Genome-Wide Identification, Characterization, Evolutionary Analysis, and Expression Pattern of the GPAT Gene Family in Barley and Functional Analysis of HvGPAT18 under Abiotic Stress
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification and Analysis of HvGPAT Genes
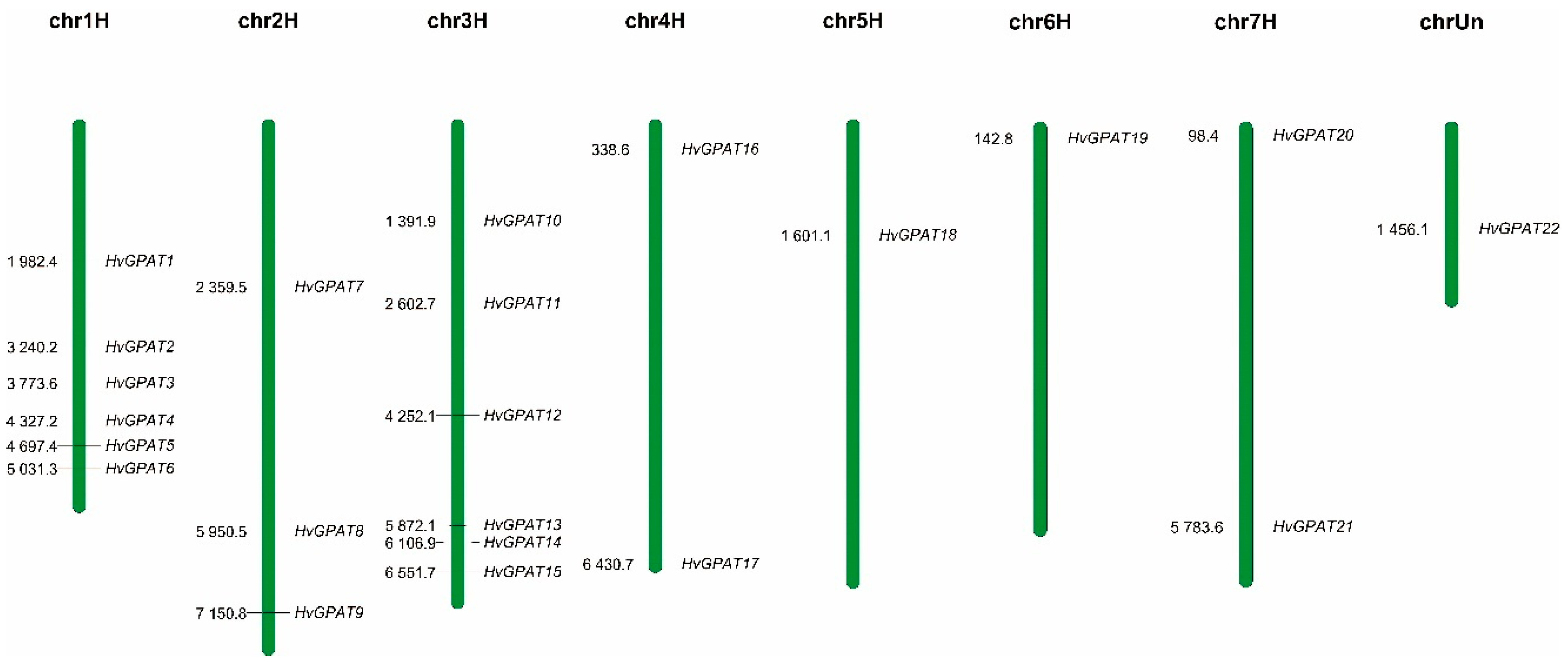
2.2. Chromosome Localization Analysis of HvGPAT Genes
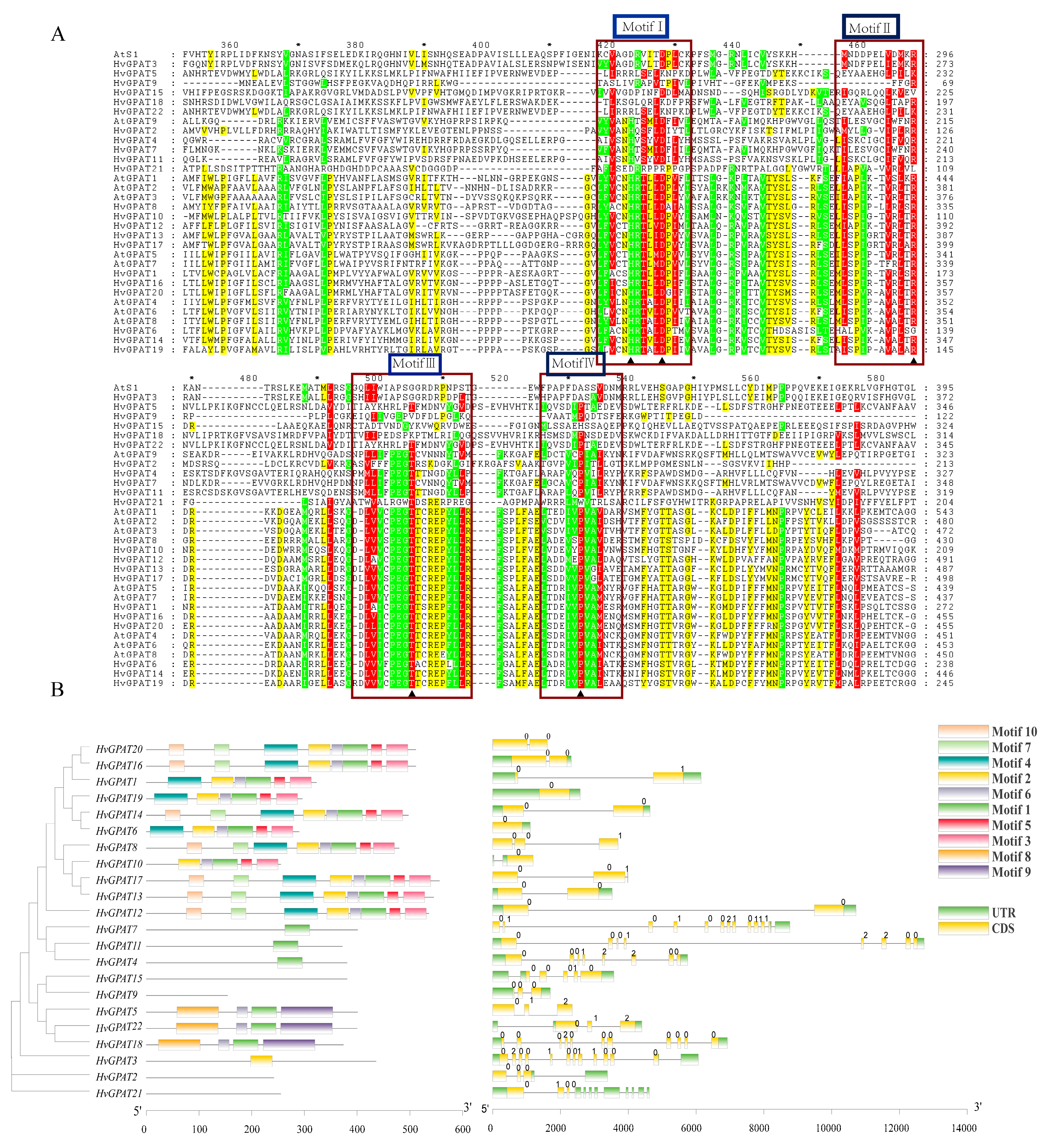
2.3. Phylogenetic and Evolutionary Analysis of the HvGPAT Gene Family
2.4. Intron–Exon Patterns and Conserved Motifs Analysis of HvGPAT Genes
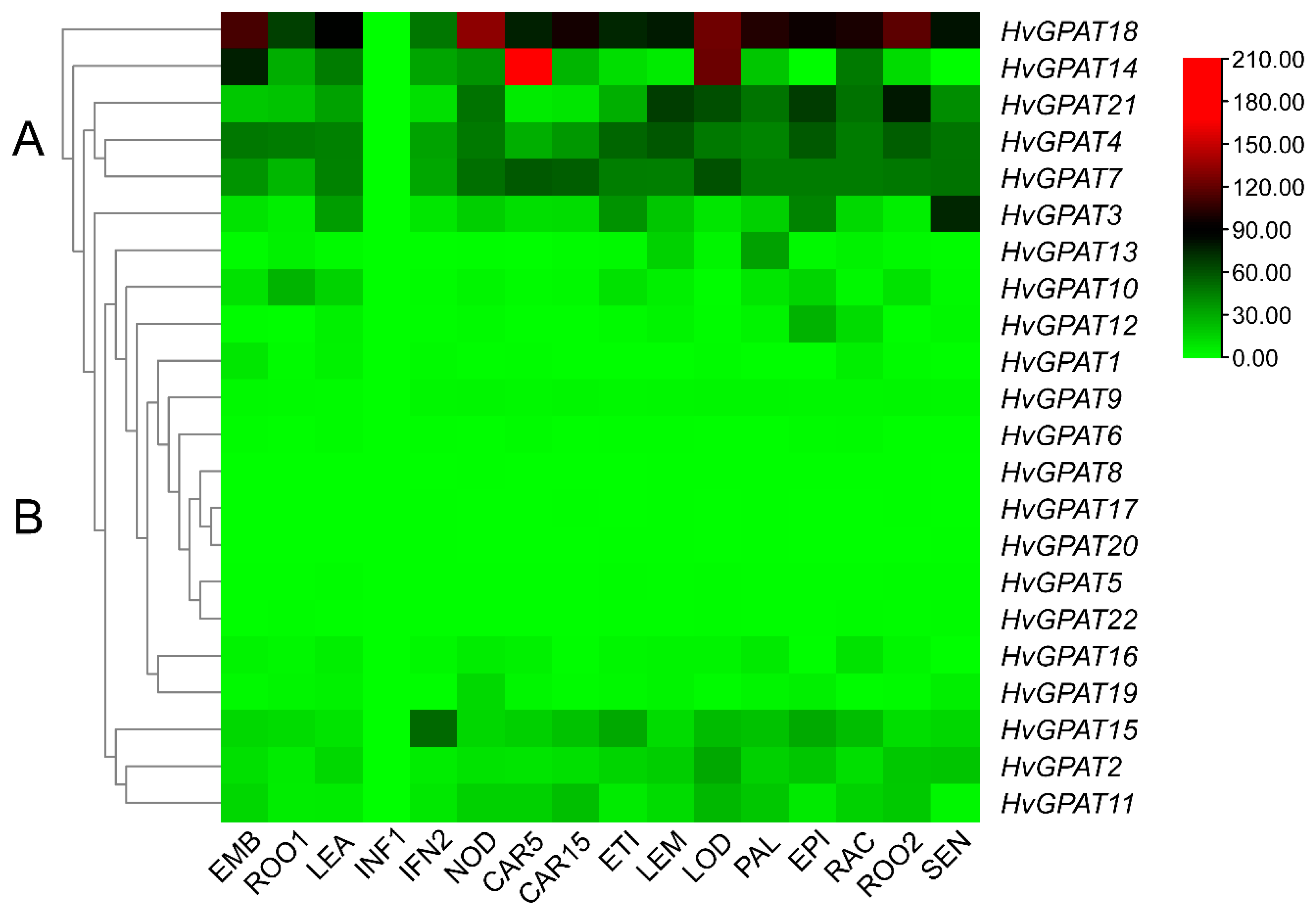
2.5. Expression Profiles of HvGPAT Genes in Different Tissues
2.6. The Promoter Analysis of HvGPAT Genes
2.7. Expression Profiles of HvGPAT Genes under Abiotic Stress
2.8. Overexpression of HvGPAT18 in Arabidopsis and Its Response to Abiotic Stress
3. Discussion
3.1. Classification of HvGPAT Genes
3.2. Relationship between HvGPAT Gene and Plant Stress Resistance
4. Materials and Methods
4.1. Identification and Chromosomal Localization of GPAT Gene Family Members in Barley Genome
4.2. Multiple Sequence Alignment, Phylogenetic, and Evolutionary Analysis of Barley GPAT Protein
4.3. Chromosomal Localization, Gene Structure, and Protein Conserved Motif Analysis
4.4. Protein Three-Dimensional Structure Construction
4.5. Cis-Acting Elements Analysis
4.6. Tissue Expression Profile Analysis
4.7. Plant Materials and Abiotic Stress Treatments for qPCR
4.8. Arabidopsis Transformation, Plant Growth, and Abiotic Stress Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Snyder, C.L.; Truksa, M.; Shah, S.; Weselake, R.J. sn-Glycerol-3-phosphate acyltransferases in plants. Plant Signal. Behav. 2011, 6, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Tasaka, Y. Glycerol-3-phosphate acyltransferase in plants. Biochim. Et Biophys. Acta 1997, 1348, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhane, K.N.; Singer, S.D.; Weselake, R.J.; Chen, G. Plant sn-Glycerol-3-Phosphate Acyltransferases: Biocatalysts Involved in the Biosynthesis of Intracellular and Extracellular Lipids. Lipids 2018, 53, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Beisson, F.; Koo, A.J.; Molina, I.; Pollard, M.; Ohlrogge, J. Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA 2007, 104, 18339–18344. