Photodegradation of a Broad-Spectrum Antibiotic Azithromycin Using H2O2 under Ultraviolet Irradiation
Abstract
1. Introduction
2. Results and Discussion
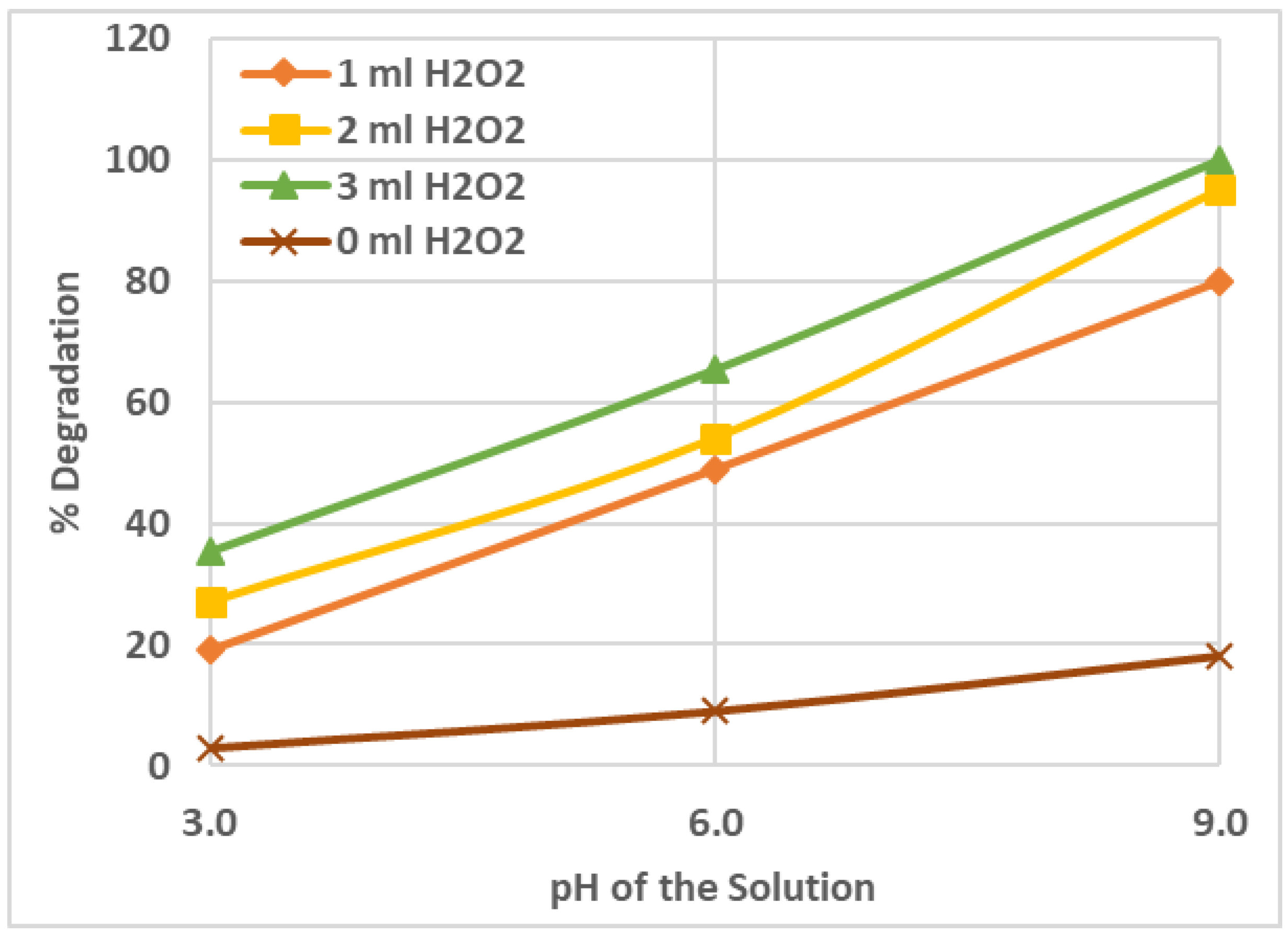
2.1. Effect of Solution pH on the AZT Degradation Efficiency
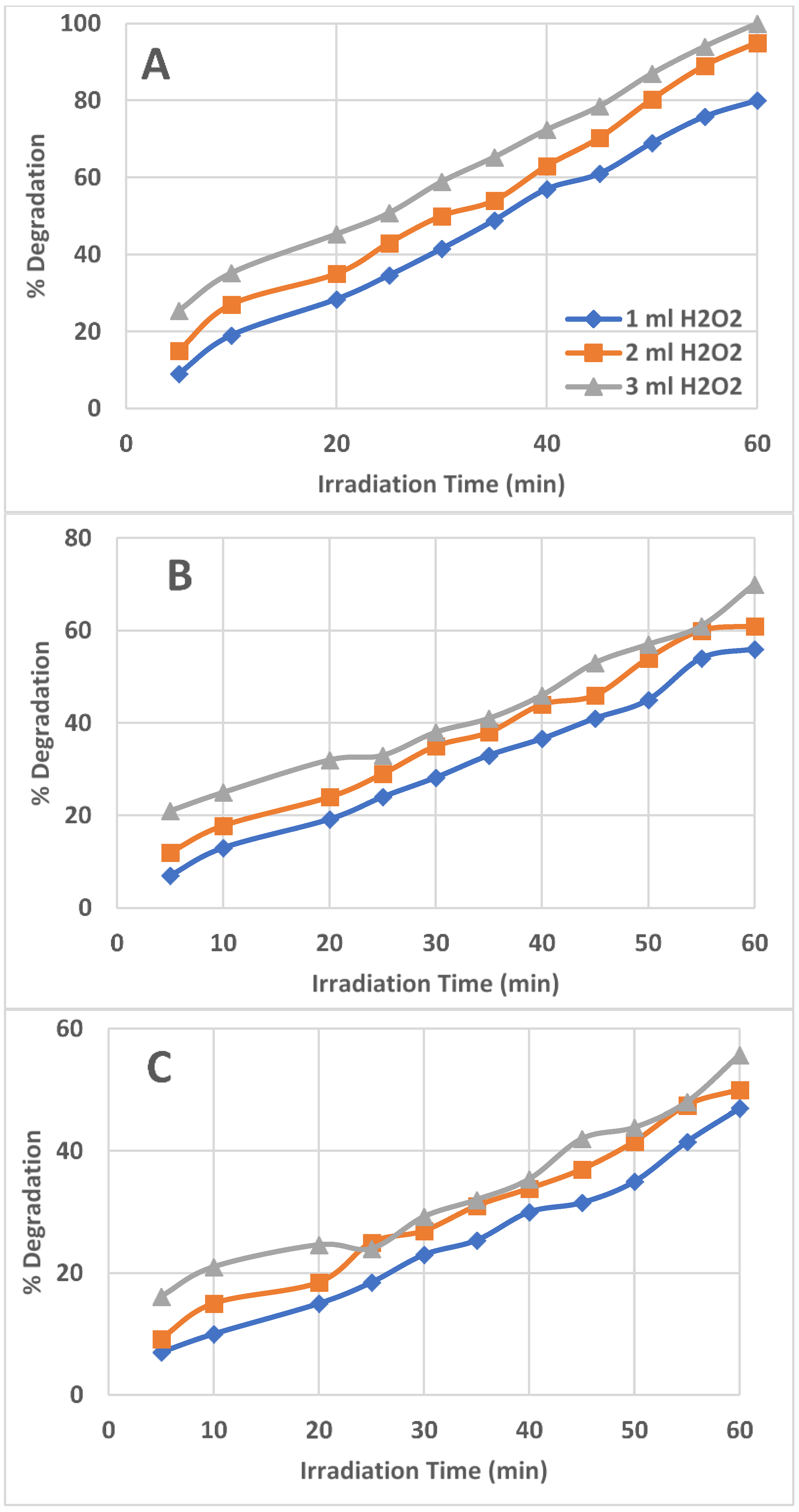
2.2. Effect of Amount/Concentration of H2O2 in the Reacting Solution
2.3. Effect of Initial AZT Concentration on AZT Degradation
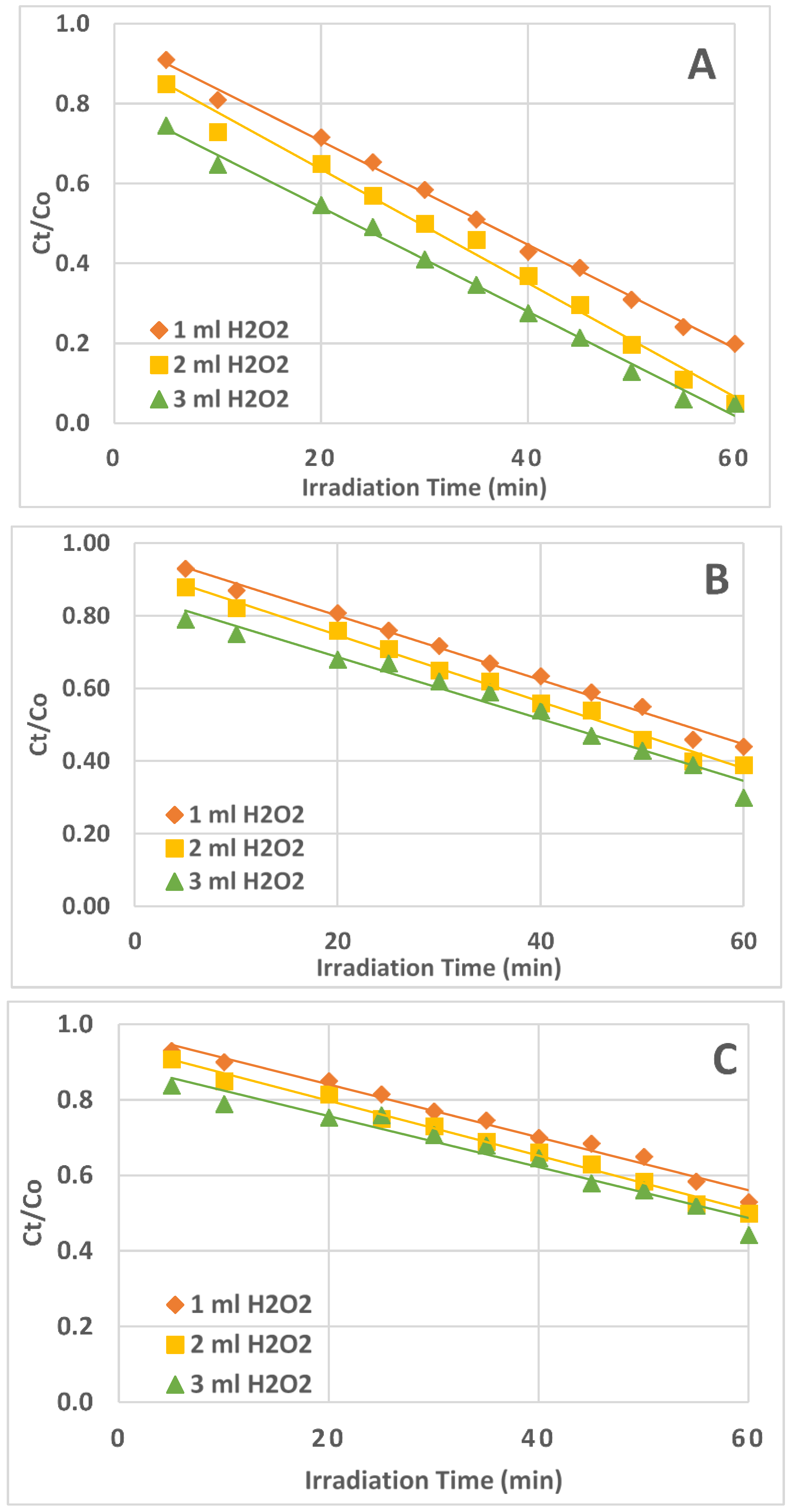
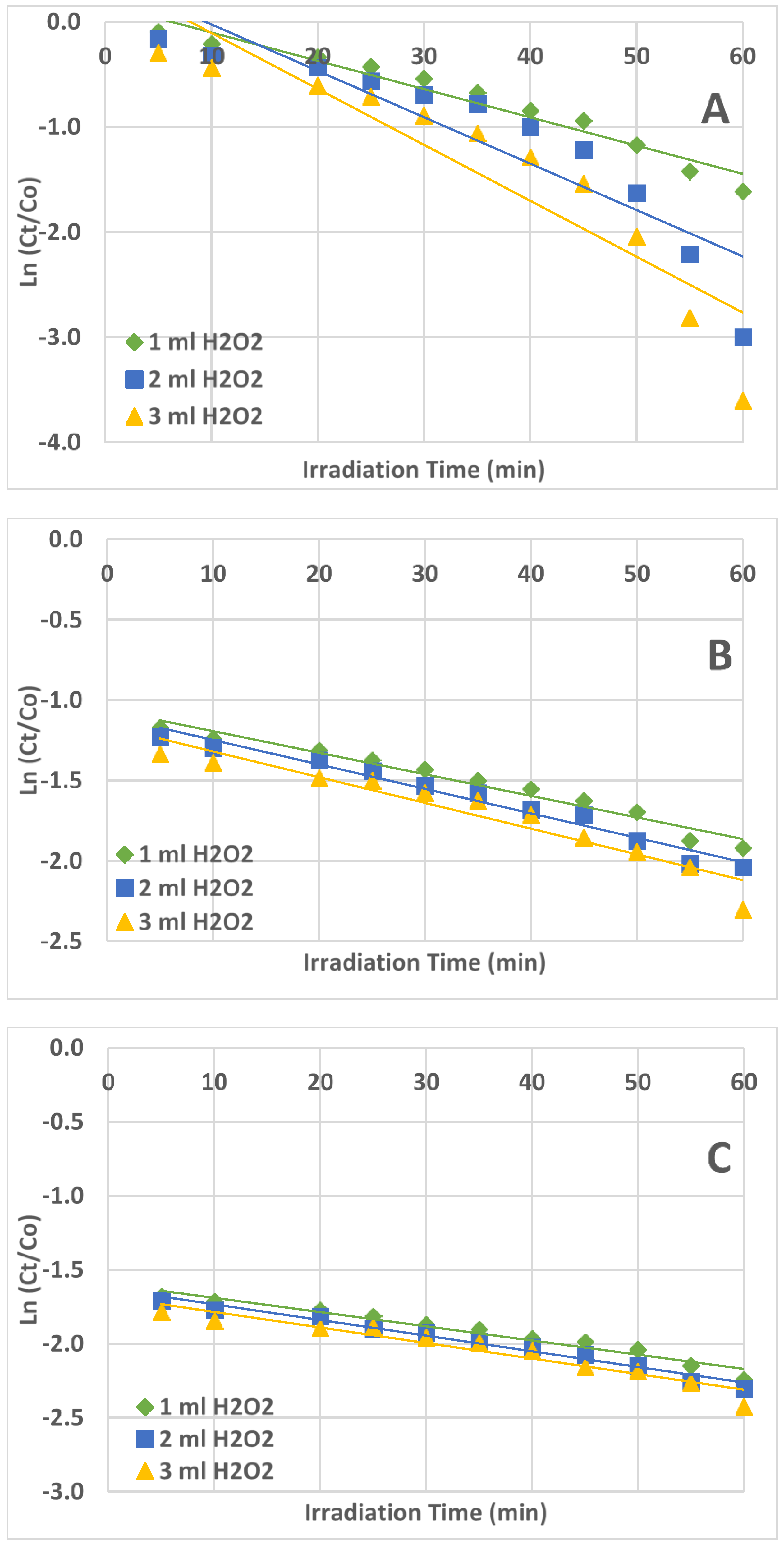
2.4. Kinetics of AZT Photodegradation
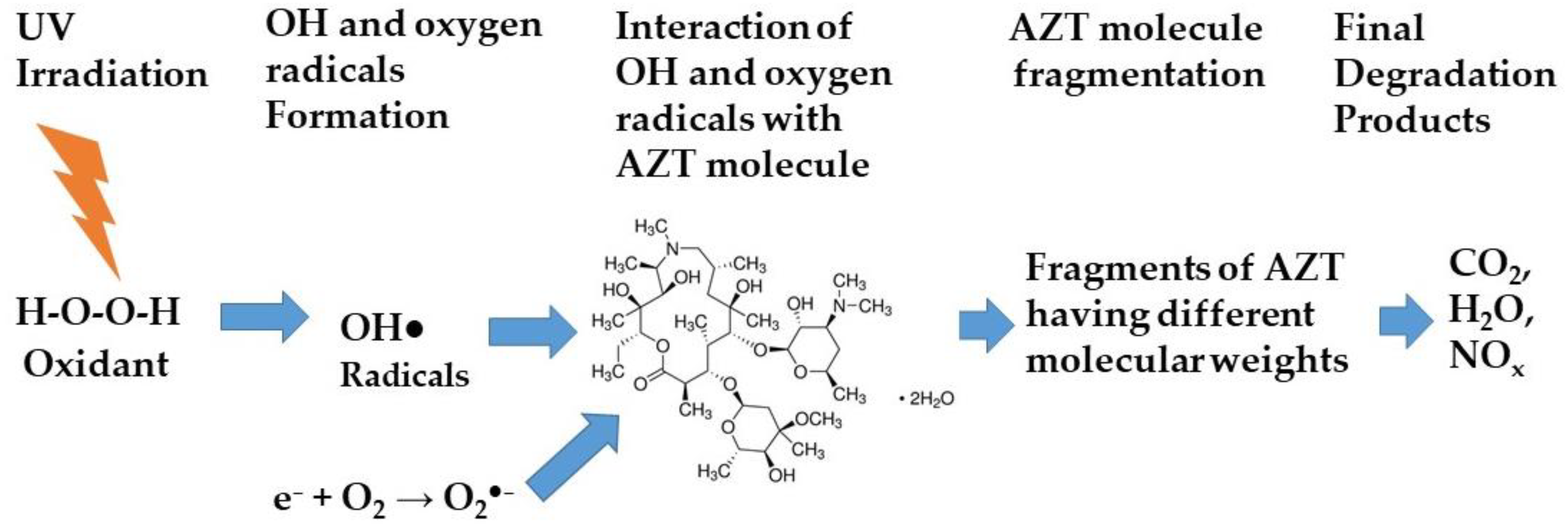
2.5. Photodegradation Mechanism
2.6. Comparison of This Work with the Literature
3. Materials and Methods
3.1. Materials
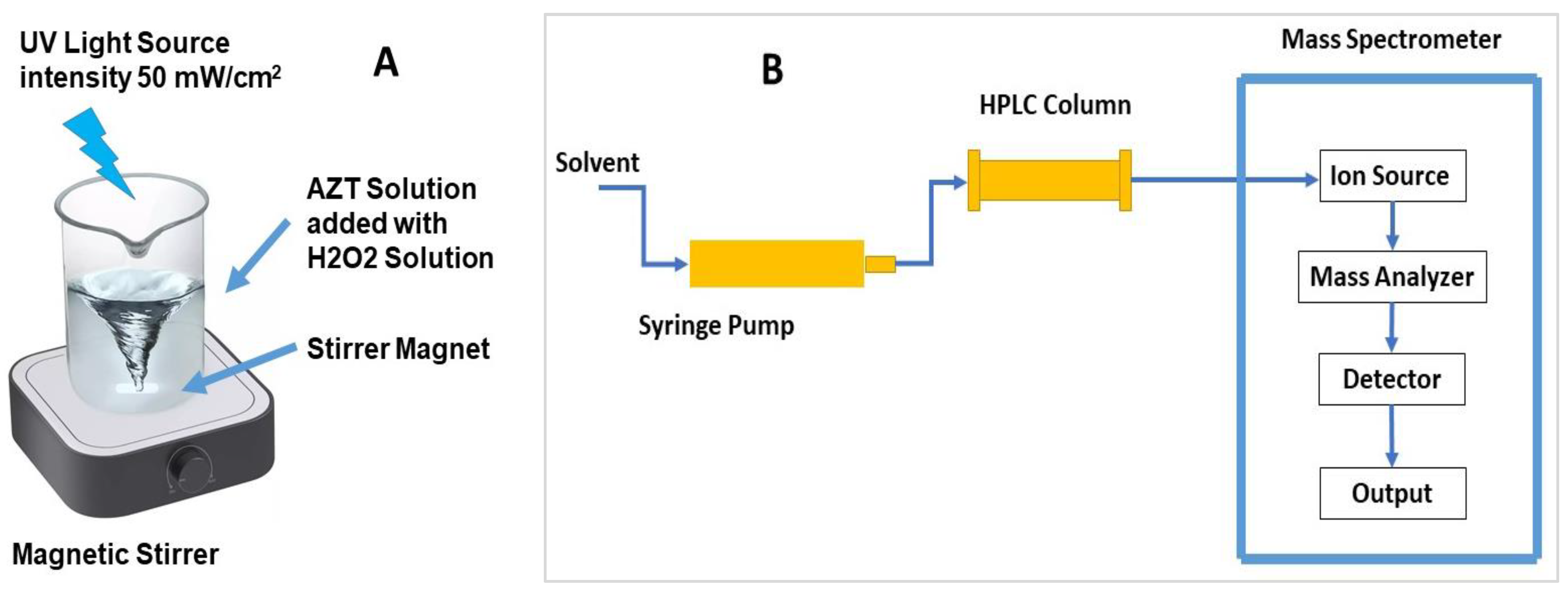
3.2. Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javed, M.; Iqbal, S.; Qamar, M.A.; Shariq, M.; Ahmed, I.A.; BaQais, A.; Alzahrani, H.; Ali, S.K.; Masmali, N.A.; Althagafi, T.M.; et al. Fabrication of Effective Co-SnO2/SGCN Photocatalysts for the Removal of Organic Pollutants and Pathogen Inactivation. Crystals 2023, 13, 163. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Venugopal, D.; Thangaraju, D.; Marnadu, R.; Palanivel, B.; Imran, M.; Shkir, M.; Ubaidullah, M.; AlFaify, S. A facile co-precipitation synthesis of novel WO3/NiWO4 nanocomposite with improved photocatalytic activity. Mater. Sci. Semicond. Process. 