A Narrative Review of Intestinal Microbiota’s Impact on Migraine with Psychopathologies
Abstract
:1. Introduction
2. Material and Methods
3. The Microbiota–Gut–Brain Axis
4. Gut Microbiota Composition in Migraine: Clinical and Preclinical Data
5. Gut Microbiota Composition in Depression and Anxiety: Clinical and Preclinical Data
6. Migraine and Psychological Disorders Share an Altered Gut Microbiota Composition
7. Potential Mechanisms Related to Microbiota and Relevant to the Drug Response in Migraine with Psychological Disorders
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.M.J.M.; Dodick, D.W. Migraine. Nat. Rev. Dis. Primers 2022, 8, 2. [Google Scholar] [CrossRef]
- GBD 2021 Nervous System Disorders Collaborators. Global, Regional, and National Burden of Disorders Affecting the Nervous System, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Evers, S. International Classification of Headache Disorders—ICHD-4 Alpha. Cephalalgia 2020, 40, 887–888. [Google Scholar] [CrossRef]
- May, A.; Schulte, L.H. Chronic Migraine: Risk Factors, Mechanisms and Treatment. Nat. Rev. Neurol. 2016, 12, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Lipton, R.B. Overuse of Acute Migraine Medications and Migraine Chronification. Curr. Pain. Headache Rep. 2009, 13, 301–307. [Google Scholar] [CrossRef]
- Dodick, D.W. Review of Comorbidities and Risk Factors for the Development of Migraine Complications (Infarct and Chronic Migraine). Cephalalgia 2009, 29 (Suppl. S3), 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cuciureanu, D.I.; Bistriceanu, C.E.; Vulpoi, G.-A.; Cuciureanu, T.; Antochi, F.; Roceanu, A.-M. Migraine Comorbidities. Life 2024, 14, 74. [Google Scholar] [CrossRef]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Peres, M.F.P.; Mercante, J.P.P.; Tobo, P.R.; Kamei, H.; Bigal, M.E. Anxiety and Depression Symptoms and Migraine: A Symptom-Based Approach Research. J. Headache Pain. 2017, 18, 37. [Google Scholar] [CrossRef]
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo, A.; Filippi, M.; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological Bases of Comorbidity in Migraine. Front. Hum. Neurosci. 2021, 15, 640574. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The Neurobiology of Depression and Antidepressant Action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Fawcett, J. The Importance of Anxiety in Both Major Depression and Bipolar Disorder. Depress. Anxiety 2012, 29, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Maron, E.; Nutt, D. Biological Markers of Generalized Anxiety Disorder. Dialogues Clin. Neurosci. 2017, 19, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Asif, S.; Bali, A.; Dang, A.K.; Gonzalez, D.A. The Development and Impact of Anxiety With Migraines: A Narrative Review. Cureus 2022, 14, e26419. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sundaram Sivamaruthi, B. Influence of Probiotic Supplementation on Brain Function: Involvement of Gut Microbiome, Inflammation, and Stress Pathway. In Gut Microbiota—Brain Axis; IntechOpen: London, UK, 2018; Volume 1, pp. 19–33. [Google Scholar]
- Ramachandran, R. Neurogenic Inflammation and Its Role in Migraine. Semin. Immunopathol. 2018, 40, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal Relationship between Stressful Life Events and the Onset of Major Depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Stubberud, A.; Buse, D.C.; Kristoffersen, E.S.; Linde, M.; Tronvik, E. Is There a Causal Relationship between Stress and Migraine? Current Evidence and Implications for Management. J. Headache Pain. 2021, 22, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shao, A.; Jiang, Z.; Tsai, H.; Liu, W. The Exploration of Mechanisms of Comorbidity between Migraine and Depression. J. Cell Mol. Med. 2019, 23, 4505–4513. [Google Scholar] [CrossRef]
- Rosignoli, C.; Ornello, R.; Onofri, A.; Caponnetto, V.; Grazzi, L.; Raggi, A.; Leonardi, M.; Sacco, S. Applying a Biopsychosocial Model to Migraine: Rationale and Clinical Implications. J. Headache Pain. 2022, 23, 100. [Google Scholar] [CrossRef]