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid Metabolism: Critical Roles in Male Fertility and Other Aspects of Reproductive Development in Plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Simpson, J.P.; Li-Beisson, Y.; Beisson, F.; Pollard, M.; Ohlrogge, J.B. A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: Substrate specificity, sn-2 preference, and evolution. Plant Physiol. 2012, 160, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Slabas, A.R.; Simon, W.R.; Schierer, T.; Kroon, J.; Fawcett, T.; Hayman, M.; Gilroy, J.; Nishida, I.; Murata, N.; Rafferty, J.; et al. Plant glycerol-3-phosphate-1-acyltransferase (GPAT): Structure selectivity studies. Biochem. Soc. Trans. 2000, 28, 677–679. [Google Scholar] [CrossRef]
- Waschburger, E.; Kulcheski, F.R.; Veto, N.M.; Margis, R.; Margis-Pinheiro, M.; Turchetto-Zolet, A.C. Genome-wide analysis of the Glycerol-3-Phosphate Acyltransferase (GPAT) gene family reveals the evolution and diversification of plant GPATs. Genet. Mol. Biol. 2018, 41, 355–370. [Google Scholar] [CrossRef]
- Wang, J.; Singh, S.K.; Geng, S.; Zhang, S.; Yuan, L. Genome-wide analysis of glycerol-3-phosphate O-acyltransferase gene family and functional characterization of two cutin group GPATs in Brassica napus. Planta 2020, 251, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, S.; Zhang, D.; Li, Z.; Xie, K.; An, X.; Ma, B.; Hou, Q.; Dong, Z.; Tian, Y.; et al. Genome-wide analysis of maize GPAT gene family and cytological characterization and breeding application of ZmMs33/ZmGPAT6 gene. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 2019, 132, 2137–2154. [Google Scholar] [CrossRef] [PubMed]
- Tank, D.C.; Sang, T. Phylogenetic utility of the glycerol-3-phosphate acyltransferase gene: Evolution and implications in Paeonia (Paeoniaceae). Mol. Phylogenetics Evol. 2001, 19, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ma, J.; Liu, G.; Wang, N.; Pei, W.; Wu, M.; Li, X.; Zhang, J.; Yu, J. Genome-Wide Identification, Sequence Variation, and Expression of the Glycerol-3-Phosphate Acyltransferase (GPAT) Gene Family in Gossypium. Front. Genet. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Hu, T.; Chen, S.; Wang, Y.; Shi, X.; Yin, M.; Li, R.; Wang, J.; Jia, X. Genome-Wide Analysis of Glycerol-3-Phosphate Acyltransferase (GPAT) Family in Perilla frutescens and Functional Characterization of PfGPAT9 Crucial for Biosynthesis of Storage Oils Rich in High-Value Lipids. Int. J. Mol. Sci. 2023, 24, 15106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xia, Q.; Dauk, M.; Shen, W.; Selvaraj, G.; Zou, J. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 2003, 15, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Zhu, J.; Yang, J.; Zhang, G.R.; Xing, W.F.; Zhang, S.; Yang, Z.N. Glycerol-3-phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol. Plant 2012, 5, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Truksa, M.; Snyder, C.L.; Shah, S.; Weselake, R.J. Glycerol-3-phosphate acyltransferase 4 is essential for the normal development of reproductive organs and the embryo in Brassica napus. J. Exp. Bot. 2014, 65, 4201–4215. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Shi, J.; Liang, W.; Zhang, Q.; Lian, G.; Quan, S.; Zhu, L.; Luo, Z.; Chen, M.; Zhang, D. Glycerol-3-Phosphate Acyltransferase 3 (OsGPAT3) is required for anther development and male fertility in rice. J. Exp. Bot. 2017, 68, 513–526. [Google Scholar] [CrossRef]
- Sun, L.; Xiang, X.; Yang, Z.; Yu, P.; Wen, X.; Wang, H.; Abbas, A.; Muhammad Khan, R.; Zhang, Y.; Cheng, S.; et al. OsGPAT3 Plays a Critical Role in Anther Wall Programmed Cell Death and Pollen Development in Rice. Int. J. Mol. Sci. 2018, 19, 4017. [Google Scholar] [CrossRef]
- Xie, K.; Wu, S.; Li, Z.; Zhou, Y.; Zhang, D.; Dong, Z.; An, X.; Zhu, T.; Zhang, S.; Liu, S.; et al. Map-based cloning and characterization of Zea mays male sterility33 (ZmMs33) gene, encoding a glycerol-3-phosphate acyltransferase. TAG. Theor. Appl. Genet. Theor. Und Angew. Genet. 2018, 131, 1363–1378. [Google Scholar] [CrossRef] [PubMed]
- Beisson, F.; Li, Y.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Paya-Milans, M.; Aznar-Moreno, J.A.; Balbuena, T.S.; Haslam, R.P.; Gidda, S.K.; Perez-Hormaeche, J.; Mullen, R.T.; Thelen, J.J.; Napier, J.A.; Salas, J.J.; et al. Sunflower HaGPAT9-1 is the predominant GPAT during seed development. Plant Sci. Int. J. Exp. Plant Biol. 2016, 252, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Khan, K.; Niranjan, A.; Kumar, V.; Sane, V.A. Heterologous expression of two GPATs from Jatropha curcas alters seed oil levels in transgenic Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2017, 263, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.U.; Kim, J.; Kim, H.; Suh, M.C. Functional Characterization of Physcomitrella patens Glycerol-3-Phosphate Acyltransferase 9 and an Increase in Seed Oil Content in Arabidopsis by Its Ectopic Expression. Plants 2019, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, X.; Luo, L.; Yang, H.; Li, P.; Zhang, K.; Liu, F.; Wan, Y. Characterization of glycerol-3-phosphate acyltransferase 9 (AhGPAT9) genes, their allelic polymorphism and association with oil content in peanut (Arachis hypogaea L.). Sci. Rep. 2020, 10, 14648. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Shen, Y.; Zhang, Z.; Jia, Q.; Xu, M.; Zhang, T.; Fang, H.; Yu, X.; Li, L.; Liu, D.; et al. A GPAT1 Mutation in Arabidopsis Enhances Plant Height but Impairs Seed Oil Biosynthesis. Int. J. Mol. Sci. 2021, 22, 785. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, L.; Zhu, J.; Zhang, B.; Gan, Y.; Zheng, Y. Identification of GmGPATs and their effect on glycerolipid biosynthesis through seed-specific expression in soybean. Mol. Biol. Rep. 2022, 49, 9585–9592. [Google Scholar] [CrossRef]
- Gong, W.; Chen, W.; Gao, Q.; Qian, L.; Yuan, X.; Tang, S.; Hong, Y. Glycerol-3-Phosphate Acyltransferase GPAT9 Enhanced Seed Oil Accumulation and Eukaryotic Galactolipid Synthesis in Brassica napus. Int. J. Mol. Sci. 2023, 24, 16111. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Zhang, B.; Li, Q.; Liu, C.; Huang, Q.; Cui, P. The functional divergence of homologous GPAT9 genes contributes to the erucic acid content of Brassica napus seeds. BMC Plant Biol. 2024, 24, 69. [Google Scholar] [CrossRef]
- Moon, B.Y.; Higashi, S.; Gombos, Z.; Murata, N. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. USA 1995, 92, 6219–6223. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, T.; Kishitani, S.; Inatsugi, R.; Nishida, I.; Murata, N.; Toriyama, K. An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol. 2002, 43, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Li, M.; Zhao, S.J.; Li, F.; Liang, H.; Meng, Q.W. Overexpression of glycerol-3-phosphate acyltransferase gene improves chilling tolerance in tomato. Planta 2007, 226, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, N.; Qu, Y.Y.; Dong, X.C.; Meng, Q.W.; Zhao, S.J. Overexpression of sweet pepper glycerol-3-phosphate acyltransferase gene enhanced thermotolerance of photosynthetic apparatus in transgenic tobacco. J. Integr. Plant Biol. 2008, 50, 613–621. [Google Scholar] [CrossRef]