2021, 133, 105970. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Shariq, M.; Fadhali, M.M.; Madkhali, O.; Ali, S.K.; Syed, I.S.; Awaji, M.Y.; Shakir Khan, M.; et al. Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12, 1388. [Google Scholar] [CrossRef]
- Helmy, E.T.; Soliman, U.A.; Elbasiony, A.M.; Nguyen, B.S. CuCe-Ferrite/TiO2 Nanocomposite as an Efficient Magnetically Separable Photocatalyst for Dye Pollutants Decolorization. Top. Catal. 2023, 66, 53–63. [Google Scholar] [CrossRef]
- Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, A.M.; Alahmari, S.D.; Farid, H.M.T. Facile Synthesis of the MOF Derived ZnMn2O4 Nanorods for Dyes Degradation in Water. J. Mater. Sci. Mater. Electron. 2023, 34, 1630. [Google Scholar] [CrossRef]
- Dilawar, S.; Albalawi, K.; Khan, A.U.; Tahir, K.; Zaki, M.E.; Saleh, E.A.M.; Almarhoon, Z.M.; Althagafi, T.M.; El-Zahhar, A.A.; El-Bialy, E. Rapid photodegradation of toxic organic compounds and photoinhibition of bacteria in the presence of novel hydrothermally synthesized Ag/Mn–ZnO nanomaterial. Environ. Res. 2023, 231, 116093. [Google Scholar] [CrossRef] [PubMed]