- Cahill, C.M.; Murphy, K.C. Migraine: Another Headache for Psychiatrists? Br. J. Psychiatry 2004, 185, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Dresler, T.; Caratozzolo, S.; Guldolf, K.; Huhn, J.-I.; Loiacono, C.; Niiberg-Pikksööt, T.; Puma, M.; Sforza, G.; Tobia, A.; Ornello, R.; et al. Understanding the Nature of Psychiatric Comorbidity in Migraine: A Systematic Review Focused on Interactions and Treatment Implications. J. Headache Pain. 2019, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; De Icco, R.; Vaghi, G.; Pazzi, S.; Guaschino, E.; Allena, M.; Ghiotto, N.; Martinelli, D.; Tassorelli, C.; Sances, G. Psychological Predictors of Negative Treatment Outcome with Erenumab in Chronic Migraine: Data from an Open Label Long-Term Prospective Study. J. Headache Pain. 2021, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Allena, M.; Sances, G.; De Icco, R.; Avenali, M.; Fadic, R.; Katsarava, Z.; Lainez, M.J.; Goicochea, M.T.; Bendtsen, L.; et al. Psychological, Clinical, and Therapeutic Predictors of the Outcome of Detoxification in a Large Clinical Population of Medication-Overuse Headache: A Six-Month Follow-up of the COMOESTAS Project. Cephalalgia 2019, 39, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Galli, F.; Viana, M.; De Icco, R.; Bitetto, V.; Allena, M.; Pazzi, S.; Sances, G.; Tassorelli, C. Negative Short-Term Outcome of Detoxification Therapy in Chronic Migraine With Medication Overuse Headache: Role for Early Life Traumatic Experiences and Recent Stressful Events. Front. Neurol. 2019, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, W.; Xiong, Y.; Tao, C.; Ma, L.; Ma, J.; You, C.; International Headache Genetics Consortium. A Causal Effects of Gut Microbiota in the Development of Migraine. J. Headache Pain 2023, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut-Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Lechuga, S.; Braga-Neto, M.B.; Naydenov, N.G.; Rieder, F.; Ivanov, A.I. Understanding Disruption of the Gut Barrier during Inflammation: Should We Abandon Traditional Epithelial Cell Lines and Switch to Intestinal Organoids? Front. Immunol. 2023, 14, 1108289. [Google Scholar] [CrossRef] [PubMed]
- Rorato, R.; Borges, B.d.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Elias, C.F.; Elias, L.L.K. LPS-Induced Low-Grade Inflammation Increases Hypothalamic JNK Expression and Causes Central Insulin Resistance Irrespective of Body Weight Changes. Int. J. Mol. Sci. 2017, 18, 1431. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Wang, A.; Lin, Z. Structural and Functional Characterization of the Gut Microbiota in Elderly Women With Migraine. Front. Cell Infect. Microbiol. 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhou, X.-Y.; Zhang, G.-X. Association between Helicobacter Pylori Infection and Migraine: A Meta-Analysis. World J. Gastroenterol. 2014, 20, 14965–14972. [Google Scholar] [CrossRef]
- Yong, D.; Lee, H.; Min, H.-G.; Kim, K.; Oh, H.-S.; Chu, M.K. Altered Gut Microbiota in Individuals with Episodic and Chronic Migraine. Sci. Rep. 2023, 13, 626. [Google Scholar] [CrossRef]
- Aamodt, A.H.; Stovner, L.J.; Hagen, K.; Zwart, J.-A. Comorbidity of Headache and Gastrointestinal Complaints. The Head-HUNT Study. Cephalalgia 2008, 28, 144–151. [Google Scholar] [CrossRef]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The Effects of a Multispecies Probiotic Supplement on Inflammatory Markers and Episodic and Chronic Migraine Characteristics: A Randomized Double-Blind Controlled Trial. Cephalalgia 2019, 39, 841–853. [Google Scholar] [CrossRef]
- Kopchak, O.; Hrytsenko, O. Feature of Gut Mcrobiota in Patients with Migraine and Healthy Individuals. Georgian Med. News 2022, 327, 13–17. [Google Scholar]