- Sun, X.L.; Yang, S.; Wang, L.Y.; Zhang, Q.Y.; Zhao, S.J.; Meng, Q.W. The unsaturation of phosphatidylglycerol in thylakoid membrane alleviates PSII photoinhibition under chilling stress. Plant Cell Rep. 2011, 30, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.K.; Yang, N.N.; Chen, L.J.; Irfan, M.; Zhao, X.H.; Li, T.L. Characterization of LpGPAT gene in Lilium pensylvanicum and response to cold stress. BioMed Res. Int. 2015, 2015, 792819. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Tian, S.; Wang, W.; Wang, M.; Fan, H. Overexpression of Glycerol-3-Phosphate Acyltransferase from Suaeda salsa Improves Salt Tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, P.; Yang, P.; Fan, C.; Sun, X. Characterization of the glycerol-3-phosphate acyltransferase gene and its real-time expression under cold stress in Paeonia lactiflora Pall. PLoS ONE 2018, 13, e0202168. [Google Scholar] [CrossRef] [PubMed]
- Fawke, S.; Torode, T.A.; Gogleva, A.; Fich, E.A.; Sorensen, I.; Yunusov, T.; Rose, J.K.C.; Schornack, S. Glycerol-3-phosphate acyltransferase 6 controls filamentous pathogen interactions and cell wall properties of the tomato and Nicotiana benthamiana leaf epidermis. New Phytol. 2019, 223, 1547–1559. [Google Scholar] [CrossRef]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W.; Wang, M. Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. PPB 2019, 143, 375–387. [Google Scholar] [CrossRef]
- Hsu, Y.F.; Yan, J.; Song, Y.; Zheng, M. Sarracenia purpurea glycerol-3-phosphate acyltransferase 5 confers plant tolerance to high humidity in Arabidopsis thaliana. Physiol. Plant. 2021, 173, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, Z.; Zhang, F.; Zheng, H.; Li, S.; Gao, Y.; Yang, J.; Sui, N. Identification and Transcriptome Analysis of Genes Related to Membrane Lipid Regulation in Sweet Sorghum under Salt Stress. Int. J. Mol. Sci. 2022, 23, 5465. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, G.; Ke, X.; Zheng, Z.; Zheng, Y. Loss-of-Function of ATS1 Enhances Arabidopsis Salt Tolerance. Plants 2023, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Beisson, F.; Ohlrogge, J.; Pollard, M. Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiol. 2007, 144, 1267–1277. [Google Scholar] [CrossRef]
- Manas-Fernandez, A.; Li-Beisson, Y.; Alonso, D.L.; Garcia-Maroto, F. Cloning and molecular characterization of a glycerol-3-phosphate O-acyltransferase (GPAT) gene from Echium (Boraginaceae) involved in the biosynthesis of cutin polyesters. Planta 2010, 232, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Mauxion, J.P.; Tai, F.W.; Martin, L.B.; Fich, E.A.; Joubes, J.; Rose, J.K.; Domergue, F.; Rothan, C. The Glycerol-3-Phosphate Acyltransferase GPAT6 from Tomato Plays a Central Role in Fruit Cutin Biosynthesis. Plant Physiol. 