Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level
Abstract
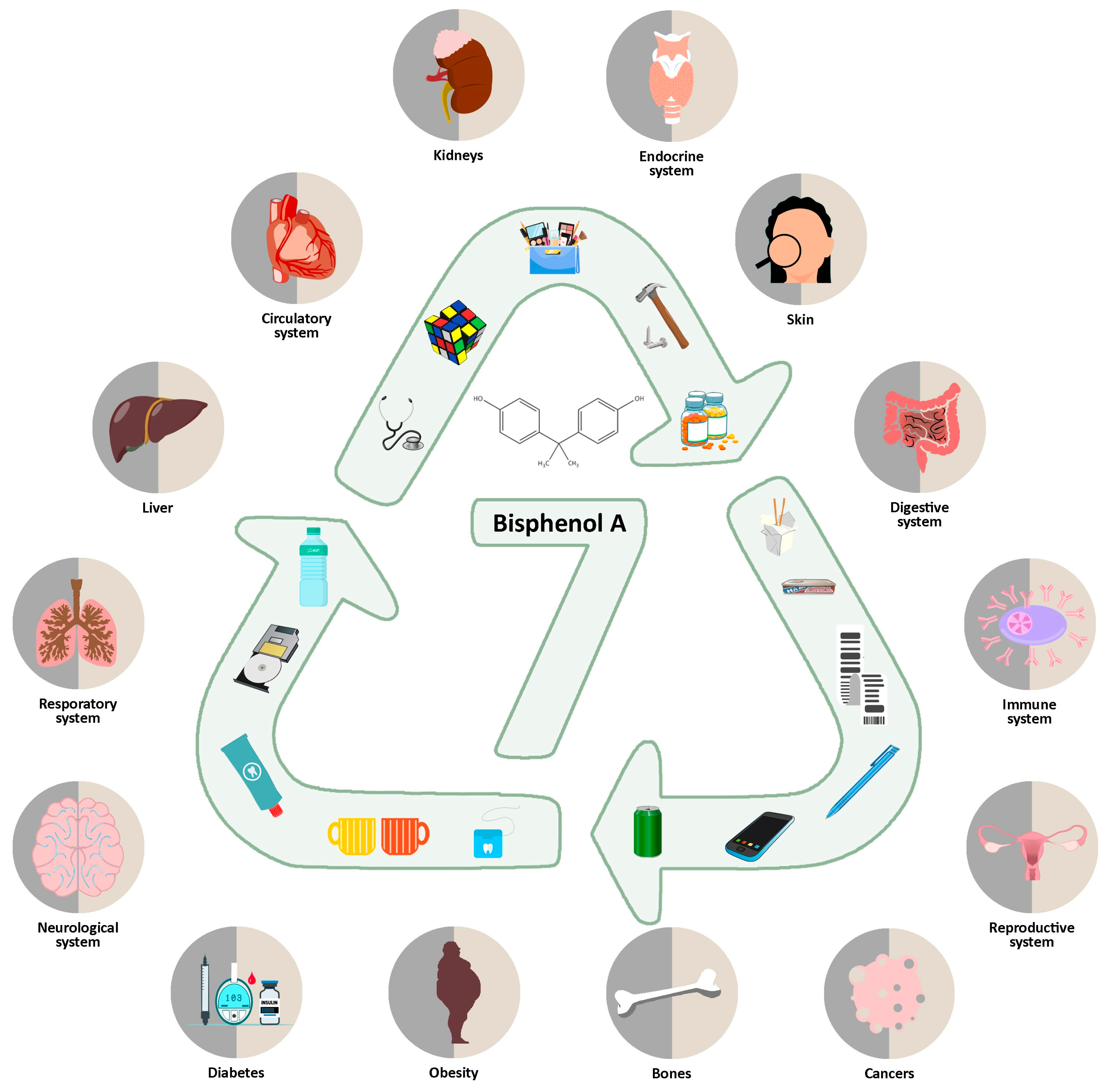
:1. Introduction
2. Health Effects
2.1. The Digestive System
2.2. Respiratory System
2.3. Reproductive System
2.4. Development and Its Potential Disorders
2.5. The Immune System
2.6. Neuroendocrine System
2.7. Cardiovascular System
2.8. Metabolic and Other Diseases
2.9. Skeletal System
2.10. Skin
2.11. Cancer
3. Sources in Food/Packaging
4. Environment
5. Guidance from Various International Organizations
6. Prevention
- -
- Choose glass, porcelain or steel packaging instead of plastic, especially for hot foods;
- -
- Try to choose only BPA-free containers;
- -
- Instead of canned products, use dried, such as seeds;
- -
- Do not choose plastic containers with recycling codes at the bottom of three or seven, they may be made of BPA;
- -
- Reduce your use of canned foods and beverages;
- -
- If you can, use BPA-free baby bottles;
- -
- Use wooden or other safe BPA free toys;
- -
- Avoid heating BPA products in the microwave or dishwasher, as it will seep into food;
- -
- Read the labels and composition of each product;
- -
- Choose natural, organic products made from safe and non-toxic ingredients;
- -
- Do not accept paper receipts, bills or tickets;
- -
- If you work in a store wash your hands at every possible opportunity;
- -
- Check your products online to see if they contain BPA and can be avoided, including medical products (ask your doctor or other medical professional);
- -
- Parents can ask schools to give their children educational lessons to get them in the habit of making good choices from an early age;
- -
- Change your exercise clothes, as there is a chance they may contain polycarbonate and when exercising in warm weather, BPA will be absorbed faster through the skin.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sampson, J.; de Korte, D. DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 2011, 21, 73–83. [Google Scholar] [CrossRef]