- Meng, X.; Li, Q.; Wang, D.; Li, J.; Cui, Y.; Sun, Z.; Yin, H. Exploring the Role of Gut Microbiota in Migraine Risk: A Two-Sample Mendelian Randomization Study. Scand. J. Gastroenterol. 2024, 59, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, T.; Liu, C.; Deng, M.; Ren, X.; Wang, F.; Zhang, Y.; Yu, X.; Yao, L.; Wang, Y. A 16S RRNA Gene Sequencing Based Study of Oral Microbiota in Migraine Patients in China. Bioengineered 2021, 12, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Sances, G.; De Icco, R.; Borsook, D.; Tassorelli, C. Nitroglycerin as a Comparative Experimental Model of Migraine Pain: From Animal to Human and Back. Prog. Neurobiol. 2019, 177, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Greco, R.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Modelling Migraine-Related Features in the Nitroglycerin Animal Model: Trigeminal Hyperalgesia Is Associated with Affective Status and Motor Behavior. Physiol. Behav. 2022, 256, 113956. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Liu, S.; Tao, F. Gut Microbiota and Migraine. Neurobiol. Pain. 2022, 11, 100090. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Tang, W.; Li, H.; Li, B.; Yang, C.; Xie, W.; Wang, T.; Bai, W.; Gong, Z.; Dong, Z.; et al. Repeated Inflammatory Dural Stimulation-Induced Cephalic Allodynia Causes Alteration of Gut Microbial Composition in Rats. J. Headache Pain. 2022, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Filippone, A.; Casili, G.; Giuffrè, L.; Scuderi, S.A.; Paterniti, I.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Supplementation with SCFAs Re-Establishes Microbiota Composition and Attenuates Hyperalgesia and Pain in a Mouse Model of NTG-Induced Migraine. Int. J. Mol. Sci. 2022, 23, 4847. [Google Scholar] [CrossRef]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of Probiotics on Gut Microbiota: Mechanisms of Intestinal Immunomodulation and Neuromodulation. Therap Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Sensenig, J.; Johnson, M.; Staverosky, T. Treatment of Migraine with Targeted Nutrition Focused on Improved Assimilation and Elimination. Altern. Med. Rev. 2001, 6, 488–494. [Google Scholar] [PubMed]
- Wen, Z.; He, M.; Peng, C.; Rao, Y.; Li, J.; Li, Z.; Du, L.; Li, Y.; Zhou, M.; Hui, O.; et al. Metabolomics and 16S RRNA Gene Sequencing Analyses of Changes in the Intestinal Flora and Biomarkers Induced by Gastrodia-Uncaria Treatment in a Rat Model of Chronic Migraine. Front. Pharmacol. 2019, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Mayer, E.A.; Gupta, A.; Gill, Z.; Brazeilles, R.; Le Nevé, B.; van Hylckama Vlieg, J.E.T.; Guyonnet, D.; Derrien, M.; Labus, J.S. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom. Med. 2017, 79, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, X.; Shi, S.; Ren, T.; Wang, W. Gut Microbiota and Metabolite Variations in a Migraine Mouse Model. Front. Cell Infect. Microbiol. 2023, 13, 1322059. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia Muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut-Liver-Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef] [PubMed]
- Nan, N.; Gong, M.-X.; Wang, Q.; Li, M.-J.; Xu, R.; Ma, Z.; Wang, S.-H.; Zhao, H.; Xu, Y.-S. Wuzhuyu Decoction Relieves Hyperalgesia by Regulating Central and Peripheral 5-HT in Chronic Migraine Model Rats. Phytomedicine 2022, 96, 153905. [Google Scholar] [CrossRef]
- Nan, N.; Wang, Q.; Li, M.-J.; Xu, Y.-S.; Guo, X.-M.; Xu, R.; Ma, Z.; Wang, S.-H.; Li, J.; Zhao, H.; et al. The Microbiota-Dependent Treatment of Wuzhuyu Decoction for Chronic Migraine Model Rat Associated with Anxiety-Depression Like Behavior. Oxid. Med. Cell Longev. 2023, 2023, 2302653. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, S.; Shu, H.; Yanagisawa, L.; Tao, F. Gut Microbiota Dysbiosis Enhances Migraine-Like Pain Via TNFα Upregulation. Mol. Neurobiol. 2020, 57, 461–468. [Google Scholar] [CrossRef]
- Kang, L.; Tang, W.; Zhang, Y.; Zhang, M.; Liu, J.; Li, Y.; Kong, S.; Zhao, D.; Yu, S. The Gut Microbiome Modulates Nitroglycerin-Induced Migraine-Related Hyperalgesia in Mice. Cephalalgia 2022, 42, 490–499. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pramanik, J.; Goyal, N.; Chauhan, D.; Sivamaruthi, B.S.; Prajapati, B.G.; Chaiyasut, C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals 2023, 16, 565. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Li, J.; Cheng, J.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Gan, R.-Y.; Li, H.