2016, 171, 894–913. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.; Zhao, Y.; Chen, X.; Huang, Y.; Yan, S.; Li, S.; Liu, M.; Huang, W.; Zhang, X.; et al. Maize male sterile 33 encodes a putative glycerol-3-phosphate acyltransferase that mediates anther cuticle formation and microspore development. BMC Plant Biol. 2018, 18, 318. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Yang, S.U.; Pandey, G.; Kim, M.S.; Hyoung, S.; Choi, D.; Shin, J.S.; Suh, M.C. Occurrence of land-plant-specific glycerol-3-phosphate acyltransferases is essential for cuticle formation and gametophore development in Physcomitrella patens. New Phytol. 2020, 225, 2468–2483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, H.; Hao, P.; Wu, A.; Ma, Q.; Zhang, J.; Wang, H.; Fu, X.; Ma, L.; Lu, J.; et al. GhGPAT12/25 Are Essential for the Formation of Anther Cuticle and Pollen Exine in Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2021, 12, 667739. [Google Scholar] [CrossRef]
- Gidda, S.K.; Shockey, J.M.; Rothstein, S.J.; Dyer, J.M.; Mullen, R.T. Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: Functional divergence of the dilysine ER retrieval motif in plant cells. Plant Physiol. Biochem. 2009, 47, 867–879. [Google Scholar] [CrossRef]
- Shockey, J.; Regmi, A.; Cotton, K.; Adhikari, N.; Browse, J.; Bates, P.D. Identification of Arabidopsis GPAT9 (At5g60620) as an Essential Gene Involved in Triacylglycerol Biosynthesis. Plant Physiol. 2016, 170, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.D.; Chen, G.; Mietkiewska, E.; Tomasi, P.; Jayawardhane, K.; Dyer, J.M.; Weselake, R.J. Arabidopsis GPAT9 contributes to synthesis of intracellular glycerolipids but not surface lipids. J. Exp. Bot. 2016, 67, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S.; Zhu, T.; An, X.; Wei, X.; Zhang, J.; Wu, S.; Dong, Z.; Long, Y.; Wan, X. The Loss-Function of the Male Sterile Gene ZmMs33/ZmGPAT6 Results in Severely Oxidative Stress and Metabolic Disorder in Maize Anthers. Cells 2022, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2014, 53, 18–81. [Google Scholar] [CrossRef] [PubMed]