- Ni, M.; Li, X.; Zhang, L.; Kumar, V.; Chen, J. Bibliometric Analysis of the Toxicity of Bisphenol A. Int. J. Environ. Res. Public Health 2022, 19, 7886. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Frankowski, R.; Zgoła-Grześkowiak, A.; Smułek, W.; Grześkowiak, T. Removal of Bisphenol A and Its Potential Substitutes by Biodegradation. Appl. Biochem. Biotechnol. 2020, 191, 1100–1110. [Google Scholar] [CrossRef]
- Allard, P.; Colaiácovo, M.P. Mechanistic insights into the action of Bisphenol A on the germline using C. elegans. Cell Cycle 2011, 10, 183–184. [Google Scholar] [CrossRef]
- Panou, A.; Karabagias, I.K. Migration and Safety Aspects of Plastic Food Packaging Materials: Need for Reconsideration? Coatings 2024, 14, 168. [Google Scholar] [CrossRef]
- Nowak, K.; Jakopin, Ž. In silico profiling of endocrine-disrupting potential of bisphenol analogues and their halogenated transformation products. Food Chem. Toxicol. 2023, 173, 113623. [Google Scholar] [CrossRef]
- Cousins, I.T.; Staples, C.A.; Clecka, G.M.; Mackay, D. A Multimedia Assessment of the Environmental Fate of Bisphenol A. Hum. Ecol. Risk Assess. 2002, 8, 1107–1135. [Google Scholar] [CrossRef]
- Frenzilli, G.; Martorell-Ribera, J.; Bernardeschi, M.; Scarcelli, V.; Jönsson, E.; Diano, N.; Moggio, M.; Guidi, P.; Sturve, J.; Asker, N. Bisphenol A and Bisphenol S Induce Endocrine and Chromosomal Alterations in Brown Trout. Front. Endocrinol. 2021, 12, 645519. [Google Scholar] [CrossRef]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sakr, H.; Moqadass, M.; Das, S.; Juliana, N.; Abu, I.F. Natural Products in Mitigation of Bisphenol A Toxicity: Future Therapeutic Use. Molecules 2022, 27, 5384. [Google Scholar] [CrossRef]
- Bisphenol A (BPA) Market Size, Growth & Forecast. 2023. Available online: https://www.chemanalyst.com/industry-report/bisphenol-a-market-57 (accessed on 1 August 2023).
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidence. Environ. Sci. Pollut. Res. Int. 2021, 28, 19643–19663. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- EEA. Human Exposure to Bisphenol A in Europe. Available online: https://www.eea.europa.eu/publications/peoples-exposure-to-bisphenol-a/ (accessed on 14 September 2023).