-B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Dong, Z.; Shen, X.; Hao, Y.; Li, J.; Li, H.; Xu, H.; Yin, L.; Kuang, W. Gut Microbiome: A Potential Indicator for Differential Diagnosis of Major Depressive Disorder and General Anxiety Disorder. Front. Psychiatry 2021, 12, 651536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhang, X.; Yu, Z.-H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of Bacterial and Metabolic Signatures and Their Interaction in Major Depressive Disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Zhang, H.; Chen, X.; Zhang, Y.; Wu, J.; Zhao, L.; Wang, D.; Pu, J.; Ji, P.; et al. Toward a Deeper Understanding of Gut Microbiome in Depression: The Promise of Clinical Applicability. Adv. Sci. 2022, 9, e2203707. [Google Scholar] [CrossRef]
- Cheng, S.; Han, B.; Ding, M.; Wen, Y.; Ma, M.; Zhang, L.; Qi, X.; Cheng, B.; Li, P.; Kafle, O.P.; et al. Identifying Psychiatric Disorder-Associated Gut Microbiota Using Microbiota-Related Gene Set Enrichment Analysis. Brief. Bioinform. 2020, 21, 1016–1022. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Bai, J.; Wu, D.; Yu, S.-F.; Qiang, X.-L.; Bai, H.; Wang, H.-N.; Peng, Z.-W. Association between Fecal Microbiota and Generalized Anxiety Disorder: Severity and Early Treatment Response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-Host Systems Interactions: Protective Effects of Propionate upon the Blood-Brain Barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible Association of Bifidobacterium and Lactobacillus in the Gut Microbiota of Patients with Major Depressive Disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible Association of Firmicutes in the Gut Microbiota of Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
- Maes, M.; Vasupanrajit, A.; Jirakran, K.; Klomkliew, P.; Chanchaem, P.; Tunvirachaisakul, C.; Payungporn, S. Exploration of the Gut Microbiome in Thai Patients with Major Depressive Disorder Shows a Specific Bacterial Profile with Depletion of the Ruminococcus Genus as a Putative Biomarker. Cells 2023, 12, 1240. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, J.-J.; Wang, Y.; Shao, W.-H.; Zhou, C.-J.; Xie, P. Differential Gut Microbiota Compositions Related With the Severity of Major Depressive Disorder. Front. Cell Infect. Microbiol. 2022, 12, 907239. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef]
- Oroojzadeh, P.; Bostanabad, S.Y.; Lotfi, H. Psychobiotics: The Influence of Gut Microbiota on the Gut-Brain Axis in Neurological Disorders. J. Mol. Neurosci. 2022, 72, 1952–1964. [Google Scholar] [CrossRef]
- Ross, K. Psychobiotics: Are They the Future Intervention for Managing Depression and Anxiety? A Literature Review. Explore 2023, 19, 669–680. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.-J. Mind-Altering with the Gut: Modulation of the Gut-Brain Axis with Probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- Knudsen, J.K.; Michaelsen, T.Y.; Bundgaard-Nielsen, C.; Nielsen, R.E.; Hjerrild, S.; Leutscher, P.; Wegener, G.; Sørensen, S. Faecal Microbiota Transplantation from Patients with Depression or Healthy Individuals into Rats Modulates Mood-Related Behaviour. Sci. Rep. 2021, 11, 21869. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The Case for a Monoamine Deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 7–11. [Google Scholar] [PubMed]
- Cheng, R.; Xu, W.; Wang, J.; Tang, Z.; Zhang, M. The Outer Membrane Protein Amuc_1100 of Akkermansia Muciniphila Alleviates the Depression-like Behavior of Depressed Mice Induced by Chronic Stress. Biochem. Biophys. Res. Commun. 2021, 566, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic Paradoxical Sleep Deprivation-Induced Depression-like Behavior, Energy Metabolism and Microbial Changes in Rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia Spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A Next-Generation Probiotic: Akkermansia Muciniphila Ameliorates Chronic Stress-Induced Depressive-like Behavior in Mice by Regulating Gut Microbiota and Metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium Prausnitzii (ATCC 27766) Has Preventive and Therapeutic Effects on Chronic Unpredictable Mild Stress-Induced Depression-like and Anxiety-like Behavior in Rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Gui, S.; Zhou, C.; Chen, J.; Yang, C.; Hu, Z.; Wang, H.; Zhong, X.; Zeng, L.; et al. Comparative Metaproteomics Analysis Shows Altered Fecal Microbiota Signatures in Patients with Major Depressive Disorder. Neuroreport 2018, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Ricciotti, E. NSAID-Gut Microbiota Interactions. Front. Pharmacol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of Gut Microbiota on Depressive-like Behaviors in Mice Is Mediated by the Endocannabinoid System. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef] [PubMed]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.-C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression-Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, M.; Guerriero, C.; Cornali, K.; Albanese, M.; Costacurta, M.; Mercuri, N.B.; Di Daniele, N.; Noce, A. Linking Migraine to Gut Dysbiosis and Chronic Non-Communicable Diseases. Nutrients 2023, 15, 4327. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Okubo, R.; Katsumata, N.; Odamaki, T.; Hashimoto, N.; Kusumi, I.; Xiao, J.; Matsuoka, Y.J. Lipid and Energy Metabolism of the Gut Microbiota Is Associated with the Response to Probiotic Bifidobacterium Breve Strain for Anxiety and Depressive Symptoms in Schizophrenia. J. Pers. Med. 2021, 11, 987. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Milani, C.; Lugli, G.A.; Turroni, F.; Mancabelli, L.; Sanchez, B.; Ferrario, C.; Viappiani, A.; Mangifesta, M.; Mancino, W.; et al. Insights from Genomes of Representatives of the Human Gut Commensal Bifidobacterium bifidum. Environ. Microbiol. 2015, 17, 2515–2531. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic Potential of Pectin and Pectic Oligosaccharides to Promote Anti-Inflammatory Commensal Bacteria in the Human Colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef]
- Khoroshkin, M.S.; Leyn, S.A.; Van Sinderen, D.; Rodionov, D.A. Transcriptional Regulation of Carbohydrate Utilization Pathways in the Bifidobacterium Genus. Front. Microbiol. 2016, 7, 178239. [Google Scholar] [CrossRef]
- Özcan, E.; Sun, J.; Rowley, D.C.; Sela, D.A. A Human Gut Commensal Ferments Cranberry Carbohydrates to Produce Formate. Appl. Environ. Microbiol. 2017, 83, e01097-17. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wang, M.; Zheng, L.; Cen, Q.; Wang, F.; Zhu, L.; Pang, R.; Zhang, A. Bifidobacterium: A Probiotic for the Prevention and Treatment of Depression. Front. Microbiol. 2023, 14, 1174800. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal Microbiota Is Fundamental for the Development of Inflammatory Pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.M.; Alviña, K. The Role of Inflammation and the Gut Microbiome in Depression and Anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Aydemir, C.; Yalcin, E.S.; Aksaray, S.; Kisa, C.; Yildirim, S.G.; Uzbay, T.; Goka, E. Brain-Derived Neurotrophic Factor (BDNF) Changes in the Serum of Depressed Women. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1256–1260. [Google Scholar] [CrossRef]
- Smith, M.; Makino, S.; Kvetnansky, R.; Post, R. Stress and Glucocorticoids Affect the Expression of Brain-Derived Neurotrophic Factor and Neurotrophin-3 MRNAs in the Hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.M.; de Jong, J.; de Kloet, E.R.; Vreugdenhil, E. Downregulation of BDNF MRNA and Protein in the Rat Hippocampus by Corticosterone. Brain Res. 1998, 813, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.-F.; Young, L.T. Increased Hippocampal Bdnf Immunoreactivity in Subjects Treated with Antidepressant Medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Deen, M.; Christensen, C.E.; Hougaard, A.; Hansen, H.D.; Knudsen, G.M.; Ashina, M. Serotonergic Mechanisms in the Migraine Brain—A Systematic Review. Cephalalgia 2017, 37, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Bayrer, J.R.; Castro, J.; Venkataraman, A.; Touhara, K.K.; Rossen, N.D.; Morrie, R.D.; Maddern, J.; Hendry, A.; Braverman, K.N.; Garcia-Caraballo, S.; et al. Gut Enterochromaffin Cells Drive Visceral Pain and Anxiety. Nature 2023, 616, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Turovskaya, O.; Kim, G.; Madan, R.; Karp, C.L.; Cheroutre, H.; Kronenberg, M. Interleukin 10 Acts on Regulatory T Cells to Maintain Expression of the Transcription Factor Foxp3 and Suppressive Function in Mice with Colitis. Nat. Immunol. 