- Bessy, T.C.; Sarojoni, V.; El Manna, C.; Sasi, F.S.; Bakri, M.; Johnson, J.; Bindhu, M.R. Optical, Structural, Morphological, Antibacterial, and Photodegradation Characteristics of BaxMg0.8−xFe2O4 (x = 0.2, 0.4, and 0.6) Nanocrystalline Powders Synthesized by Combustion Method. Phys. Status Solidi (a)—Appl. Mater. Sci. (PSSA) 2022, 219, 1–8. [Google Scholar] [CrossRef]
- Husain, A.; Khan, H.A.A.; Nadeem, A.K.; Ahmed, S.; Mehtab, M.S.; Vambol, S.; Vambol, V.; Changani, F.; Islam, S. Pharmaceuticals of emerging concern in hospital wastewater: Removal of Ibuprofen and Ofloxacin drugs using M BBR method. Int. J. Environ. Anal. Chem. 2023, 103, 140–154. [Google Scholar] [CrossRef]
- Ling, Y.; Hai, L.; Biqing, L.; Biaojun, Z.; Yixiao, W.; Heping, H.; Deyou, Y.; Shaobin, H. Efficient photocatalytic ozonation of azithromycin by three-dimensional g-C3N4 nanosheet loaded magnetic Fe-MCM-48 under simulated solar light. Appl. Catal. B Environ. 2023, 324, 122208. [Google Scholar]
- Sharma, M.; Deepanshi, R.; Vinod, K.; Indu, J.; Tejraj, M.A.; Gunda, M.; Ravi, K.; Kashyap, K.D. Photocatalytic degradation of four emerging antibiotic contaminants and toxicity assessment in wastewater: A comprehensive study. Environ. Res. 2023, 231 Pt 2, 116132. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, S.; Abu Haija, M. Effective removal of azithromycin by novel g-C3N4/CdS/CuFe2O4 nanocomposite under visible light irradiation. Chemosphere 2023, 337, 139372. [Google Scholar] [CrossRef] [PubMed]