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Aysin, F. Bisphenol A promotes cell death in healthy respiratory system cells through inhibition of cell proliferation and induction of G2/M cell cycle arrest. Environ. Toxicol. 2024, 39, 3264–3273. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Lorigo, M.; Cairrao, E. Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. J. Xenobiot. 2022, 12, 181–213. [Google Scholar] [CrossRef]
- Dias, P.; Tvrdý, V.; Jirkovský, E.; Dolenc, M.S.; Peterlin Mašič, L.; Mladěnka, P. The effects of bisphenols on the cardiovascular system. Crit. Rev. Toxicol. 2022, 52, 66–87. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Charkiewicz, A.E.; Garley, M.; Ratajczak-Wrona, W.; Czerniecki, J.; Jabłońska, E.; Cechowska-Pasko, M.; Miltyk, W. Bisphenol A: Potential Factor of Miscarriage in Women in the Context of the Phenomenon of Neutrophil Extracellular Traps. Arch. Immunol. Ther. Exp. 2022, 70, 24. [Google Scholar] [CrossRef]
- Tsai, C.S.; Chou, W.J.; Lee, S.Y.; Lee, M.J.; Chou, M.C.; Wang, L.J. Phthalates, Para-Hydroxybenzoic Acids, Bisphenol-A, and Gonadal Hormones’ Effects on Susceptibility to Attention-Deficit/Hyperactivity Disorder. Toxics 2020, 8, 57. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Jaimes, R., 3rd; Swiercz, A.; Sherman, M.; Muselimyan, N.; Marvar, P.J.; Posnack, N.G. Plastics and cardiovascular health: Phthalates may disrupt heart rate variability and cardiovascular reactivity. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1044–H1053. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, R., 3rd; McCullough, D.; Siegel, B.; Swift, L.; McInerney, D.; Hiebert, J.; Perez-Alday, E.A.; Trenor, B.; Sheng, J.; Saiz, J.; et al. Plasticizer Interaction with the Heart: Chemicals Used in Plastic Medical Devices Can Interfere with Cardiac Electrophysiology. Circ. Arrhythm. Electrophysiol. 2019, 12, e007294. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Nassan, F.L.; Coull, B.A.; Gaskins, A.J.; Williams, M.A.; Skakkebaek, N.E.; Ford, J.B.; Ye, X.; Calafat, A.M.; Braun, J.M.; Hauser, R. Personal Care Product Use in Men and Urinary Concentrations of Select Phthalate Metabolites and Parabens: Results from the Environment and Reproductive Health (EARTH) Study. Environ. Health Perspect. 2017, 125, 087012. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, Z.; Nasser, S.A.; El-Yazbi, A.; Nasreddine, S.; Eid, A.H. Estrogen and Bisphenol A in Hypertension. Curr. Hypertens. Rep. 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. NF-κB—An Important Player in Xenoestrogen Signaling in Immune Cells. Cells 2021, 10, 1799. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.Y.; Choi, J.W.; Lee, S.; Kim, S.; Kho, Y.; Choi, K.; Kim, S. Pharmacokinetics of transdermal methyl-, ethyl-, and propylparaben in humans following single dermal administration. Chemosphere 2023, 310, 136689. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Bisphenol A in Food is a Health Risk. Available online: https://www.efsa.europa.eu/en/news/bisphenol-food-health-risk (accessed on 19 April 2023).
- Sun, Y.; Sha, M.; Qin, Y.; Xiao, J.; Li, W.; Li, S.; Chen, S. Bisphenol A induces placental ferroptosis and fetal growth restriction via the YAP/TAZ-ferritinophagy axis. Free Radic. Biol. Med. 2024, 211, 127–144, Erratum in Free Radic Biol Med. 2024, 213, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Ricker, K.; Cheng, V.; Hsieh, C.J.; Tsai, F.C.; Osborne, G.; Li, K.; Yilmazer-Musa, M.; Sandy, M.S.; Cogliano, V.J.; Schmitz, R.; et al. Application of the Key Characteristics of Carcinogens to Bisphenol A. Int. J. Toxicol. 2024, 43, 253–290. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Jing, D.; Zhang, K.; Shi, J.; Qiu, H.; Kan, C.; Han, F.; Wu, C.; Sun, X. Environmental toxicology of bisphenol A: Mechanistic insights and clinical implications on the neuroendocrine system. Behav. Brain Res. 2024, 460, 114840. [Google Scholar] [CrossRef]
- Han, C.; Hong YCBisphenol, A. Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Sci. Total Environ. 2021, 763, 142941. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef]
- Kang, J.-H.; Asai, D.; Toita, R. Bisphenol A (BPA) and Cardiovascular or Cardiometabolic Diseases. J. Xenobiot. 2023, 13, 775–810. [Google Scholar] [CrossRef] [PubMed]
- CDC. Bisphenol A Biomonitoring Summary. Available online: https://www.cdc.gov/biomonitoring/BisphenolA_BiomonitoringSummary.html (accessed on 7 April 2017).