- Dormann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Paya-Milans, M.; Venegas-Caleron, M.; Salas, J.J.; Garces, R.; Martinez-Force, E. Cloning, heterologous expression and biochemical characterization of plastidial sn-glycerol-3-phosphate acyltransferase from Helianthus annuus. Phytochemistry 2015, 111, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Kaneko, M.; Akter, K.; Murai, S.; Komatsu, K.; Ishizaki, K.; Yamato, K.T.; Kohchi, T.; Takezawa, D. Abscisic acid-induced gene expression in the liverwort Marchantia polymorpha is mediated by evolutionarily conserved promoter elements. Physiol. Plant. 2016, 156, 407–420. [Google Scholar] [CrossRef]
- Guo, L.; Yang, H.; Zhang, X.; Yang, S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 2013, 64, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Qing, D.J.; Lu, H.F.; Li, N.; Dong, H.T.; Dong, D.F.; Li, Y.Z. Comparative profiles of gene expression in leaves and roots of maize seedlings under conditions of salt stress and the removal of salt stress. Plant Cell Physiol. 2009, 50, 889–903. [Google Scholar] [CrossRef]
- Campo, S.; Baldrich, P.; Messeguer, J.; Lalanne, E.; Coca, M.; San Segundo, B. Overexpression of a Calcium-Dependent Protein Kinase Confers Salt and Drought Tolerance in Rice by Preventing Membrane Lipid Peroxidation. Plant Physiol. 2014, 165, 688–704. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]




| Accession Number | Gene | Chro | Length | Molecular | Isoelectric | Int Loc b | Transmembrane Region | ||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Dist a | (Amino Acid) | Weight (Da) | Point | Instability Index | Grand Average of Hydropathicity (GRAVY) | |||
| HORVU1Hr1G050900.1 | HvGPAT1 | 1H | 436 | 48,648.90 | 7.31 | ER | 71-93-bp | 44.14 | 0.178 |
| HORVU1Hr1G059350.1 | HvGPAT2 | 1H | 381 | 41,946.90 | 9.23 | Chl | 13-35bp,106-128bp | 42.61 | 0.295 |
| HORVU1Hr1G044570.1 | HvGPAT3 | 1H | 242 | 26,958.60 | 10.2 | Chl | no | 50.46 | −0.337 |
| HORVU1Hr1G066030.1 | HvGPAT4 | 1H | 401 | 45,975.50 | 8.53 | Chl, ER | no | 47.11 | −0.086 |
| HORVU1Hr1G073340.1 | HvGPAT5 | 1H | 290 | 31,761.60 | 9.02 | ER | 53-75,90-107,346-365,369-391 | 45.78 | 0.040 |
| HORVU1Hr1G032150.1 | HvGPAT6 | 1H | 323 | 35,143.70 | 9.82 | ER | 37-59 | 41.33 | −0.007 |
| HORVU2Hr1G081970.1 | HvGPAT7 | 2H | 480 | 52,043.10 | 10.09 | ER, Chl | 105-127,132-154 | 53.45 | −0.213 |
| HORVU2Hr1G045130.1 | HvGPAT8 | 2H | 401 | 46,548.80 | 9.97 | ER, Mit | 91-113,235-257 | 36.82 | 0.210 |
| HORVU2Hr1G108810.1 | HvGPAT9 | 2H | 154 | 17,324 | 9.19 | Mit | no | 52.65 | −0.312 |