- Mehrdoost, A.; Reza, J.Y.; Mohammad, K.M.; Azadeh, H.; Ali, A.B. Adsorption removal and photocatalytic degradation of azithromycin from aqueous solution using PAC/Fe/Ag/Zn nanocomposite. Environ. Sci. Pollut. Res. 2022, 29, 33514–33527. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.T.; Alasedi, K.K.; Ruyid, R. ZnO/Co3O4 Nanocomposites: Novel Preparation, Characterization, and Their Performance toward Removal of Antibiotics from Wastewater. J. Nanostruct. 2022, 12, 503–509. [Google Scholar]
- Shukla, S.; Pandey, H.; Singh, P.; Tiwari, A.K.; Baranwal, V.; Singh, J.; Pandey, A.C. Pandey. Time and Concentration Dependent; UV Light–Mediated Photocatalytic Degradation of Major Antibiotic Consortium Using ZnO. Braz. J. Phys. 2022, 52, 183. [Google Scholar] [CrossRef]
- Kumar, A.; Anamika, R.; Changsheng, G.; Gaurav, S.; Khadijah, M.S.M.K.; Fatimah, M.A.; Mu, N.; Mika, S.; Pooja, D.; Florian, J.S. Acceleration of photo-reduction and oxidation capabilities of Bi4O5I2/SPION@calcium alginate by metallic Ag: Wide spectral removal of nitrate and azithromycin. Chem. Eng. J. 2021, 423, 130173. [Google Scholar] [CrossRef]