- Sirasanagandla, S.R.; Al-Huseini, I.; Al Mushaiqri, M.; Al-Abri, N.; Al-Ghafri, F. Maternal resveratrol supplementation ameliorates bisphenol A-induced atherosclerotic lesions formation in adult offspring ApoE−/− mice. 3 Biotech 2022, 12, 36. [Google Scholar] [CrossRef]
- Amjad, S.; Rahman, M.S.; Pang, M.G. Role of Antioxidants in Alleviating Bisphenol A Toxicity. Biomolecules 2020, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 2023, 315, 137763. [Google Scholar] [CrossRef]
- Toxicological and Health Aspects of Bisphenol A. Report of Joint FAO/WHO Expert Meeting. 2–5 November 2010 and Report of Stakeholder Meeting on Bisphenol A 1 November 2010. Ottawa, Canada. Available online: https://www.who.int/publications/i/item/toxicological-and-health-aspects-of-bisphenol-a (accessed on 5 November 2011).
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Bisphenol A (BPA)-Induced Changes in the Number of Serotonin-Positive Cells in the Mucosal Layer of Porcine Small Intestine-the Preliminary Studies. Int. J. Mol. Sci. 2020, 21, 1079. [Google Scholar] [CrossRef]
- Makowska, K.; Szymańska, K.; Całka, J.; Gonkowski, S. The Influence of Bisphenol A (BPA) on the Occurrence of Selected Active Substances in Neuregulin 1 (NRG1)-Positive Enteric Neurons in the Porcine Large Intestine. Int. J. Mol. Sci. 2021, 22, 10308. [Google Scholar] [CrossRef]
- Gorecki, S.; Bemrah, N.; Roudot, A.C.; Marchioni, E.; Le Bizec, B.; Faivre, F.; Kadawathagedara, M.; Botton, J.; Rivière, G.; EDEN Mother-Child Cohort Study Group. Human health risks related to the consumption of foodstuffs of animal origin contaminated by bisphenol A. Food Chem. Toxicol. 2017, 110, 333–339. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials & Aids. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 397. [Google Scholar] [CrossRef]
- Vacca, M.; Calabrese, F.M.; Loperfido, F.; Maccarini, B.; Cerbo, R.M.; Sommella, E.; Salviati, E.; Voto, L.; De Angelis, M.; Ceccarelli, G.; et al. Maternal Exposure to Endocrine-Disrupting Chemicals: Analysis of Their Impact on Infant Gut Microbiota Composition. Biomedicines 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, Z.; Luo, X.; Li, Y.; Yuan, P.; Long, J.; Shen, N.; Lu, Q.; Zeng, Q.; Zhong, R.; et al. Urinary bisphenol A and its interaction with ESR1 genetic polymorphism associated with non-small cell lung cancer: Findings from a case-control study in Chinese population. Chemosphere 2020, 254, 126835. [Google Scholar] [CrossRef] [PubMed]
- Ryszawy, D.; Pudełek, M.; Kochanowski, P.; Janik-Olchawa, N.; Bogusz, J.; Rąpała, M.; Koczurkiewicz, P.; Mikołajczyk, J.; Borek, I.; Kędracka-Krok, S.; et al. High bisphenol A concentrations augment the invasiveness of tumor cells through Snail-1/Cx43/ERRγ-dependent epithelial-mesenchymal transition. Toxicol. In Vitro 2020, 62, 104676. [Google Scholar] [CrossRef]
- Liu, X.; Miao, M.; Zhou, Z.; Gao, E.; Chen, J.; Wang, J.; Sun, F.; Yuan, W.; Li, D.K. Exposure to bisphenol-A and reproductive hormones among male adults. Environ. Toxicol. Pharmacol. 2015, 39, 934–941. [Google Scholar] [CrossRef]
- Feng, M.J.; Wu, X.Q.; Li, J.; Ding, L.; Wang, Z.Q.; Shen, Y.; Song, Z.C.; Wang, L.; Yang, Q.; Wang, X.P.; et al. Relationship between daily exposure to bisphenol A and male sexual function-a study from the reproductive center. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 836–840. (In Chinese) [Google Scholar] [CrossRef]
- Hart, R.J.; Doherty, D.A.; Keelan, J.A.; Minaee, N.S.; Thorstensen, E.B.; Dickinson, J.E.; Pennell, C.E.; Newnham, J.P.; McLachlan, R.; Norman, R.J.; et al. The impact of antenatal Bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod. Biomed. Online 2018, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Vitku, J.; Sosvorova, L.; Chlupacova, T.; Hampl, R.; Hill, M.; Sobotka, V.; Heracek, J.; Bicikova, M.; Starka, L. Differences in bisphenol A and estrogen levels in the plasma and seminal plasma of men with different degrees of infertility. Physiol. Res. 2015, 64 (Suppl. 2), S303–S311. [Google Scholar] [CrossRef]