2009, 10, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A Link between Stress and Depression: Shifts in the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism and the Etiology and Pathophysiology of Depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Maes, M.; Song, C.; Lin, A.; De Jongh, R.; Van Gastel, A.; Kenis, G.; Bosmans, E.; De Meester, I.; Benoy, I.; Neels, H.; et al. The Effects of Psychological Stress on Humans: Increased Production of pro-Inflammatory Cytokines and Th-1like Response in Stress-Induced Anxiety. Cytokine 1998, 10, 313–318. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Fragas, M.G.; de Oliveira, D.M.; Hiyane, M.I.; Braga, T.T.; Camara, N.O.S. The Dual Effect of Acetate on Microglial TNF-α Production. Clinics 2022, 77, 100062. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef] [PubMed]
- Maini Rekdal, V.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and Inhibition of an Interspecies Gut Bacterial Pathway for Levodopa Metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef]
- Alexander, M.; Ang, Q.Y.; Nayak, R.R.; Bustion, A.E.; Sandy, M.; Zhang, B.; Upadhyay, V.; Pollard, K.S.; Lynch, S.V.; Turnbaugh, P.J. Human Gut Bacterial Metabolism Drives Th17 Activation and Colitis. Cell Host Microbe 2022, 30, 17–30.e9. [Google Scholar] [CrossRef] [PubMed]
- Asif, N.; Patel, A.; Vedantam, D.; Poman, D.S.; Motwani, L. Migraine With Comorbid Depression: Pathogenesis, Clinical Implications, and Treatment. Cureus 2022, 14, e25998. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-T.; Liang, C.-S.; Lee, J.-T.; Yeh, T.-C.; Lee, M.-S.; Sung, Y.-F.; Yang, F.-C. Associations Between Depression/Anxiety and Headache Frequency in Migraineurs: A Cross-Sectional Study. Headache 2018, 58, 407–415. [Google Scholar] [CrossRef]
- Viana, M.; Bottiroli, S.; Sances, G.; Ghiotto, N.; Allena, M.; Guaschino, E.; Nappi, G.; Tassorelli, C. Factors Associated to Chronic Migraine with Medication Overuse: A Cross-Sectional Study. Cephalalgia 2018, 38, 2045–2057. [Google Scholar] [CrossRef]
- Bottiroli, S.; Viana, M.; Sances, G.; Ghiotto, N.; Guaschino, E.; Galli, F.; Vegni, E.; Pazzi, S.; Nappi, G.; Tassorelli, C. Psychological Factors Associated with Failure of Detoxification Treatment in Chronic Headache Associated with Medication Overuse. Cephalalgia 2016, 36, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Renzi, A.; Ballante, E.; De Icco, R.; Sances, G.; Tanzilli, A.; Vecchi, T.; Tassorelli, C.; Galli, F. Personality in Chronic Headache: A Systematic Review with Meta-Analysis. Pain. Res. Manag. 2023, 2023, 6685372. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef] [PubMed]
- Buse, D.C.; Silberstein, S.D.; Manack, A.N.; Papapetropoulos, S.; Lipton, R.B. Psychiatric Comorbidities of Episodic and Chronic Migraine. J. Neurol. 2013, 260, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Seng, E.K.; Chu, M.K.; Reed, M.L.; Fanning, K.M.; Adams, A.M.; Buse, D.C. The Effect of Psychiatric Comorbidities on Headache-Related Disability in Migraine: Results From the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache J. Head Face Pain 2020, 60, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Begasse De Dhaem, O.; Kroon Van Diest, A.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and Its Psychiatric Comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Nelson, E.A.; Palombo, E.A. Investigating the Role of Perceived Stress on Bacterial Flora Activity and Salivary Cortisol Secretion: A Possible Mechanism Underlying Susceptibility to Illness. Biol. Psychol. 