| HORVU3Hr1G080190.1 | HvGPAT10 | 3H | 545 | 59,545.20 | 9.59 | Mit | no | 42.25 | −0.115 |
| HORVU3Hr1G056830.1 | HvGPAT11 | 3H | 536 | 59,231.50 | 9.3 | ER, Chl | 74-96,131-148 | 52.42 | −0.067 |
| HORVU3Hr1G097070.2 | HvGPAT12 | 3H | 381 | 42,426.90 | 6.61 | ER | 5-24,285-307,312-334 | 40.49 | 0.169 |
| HORVU3Hr1G029770.10 | HvGPAT13 | 3H | 255 | 28,932.30 | 9.27 | ER | 96-118 | 41.06 | 0.099 |
| HORVU3Hr1G084990.1 | HvGPAT14 | 3H | 497 | 55,373.30 | 9.47 | Mit | 71-93,247-269 | 44.71 | 0.082 |
| HORVU3Hr1G041560.1 | HvGPAT15 | 3H | 372 | 40,775.40 | 7.2 | Chl, ER | no | 49.47 | −0.433 |
| HORVU4Hr1G011110.1 | HvGPAT16 | 4H | 511 | 55,592.50 | 9.42 | Mit | 62-84,255-277 | 36.62 | 0.171 |
| HORVU4Hr1G089610.1 | HvGPAT17 | 4H | 556 | 61,130.50 | 9.43 | ER, Mit | no | 38.34 | 0.041 |
| HORVU5Hr1G027810.1 | HvGPAT18 | 5H | 374 | 42,490.80 | 10.3 | ER | 15-37,311-330,340-362 | 43.37 | 0.286 |
| HORVU6Hr1G006810.1 | HvGPAT19 | 6H | 296 | 31,952.80 | 9.94 | ER, Mit | 45-67 | 40.47 | 0.092 |
| HORVU7Hr1G095010.1 | HvGPAT20 | 7H | 255 | 28,011.70 | 10.07 | Mit | 61-83,254-276 | 34.45 | 0.218 |
| HORVU7Hr1G007570.1 | HvGPAT21 | 7H | 511 | 56,464.90 | 9.04 | ER, Mit | 108-130 | 43.39 | −0.162 |
| HORVU0Hr1G027290.1 | HvGPAT22 | Un | 400 | 45,872.30 | 8.6 | ER | 52-74,89-106,345-364,368-390 | 45.06 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Ma, J.; Qi, C.; Ma, Y.; Xiong, H.; Duan, R. Genome-Wide Identification, Characterization, Evolutionary Analysis, and Expression Pattern of the GPAT Gene Family in Barley and Functional Analysis of HvGPAT18 under Abiotic Stress. Int. J. Mol. Sci. 2024, 25, 6101. https://doi.org/10.3390/ijms25116101
Yang C, Ma J, Qi C, Ma Y, Xiong H, Duan R. Genome-Wide Identification, Characterization, Evolutionary Analysis, and Expression Pattern of the GPAT Gene Family in Barley and Functional Analysis of HvGPAT18 under Abiotic Stress. International Journal of Molecular Sciences. 2024; 25(11):6101. https://doi.org/10.3390/ijms25116101
Chicago/Turabian StyleYang, Chenglan, Jianzhi Ma, Cunying Qi, Yinhua Ma, Huiyan Xiong, and Ruijun Duan. 2024. "Genome-Wide Identification, Characterization, Evolutionary Analysis, and Expression Pattern of the GPAT Gene Family in Barley and Functional Analysis of HvGPAT18 under Abiotic Stress" International Journal of Molecular Sciences 25, no. 11: 6101. https://doi.org/10.3390/ijms25116101