- Carranza-Diaz, O.; Schultze-Nobre, L.; Moeder, M.; Nivala, J.; Kuschk, P.; Koeser, H. Removal of selected organic micropollutants in planted and unplanted pilot-scale horizontal flow constructed wetlands under conditions of high organic load. Ecol. Eng. 2014, 71, 234–245. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; Ocón-Hernandez, O.; Mínguez-Alarcón, L.; Dávila-Arias, C.; Pérez-Lobato, R.; Calvente, I.; Arrebola, J.P.; Vela-Soria, F.; Rubio, S.; Hauser, R.; et al. Bisphenol A and reproductive hormones and cortisol in peripubertal boys: The INMA-Granada cohort. Sci. Total Environ. 2018, 618, 1046–1053. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Y.; Jiang, J.; Liu, Y.; Luo, X.; Shen, Z.; Chen, X.; Wang, Y.; Dai, Y.; Zhao, J.; et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE 2015, 10, e0127886. [Google Scholar] [CrossRef] [PubMed]
- Özel, Ş.; Tokmak, A.; Aykut, O.; Aktulay, A.; Hançerlioğulları, N.; Engin Ustun, Y. Serum levels of phthalates and bisphenol-A in patients with primary ovarian insufficiency. Gynecol. Endocrinol. 2019, 35, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, M.; Saeedi, A.; Poorbaghi, S.L.; Sepehrimanesh, M.; Fattahi, M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res. Int. 2016, 23, 23546–23550. [Google Scholar] [CrossRef]
- Watkins, D.J.; Sánchez, B.N.; Téllez-Rojo, M.M.; Lee, J.M.; Mercado-García, A.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res. 2017, 159, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, W.; Zhu, W.; Chen, L.; Wang, W.; Tian, Y.; Shen, L.; Zhang, J.; Shanghai Birth Cohort Study. Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environ. Int. 2018, 117, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.; Meeker, J.D.; Hu, H.; Sánchez, B.N.; Lamadrid-Figueroa, H.; Mercado-García, A.; Fortenberry, G.Z.; Calafat, A.M.; Téllez-Rojo, M.M. Bisphenol a exposure in Mexico City and risk of prematurity: A pilot nested case control study. Environ. Health 2010, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, Q.; Feng, L.; Wu, J.; Xiong, Y.; Li, X. Maternal serum bisphenol A levels and risk of pre-eclampsia: A nested case-control study. Eur. J. Public Health 2017, 27, 1102–1107. [Google Scholar] [CrossRef]
- Perez-Lobato, R.; Mustieles, V.; Calvente, I.; Jimenez-Diaz, I.; Ramos, R.; Caballero-Casero, N.; López-Jiménez, F.J.; Rubio, S.; Olea, N.; Fernandez, M.F. Exposure to bisphenol A and behavior in school-age children. Neurotoxicology 2016, 53, 12–19. [Google Scholar] [CrossRef]
- Barrett, E.S.; Sathyanarayana, S.; Mbowe, O.; Thurston, S.W.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. First-Trimester Urinary Bisphenol A Concentration in Relation to Anogenital Distance, an Androgen-Sensitive Measure of Reproductive Development, in Infant Girls. Environ. Health Perspect. 2017, 125, 077008. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadi, M.; Roumeliotaki, T.; Myridakis, A.; Chalkiadaki, G.; Fthenou, E.; Dermitzaki, E.; Karachaliou, M.; Sarri, K.; Vassilaki, M.; Stephanou, E.G.; et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ. Res. 2016, 146, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Ding, G.; Tian, Y.; Zhou, Z.; Wang, X.; Shen, L.; Huang, H. Association between bisphenol a exposure and idiopathic central precocious puberty (ICPP) among school-aged girls in Shanghai, China. Environ. Int. 2018, 115, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 97004. [Google Scholar] [CrossRef] [PubMed]
- Ghassabian, A.; Bell, E.M.; Ma, W.L.; Sundaram, R.; Kannan, K.; Buck Louis, G.M.; Yeung, E. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ. Pollut. 2018, 243 Pt. B, 1629–1636. [Google Scholar] [CrossRef]