2008, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Aronoff, D.M. The Influence of Non-Steroidal Anti-Inflammatory Drugs on the Gut Microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Patil, K.R.; Typas, A.; Maier, L. Towards a Mechanistic Understanding of Reciprocal Drug-Microbiome Interactions. Mol. Syst. Biol. 2021, 17, e10116. [Google Scholar] [CrossRef]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of Therapeutic Drugs by Human Gut Bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The Microbial Pharmacists within Us: A Metagenomic View of Xenobiotic Metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Bala, A.; Surette, M.; Bienenstock, J.; Vigod, S.N.; Taylor, V.H. Gut Microbiome Patterns Associated With Treatment Response in Patients With Major Depressive Disorder: Changements Du Microbiote Intestinal Associés à La Réponse Au Traitement Chez Des Patients Souffrant de Trouble Dépressif Majeur. Can. J. Psychiatry 2020, 65, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tu, H.; Liu, P.; Zhang, Y.; Zhang, R.; Jing, L.; Zhang, K. Association Analysis of Gut Microbiota and Efficacy of SSRIs Antidepressants in Patients with Major Depressive Disorder. J. Affect. Disord. 2023, 330, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shen, X.; Hao, Y.; Li, J.; Xu, H.; Yin, L.; Kuang, W. Gut Microbiome: A Potential Indicator for Predicting Treatment Outcomes in Major Depressive Disorder. Front. Neurosci. 2022, 16, 813075. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.-H.; Kelly, A.G.; Pudlo, N.A.; Martens, E.C.; Boraston, A.B. Bacteria of the Human Gut Microbiome Catabolize Red Seaweed Glycans with Carbohydrate-Active Enzyme Updates from Extrinsic Microbes. Proc. Natl. Acad. Sci. USA 2012, 109, 19786–19791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chekan, J.R.; Dodd, D.; Hong, P.-Y.; Radlinski, L.; Revindran, V.; Nair, S.K.; Mackie, R.I.; Cann, I. Xylan Utilization in Human Gut Commensal Bacteria Is Orchestrated by Unique Modular Organization of Polysaccharide-Degrading Enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, E3708-17. [Google Scholar] [CrossRef] [PubMed]
- Vuralli, D.; Ceren Akgor, M.; Gok Dagidir, H.; Gulbahar, O.; Yalinay, M.; Bolay, H. Lipopolysaccharide, VE-Cadherin, HMGB1, and HIF-1α Levels Are Elevated in the Systemic Circulation in Chronic Migraine Patients with Medication Overuse Headache: Evidence of Leaky Gut and Inflammation. J. Headache Pain 2024, 25, 23. [Google Scholar] [CrossRef]
- Breslau, N.; Davis, G.C. Migraine, Major Depression and Panic Disorder: A Prospective Epidemiologic Study of Young Adults. Cephalalgia 1992, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective Unconscious: How Gut Microbes Shape Human Behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology From the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
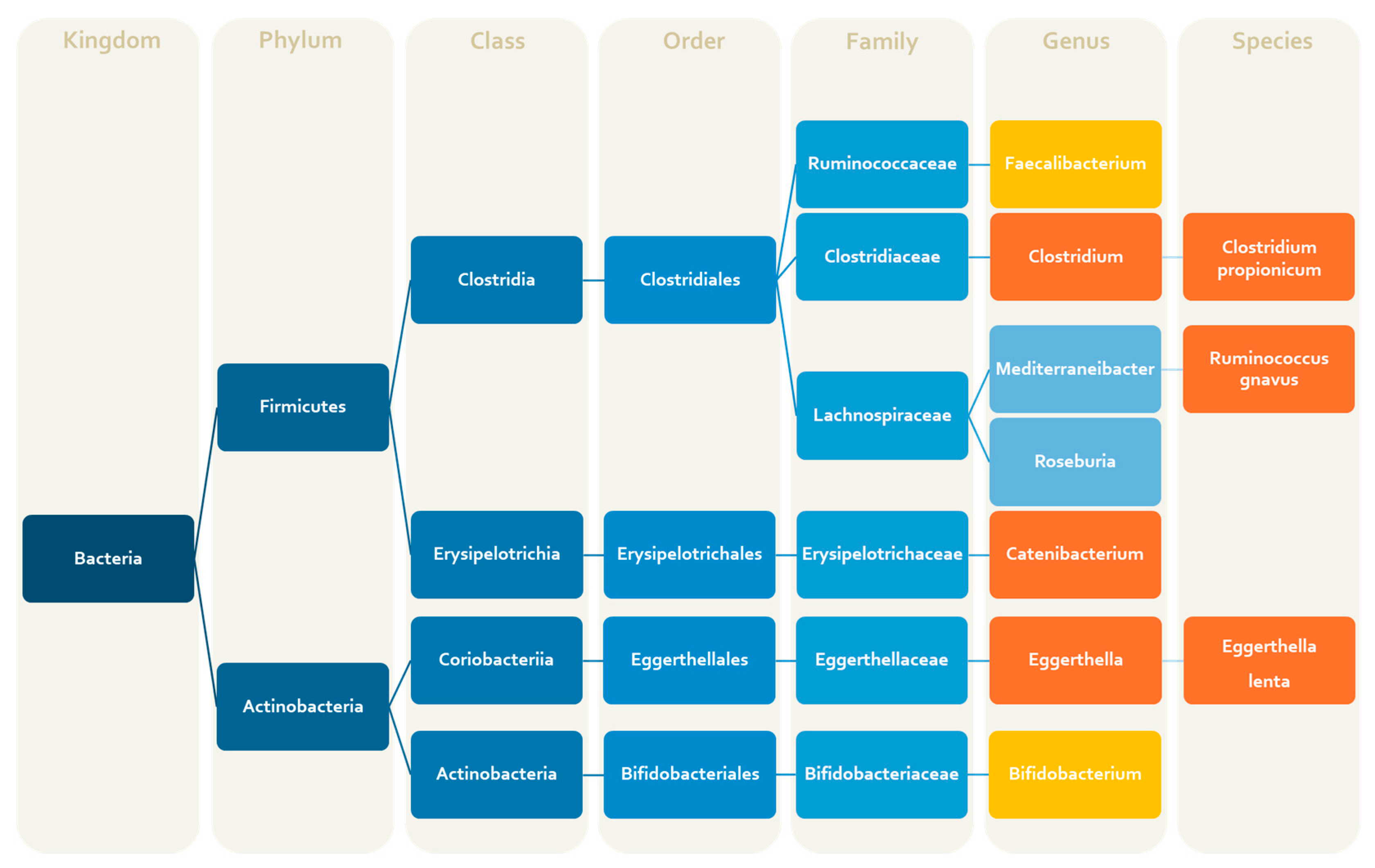

| Bacteria | Alterations | Products (Metabolites, Amino Acids, Vitamins, Neurotransmitters) | Effects | References |
|---|---|---|---|---|
| Agathobacter (genus) | ↑ c | Negative association with severe headache intensity | [49] | |
| Alcaligenes spp. (genus) | ↑ c | [52] | ||
| Alistipes (genus) | ↑ p | Indole and derivatives | Pro-inflammatory activity | [59] |
| Akkermansia muciniphila (species) | ↓ p | 5-HT | Anti-migraine activity | [66] |
| Bacteroides (genus) | ↑ p | Tryptophan pathway metabolites, 5-HT, GABA, SCFAs | [63] | |
| Bifidobacterium (genus) | ↓ c | Tryptophan, Folate, Pyroxidine, riboflavin, folate, niacin | Anti-migraine activity | [29] |
| Bifidobacterium adolescentis (species) | ↓ c | SCFAs (mainly acetate), GABA | Anti-inflammatory, antinociceptive activities | [47] |
| Catenibacterium (genus) | ↑ c | SCFAs | [29] | |
| Clostrium spp. (genus) | ↑ c | Tryptophan | Pro-infalmmatoy activity | [47] |
| Clostridium coccoides (species) | ↑ c | Association with the severity of migraine symptoms | [52] | |
| Clostridium propionicum (species) | ↑ c | Propionate | [52] | |
| Coprococcus (genus) | ↓ p | SCFAs (mainly butyrate) | Anti-inflammatory activity | [63] |
| Desulfovibrio (genus) | ↓ p | Ammonia production, amino acid breakdown, 5-HT | Pro-inflammatory effects | [63] |
| Eggerthella lenta (species) | ↑ c | Arginine, dopamine modulation gut Th17 cells activation | Pro-nociceptive activity | [47,52] |
| Eubacterium_g4 (genus) | ↑ c | [49] | ||
| Faecalibacterium (genus) | ↓ c | SCFAs (butyrate, D-lactate) | Anti-inflammatory activity | [47] |
