Pérez-Méndez, O.; Escalante, B.; Franco, M. Involvement of neutral sphingomyelinase in the angiotensin II signaling pathway. Am. J. Physiol. Renal Physiol. 2015, 308, F1178–F1187. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Pérez, R.; Cano-Martínez, A.; Herrera-Rodríguez, M.A.; Ramos-Godinez, M.d.P.; Pérez Reyes, O.L.; Chirino, Y.I.; Rodríguez Serrano, Z.J.; López-Marure, R. Oral Exposure to Titanium Dioxide E171 and Zinc Oxide Nanoparticles Induces Multi-Organ Damage in Rats: Role of Ceramide. Int. J. Mol. Sci. 2024, 25, 5881. https://doi.org/10.3390/ijms25115881
Bautista-Pérez R, Cano-Martínez A, Herrera-Rodríguez MA, Ramos-Godinez MdP, Pérez Reyes OL, Chirino YI, Rodríguez Serrano ZJ, López-Marure R. Oral Exposure to Titanium Dioxide E171 and Zinc Oxide Nanoparticles Induces Multi-Organ Damage in Rats: Role of Ceramide. International Journal of Molecular Sciences. 2024; 25(11):5881. https://doi.org/10.3390/ijms25115881
Chicago/Turabian StyleBautista-Pérez, Rocío, Agustina Cano-Martínez, Manuel Alejandro Herrera-Rodríguez, María del Pilar Ramos-Godinez, Olga Lidia Pérez Reyes, Yolanda Irasema Chirino, Zariá José Rodríguez Serrano, and Rebeca López-Marure. 2024. "Oral Exposure to Titanium Dioxide E171 and Zinc Oxide Nanoparticles Induces Multi-Organ Damage in Rats: Role of Ceramide" International Journal of Molecular Sciences 25, no. 11: 5881. https://doi.org/10.3390/ijms25115881