- Jensen, T.K.; Mustieles, V.; Bleses, D.; Frederiksen, H.; Trecca, F.; Schoeters, G.; Andersen, H.R.; Grandjean, P.; Kyhl, H.B.; Juul, A.; et al. Prenatal bisphenol A exposure is associated with language development but not with ADHD-related behavior in toddlers from the Odense Child Cohort. Environ. Res. 2019, 170, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Yolton, K.; Stacy, S.L.; Erar, B.; Papandonatos, G.D.; Bellinger, D.C.; Lanphear, B.P.; Chen, A. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 2017, 62, 192–199. [Google Scholar] [CrossRef]
- Kharrazian, D. The Potential Roles of Bisphenol A (BPA) Pathogenesis in Autoimmunity. Autoimmune Dis. 2014, 2014, 743616. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Ladeira, C.; Zeferino, S.; Dias, A.; Faria, I.; Cristovam, E.; Gomes, M.; Ribeiro, E. Cytotoxic and genotoxic effects of environmental relevant concentrations of bisphenol A and interactions with doxorubicin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 838, 28–36. [Google Scholar] [CrossRef]
- Durovcova, I.; Spackova, J.; Puskar, M.; Galova, E.; Sevcovicova, A. Bisphenol A as an environmental pollutant with dual genotoxic and DNA-protective effects. Neuro Endocrinol. Lett. 2018, 39, 294–298. [Google Scholar]
- Shelly, S.; Boaz, M.; Orbach, H. Prolactin and autoimmunity. Autoimmun. Rev. 2012, 11, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Neutrophils life under estrogenic and xenoestrogenic control. J. Steroid Biochem. Mol. Biol. 2019, 186, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L.; Mark-Lee, W.F. A Review on the Effects of Bisphenol A and Its Derivatives on Skeletal Health. Int. J. Med. Sci. 2018, 15, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- García-Recio, E.; Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C.; de Luna-Bertos, E. Repercussions of Bisphenol A on the Physiology of Human Osteoblasts. Int. J. Mol. Sci. 2022, 23, 5349. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef]
- Tvrdý, V.; Dias, P.; Nejmanová, I.; Carazo, A.; Jirkovský, E.; Pourová, J.; Fadraersada, J.; Moravcová, M.; Peterlin Mašič, L.; Sollner Dolenc, M.; et al. The effects of bisphenols on the cardiovascular system ex vivo and in vivo. Chemosphere 2023, 313, 137565. [Google Scholar] [CrossRef]
- Warembourg, C.; Basagaña, X.; Seminati, C.; de Bont, J.; Granum, B.; Lyon-Caen, S.; Manzano-Salgado, C.B.; Pin, I.; Sakhi, A.K.; Siroux, V.; et al. Exposure to phthalate metabolites, phenols and organophosphate pesticide metabolites and blood pressure during pregnancy. Int. J. Hyg. Environ. Health 2019, 222, 446–454. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of urinary bisphenol a concentration with heart disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 2011, 218, 207–213. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Melcer, H.; Klecka, G. Treatment of wastewaters containing bisphenol A: State of the science review. Water Environ. Res. 2011, 83, 650–666. [Google Scholar] [CrossRef]
- Shu, X.; Tang, S.; Peng, C.; Gao, R.; Yang, S.; Luo, T.; Cheng, Q.; Wang, Y.; Wang, Z.; Zhen, Q.; et al. Bisphenol A is not associated with a 5-year incidence of type 2 diabetes: A prospective nested case-control study. Acta Diabetol. 2018, 55, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Levine, D.; Wertenteil, S.; Vento, S.; Xue, J.; Rajendiran, K.; Kannan, K.; Thurman, J.M.; Morrison, D.; Brody, R.; et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res. 2017, 81, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, S.G.; Wojcicki, J.M.; Perito, E.R.; Rosenthal, P. Bisphenol a increases risk for presumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003–2010. Environ. Health 2018, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Chang, H.; Huo, W.; Zhang, B.; Hu, J.; Xia, W.; Chen, Z.; Xiong, C.; Zhang, Y.; Wang, Y.; et