| Faecalibacterium prausnitzii (species) | ↓ c | Butyrate | Anti-inflammatory activity | [47] |
| Lachnospiraceae (family) | ↑ p | SCFAs | [66] | |
| PAC000195_g (genus) | ↑ c | Associated with a lower headache frequency | [49] | |
| Prevotella (genus) | ↑ p | SCFAs (mainly butyrate, propionate) | Anti-inflammatory activity | [63] |
| Rhodococcus spp. (genus) | ↑ c | [52] | ||
| Roseburia (genus) | ↑ c | SCFAs (mainly butyrate) | [49] | |
| Ruminococcus (genus) | ↑ p | Metabolizes tryptophan to tryptamine SCFAs, L-glutammate | [63] | |
| Ruminococcus gnavus (species) | ↑ c | Intestinal mucin degradation SCFAs | Pro-inflammatory activity | [47] |
| Streptococcus (genus) | ↑ p | 5-HT, dopamine, and norepinephrine | [63] | |
| Tissierellia (classis) | ↑ c | [49] |
| Bacteria | Alterations | Products (Metabolites, Amino Acids, Vitamins, Neurotransmitters) | Effects | References |
|---|---|---|---|---|
| Alistipes (genus) | ↑ c | Indole and derivatives | Pro-inflammatory activity | [78,86] |
| Akkermansia muciniphila (species) | ↓ p | 5-HT, SCFAs | Modulation of the immune system, metabolic system, and endocannabinoid system | [109] |
| Bacteroides (genus) | ↑ c | Tryptophan pathway metabolites, 5-HT, GABA, SCFAs | Susceptibility to depression-like behavior | [82] |
| Bifidobacterium (genus) | ↓ c | Tryptophan, Folate, Pyroxidine, riboflavin, folate, niacin | Anti-depressive activity | [91] |
| Catenibacterium (genus) | ↑ c | SCFAs | Associated with depression severity | [94] |
| Clostrium spp. (genus) | ↑ c | Tryptophan | Pro-infalmmatoy activity | [78] |
| Clostridium propionicum (species) | ↑ c | Propionate | [93] | |
| Coprococcus (genus) | ↓ c | SCFAs (mainly butyrate) | Anti-inflammatory activity | [84,87] |
| Desulfovibrio (genus) | ↑ c | Ammonia production, amino acid breakdown, 5-HT | Pro-inflammatory effects | [85] |
| Dialister (genus) | ↓ c | Butyrate | Anti-inflammatory activity | [79,83,87] |
| Eggerthella (genus) | ↑ c | 5-HT, GABA | [83] | |
| Faecalibacterium (genus) | ↓ c | SCFAs (butyrate, D-lactate) | Anti-inflammatory activity | [78,79] |
| Faecalibacterium prausnitzii (species) | ↓ p | Butyrate | Anti-inflammatory activity | [111] |
| Lachnospiraceae (family) | ↑ c/↓ c | SCFAs | [78,112] | |
| Prevotellaceae (family) | ↓ c | SCFAs | Anti-inflammatory activity | [83] |
| Prevotella (genus) | ↓ c | SCFAs (mainly butyrate, propionate) | Anti-inflammatory activity | [78,83] |
| Roseburia (genus) | ↓ c | SCFAs (mainly butyrate) | Anti-inflammatory activity | [77,80] |
| Ruminococcus (genus) | ↓ p/↓ c | Metabolizes tryptophan to tryptamine SCFAs, L-glutammate | [78,108] | |
| Ruminococcus gnavus (species) | ↑ c/↓ c | intestinal mucin degradation SCFAs | Pro-inflammatory activity | [78,80,81] |
| Sutterella (genus) | ↓ c | Pro-inflammatory activity | [73,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francavilla, M.; Facchetti, S.; Demartini, C.; Zanaboni, A.M.; Amoroso, C.; Bottiroli, S.; Tassorelli, C.; Greco, R. A Narrative Review of Intestinal Microbiota’s Impact on Migraine with Psychopathologies. Int. J. Mol. Sci. 2024, 25, 6655. https://doi.org/10.3390/ijms25126655
Francavilla M, Facchetti S, Demartini C, Zanaboni AM, Amoroso C, Bottiroli S, Tassorelli C, Greco R. A Narrative Review of Intestinal Microbiota’s Impact on Migraine with Psychopathologies. International Journal of Molecular Sciences. 2024; 25(12):6655. https://doi.org/10.3390/ijms25126655
Chicago/Turabian StyleFrancavilla, Miriam, Sara Facchetti, Chiara Demartini, Anna Maria Zanaboni, Chiara Amoroso, Sara Bottiroli, Cristina Tassorelli, and Rosaria Greco. 2024. "A Narrative Review of Intestinal Microbiota’s Impact on Migraine with Psychopathologies" International Journal of Molecular Sciences 25, no. 12: 6655. https://doi.org/10.3390/ijms25126655
APA StyleFrancavilla, M., Facchetti, S., Demartini, C., Zanaboni, A. M., Amoroso, C., Bottiroli, S., Tassorelli, C., & Greco, R. (2024). A Narrative Review of Intestinal Microbiota’s Impact on Migraine with Psychopathologies. International Journal of Molecular Sciences, 25(12), 6655. https://doi.org/10.3390/ijms25126655