- Ospino-Atehortúa, B.A.; Zúñiga-Benítez, H.; Peñuela, G.A. Potential Application of Persulfate and Simulated Sunlight Radiation on Azithromycin Removal. Environ. Eng. Res. 2021, 26, 200189. [Google Scholar] [CrossRef]
- Tenzin, T.; Yashas, S.R.; Anilkumar, K.M. UV–LED driven photodegradation of organic dye and antibiotic using strontium titanate nanostructures. J. Mater. Sci. Mater. Electron. 2021, 32, 21093–21105. [Google Scholar] [CrossRef]
- Li, C.; Jin, H.; Hou, Z.; Guo, Y. Study on degradation of azithromycin antibiotics by molybdenum sulfide graphene oxide composites under visible light. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012019. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; Ramírezdel, S.M.; Levchuk, I. Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Sobhani, S.; Shekari, H. Photocatalytic degradation of azithromycin using GO@Fe3O4/ZnO/SnO2 nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babić, S. Photocatalytic Degradation of Azithromycin by Nanostructured TiO2 Film: Kinetics, Degradation Products, and Toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef]
- Naraginti, S.; Yu, Y.Y.; Fang, Z.; Yong, Y.C. Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@TiO2 nanorod composite: Transformation pathways and toxicity evaluation. Proc. Saf. Environ. Prot. 2019, 125, 39–49. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic-resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Luo, X.; Hao, T.; Yue, L.; Hong, G.; Lu, Y. Azithromycin wastewater treatment with La doping titanium dioxide/active carbon composites. In International Conference on Sensors, Measurement and Intelligent Materials (ICSMIM 2015); Atlantis Press: Amsterdam, The Netherlands, 2016; pp. 861–870. [Google Scholar]
- Chen, X. Photodegradation of Azithromycin in Aqueous System under VIS/UV Irradiation. Int. J. Sci. Adv. 2022, 3, 707–710. [Google Scholar] [CrossRef]
- Cano, P.A.; Marcela, J.-B.; Henry, Z.-B.; Yudy, A.L.; Gustavo, A.P. Use of simulated sunlight radiation and hydrogen peroxide in azithromycin removal from aqueous solutions: Optimization and mineralization analysis. Emerg. Contam. 2020, 6, 53–61. [Google Scholar] [CrossRef]
- Voigt, M.; Indra, B.; Anna, N.-H.; Martin, J. Elimination of macrolides in water bodies using photochemical oxidation. AIMS Environ. Sci. 2018, 5, 372–388. [Google Scholar] [CrossRef]