al. Prenatal exposure to bisphenol A and risk of allergic diseases in early life. Pediatr. Res. 2017, 81, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martínez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; Swingle, H.M.; Christian, M.A.; Hessabi, M.; Lee, M.; Pitcher, M.R.; Campbell, S.; Mitchell, A.; Krone, R.; Loveland, K.A.; et al. Environmental Exposure to Dioxins, Dibenzofurans, Bisphenol A, and Phthalates in Children with and without Autism Spectrum Disorder Living near the Gulf of Mexico. Int. J. Environ. Res. Public Health 2017, 14, 1425. [Google Scholar] [CrossRef]
- Khalil, N.; Ebert, J.R.; Wang, L.; Belcher, S.; Lee, M.; Czerwinski, S.A.; Kannan, K. Bisphenol A and cardiometabolic risk factors in obese children. Sci. Total Environ. 2014, 470–471, 726–732. [Google Scholar] [CrossRef]
- Vitku, J.; Kolatorova, L.; Franekova, L.; Blahos, J.; Simkova, M.; Duskova, M.; Skodova, T.; Starka, L. Endocrine disruptors of the bisphenol and paraben families and bone metabolism. Physiol. Res. 2018, 67 (Suppl. 3), S455–S464. [Google Scholar] [CrossRef]
- Prasse, T.; Stratos, I.; Niehoff, A.; Christ, H.; Heck, V.; Meyer, C.; Mittlmeier, T. Bisphenol A-Related Effects on Bone Morphology and Biomechanical Properties in an Animal Model. Toxics 2022, 10, 86. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, S.; Dai, Y.; Rui, C.; Wang, Y.; Zhou, Y.; Li, Y.; Pang, Q.; Fan, R. Higher dermal exposure of cashiers to BPA and its association with DNA oxidative damage. Environ. Int. 2017, 98, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bernier, M.R.; Vandenberg, L.N. Handling of thermal paper: Implications for dermal exposure to bisphenol A and its alternatives. PLoS ONE 2017, 12, e0178449. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Zalko, D.; Jacques, C.; Duplan, H.; Bruel, S.; Perdu, E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011, 82, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Analytical method for biomonitoring of endocrine-disrupting compounds (bisphenol A, parabens, perfluoroalkyl compounds and a brominated flame retardant) in human hair by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2016, 945, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.F.; Zhang, J.; Shuai, H.L.; Guan, B.Z.; Luo, X.; Yan, R.L. IKKβ/NF-κB mediated the low doses of bisphenol A induced migration of cervical cancer cells. Arch. Biochem. Biophys. 2015, 573, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.K.; Govindarajah, V.; Cheong, A.; Veevers, J.; Song, D.; Gear, R.; Zhu, X.; Ying, J.; Kendler, A.; Medvedovic, M.; et al. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk. Endocr. Relat. Cancer 2017, 24, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Adiga, D.; Eswaran, S.; Sriharikrishnaa, S.; Nadeem, K.G. Role of epigenetic changes in reproductive inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 140–155. [Google Scholar]
- Niu, L.; Jia, J.; Yang, H.; Liu, S.; Wang, H.; Yan, Y.; Li, Q.; Dong, Q.; Zhang, H.; Zhao, G.; et al. 2024. "Bisphenol A: Unveiling Its Role in Glioma Progression and Tumor Growth. Int. J. Mol. Sci. 2024, 25, 2504. [Google Scholar] [CrossRef]
- Carwile, J.L.; Ye, X.; Zhou, X.; Calafat, A.M.; Michels, K.B. Canned soup consumption and urinary bisphenol A: A randomized crossover trial. JAMA-J. Am. Med. Assoc. 2011, 306, 2218–2220. [Google Scholar] [CrossRef]
- Peng, C.Y.; Tsai, E.M.; Kao, T.H.; Lai, T.C.; Liang, S.S.; Chiu, C.C.; Wang, T.N. Canned food intake and urinary bisphenol a concentrations: A randomized crossover intervention study. Environ. Sci. Pollut. Res. 2019, 26, 27999–28009. [Google Scholar] [CrossRef]
- Kim, S.; Lee, I.; Lim, J.-E.; Lee, A.; Moon, H.-B.; Park, J.; Choi, K. Dietary contribution to body burden of bisphenol A and bisphenol S among mother-children pairs. Sci. Total Environ. 2020, 744, 140856. [Google Scholar] [CrossRef]
- Yang, T.C.; Jovanovic, N.; Chong, F.; Worcester, M.; Sakhi, A.K.; Thomsen, C.; Garlantézec, R.; Chevrier, C.; Jensen, G.; Cingotti, N.; et al. Interventions to Reduce Exposure to Synthetic Phenols and Phthalates from Dietary Intake and Personal Care Products: A Scoping Review. Curr. Environ. Health Rep. 2023, 10, 184–214. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Bernat, K.; Bułkowska, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) in a nitrifying system with immobilized biomass. Bioresour. Technol. 2014, 171, 305–313. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M.; Bernat, K.; Bułkowska, K.; Wojnowska-Baryła, I. Insights into mechanisms of bisphenol A biodegradation in aerobic granular sludge. Bioresour. Technol. 2020, 315, 123806. [Google Scholar] [CrossRef]
- Bisfenol A|EFSA. Available online: https://www.efsa.europa.eu/entopics/topic/bisphenol (accessed on 4 May 2022).
- European Food Safety Authority. Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake. 2022. Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 20 April 2022).
- BfR; EFSA. Report on Diverging Views between EFSA and BfR on EFSA Updated Bisphenol A Assessment; German Federal Institute for Risk Assessment and European Food Safety Agency: Berlin, Germany, 2023; Available online: https://www.efsa.europa.eu/sites/default/files/2023-04/bfr-efsa-art-30.pdf (accessed on 7 June 2023).
- Winter, C.K. (Ed.) Food Safety: Other Contaminants. In Benjamin Caballero, Encyclopedia of Human Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 342–346. ISBN 9780123848857. [Google Scholar] [CrossRef]
- 24th Australian Total Diet Study. Food Standards Australia New Zealand (FSANZ). Available online: https://www.foodstandards.gov.au/sites/default/files/2023-11/24th%20Total%20Diet%20Study_Phase%202.pdf (accessed on 1 June 2024).
- Committee on Toxicity. Final Minutes of the 16 May 2023 COT Meeting. Available online: https://cot.food.gov.uk/Final%20Minutes%20of%20the%2016th%20May%202023%20COT%20Meeting (accessed on 17 May 2023).
- Plastics Europe. Available online: https://plasticseurope.org/about-us/ (accessed on 1 June 2022).
- Bisphenol A (BPA). NIEHS. Available online: https://www.niehs.nih.gov/health/topics/agents/sya-bpa (accessed on 31 August 2023).
- Kim, J.H.; Kwak, J.M.; Kang, H. Web-based behavioral intervention to reduce exposure to phthalate metabolites, bisphenol A, triclosan, and parabens in mothers with young children: A randomized controlled trial. Int. J. Hyg. Environ. Health 2021, 236, 113798. [Google Scholar] [CrossRef]
- NTP Research Report On The Consortium Linking Academic And Regulatory Insights On Bisphenol A Toxicity (Clarity-BPA): A Compendium Of Published Findings. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/results/pubs/rr/reports/rr18_508.pdf (accessed on 31 October 2021).
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkiewicz, A.E.; Omeljaniuk, W.J.; Nikliński, J. Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level. Int. J. Mol. Sci. 2024, 25, 6229. https://doi.org/10.3390/ijms25116229
Charkiewicz AE, Omeljaniuk WJ, Nikliński J. Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level. International Journal of Molecular Sciences. 2024; 25(11):6229. https://doi.org/10.3390/ijms25116229
Chicago/Turabian StyleCharkiewicz, Angelika Edyta, Wioleta Justyna Omeljaniuk, and Jacek Nikliński. 2024. "Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level" International Journal of Molecular Sciences 25, no. 11: 6229. https://doi.org/10.3390/ijms25116229
APA StyleCharkiewicz, A. E., Omeljaniuk, W. J., & Nikliński, J. (2024). Bisphenol A—What Do We Know? A Global or Local Approach at the Public Health Risk Level. International Journal of Molecular Sciences, 25(11), 6229. https://doi.org/10.3390/ijms25116229