- Tong, L.; Peter, E.; Sandra, P.; Yanxin, W.; Damià, B. Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts. Chemosphere 2011, 83, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Ferreol, M.; Coquery, M.; Choubert, J.M.; Chovelon, J.M.; Miege, C. Direct photodegradation of 36 organic micropollutants under simulated solar radiation: Comparison with free-water surface constructed wetland and influence of chemical structure. J. Hazard. Mater. 2021, 407, 124801. [Google Scholar] [CrossRef]
- Jaramillo-Baquero, M.; Zúñiga-Benítez, H.; Peñuela, G.A. Use of photo-Fenton for macrolide antibiotic azithromycin removal. Acta Period. Technol. 2020, 50, 29–37. [Google Scholar] [CrossRef]
- Orona-Návar, C.; Levchuk, I.; Moreno-Andrés, J.; Park, Y.; Mikola, A.; Mahlknecht, J.; Sillanpää, M.; Ornelas-Soto, N. Removal of pharmaceutically active compounds (PhACs) and bacteria inactivation from urban wastewater effluents by UVA-LED photocatalysis with Gd3+ doped BiVO4. J. Environ. Chem. Eng. 2020, 8, 104540. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Joudaki, S.; Banisharif, F.; Eskandari, Z.; Cho, J.; Moghadam, G.; Rezania, S. Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles. Int. J. Environ. Res. Public Health 2020, 17, 1758. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Soriano-Molina, P.; Abeledo-Lameiro, M.J.; de la Obra, I.; Proto, A.; Polo-López, M.I.; Rizzo, L. Neutral (Fe3+-NTA) and acidic (Fe2+) pH solar photo-Fenton Vs chlorination: Effective urban wastewater disinfection does not mean control of antibiotic resistance. J. Environ. Chem. Eng. 2022, 10, 108777. [Google Scholar] [CrossRef]
- Ghari, T.; Kobarfard, F.; Mortazavi, S.A. Development of a Simple RP-HPLC-UV Method for Determination of Azithromycin in Bulk and Pharmaceutical Dosage forms as an Alternative to the USP Method. Iran. J. Pharm. Res. 2013, 12, 57–63. [Google Scholar] [PubMed]

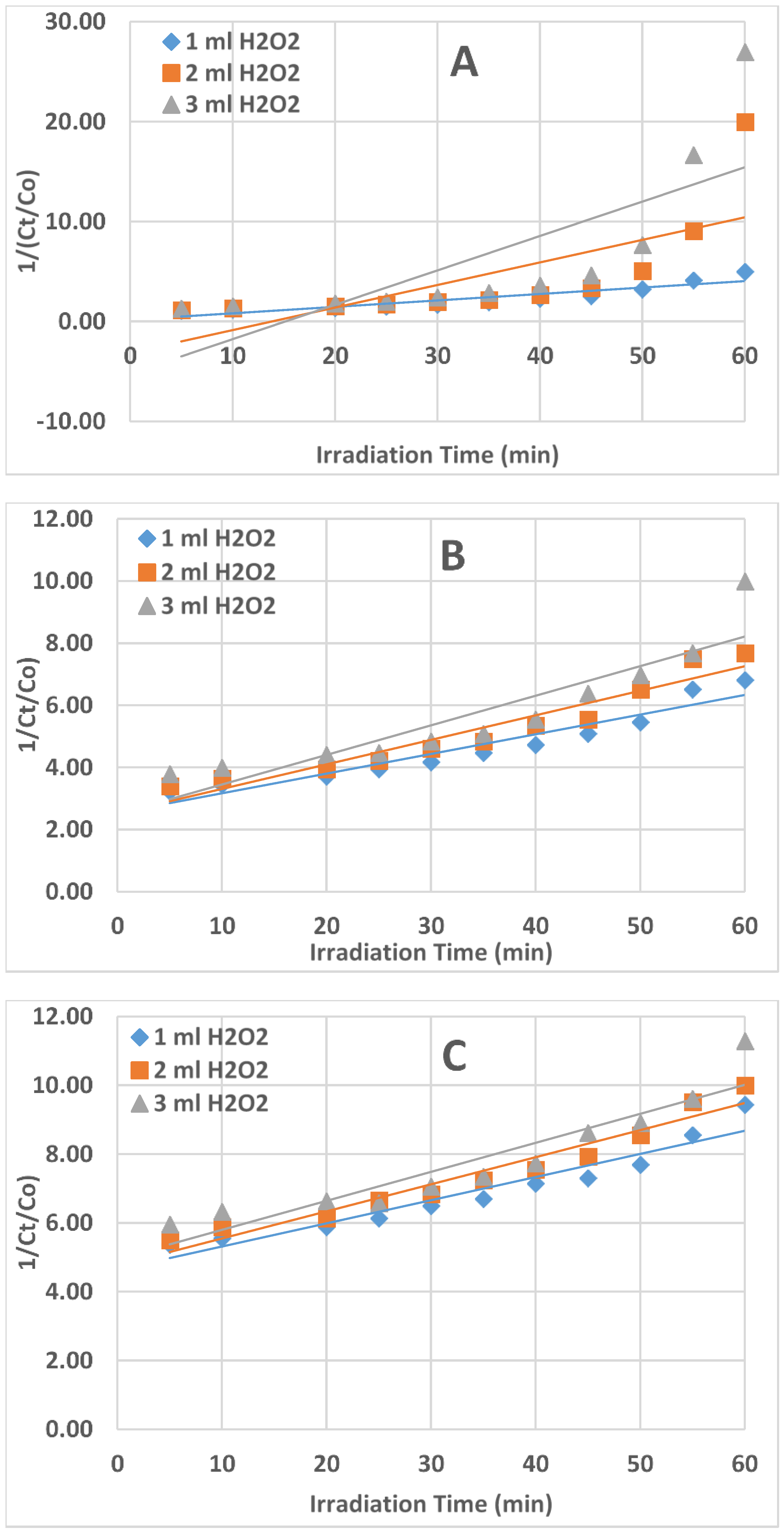
| Zero-Order Kinetics (Ct/Co) vs. t | First-Order Kinetics Ln (Ct/Co) vs. t | Second-Order Kinetics 1/(Ct/Co) vs. t | ||||
|---|---|---|---|---|---|---|
| 1 ppm AZT solution (50 mL) | R2 | k, min−1 | R2 | k, min−1 | R2 | k, min−1 |
| 1 mL 30% H2O2 | 0.9968 | 0.0130 | 0.9555 | 0.0269 | 0.8403 | 0.0643 |
| 2 mL 30% H2O2 | 0.9912 | 0.0142 | 0.8293 | 0.0442 | 0.5231 | 0.2255 |
| 3 mL 30% H2O2 | 0.9974 | 0.0134 | 0.8387 | 0.0532 | 0.5816 | 0.3440 |
| 3 ppm AZT solution (50 mL) | R2 | k, min−1 | R2 | k, min−1 | R2 | k, min−1 |
| 1 mL 30% H2O2 | 0.9925 | 0.0089 | 0.9670 | 0.0134 | 0.9188 | 0.0633 |
| 2 mL 30% H2O2 | 0.9923 | 0.0092 | 0.9697 | 0.0152 | 0.9229 | 0.0792 |
| 3 mL 30% H2O2 | 0.9760 | 0.0085 | 0.9207 | 0.0160 | 0.8311 | 0.0952 |
| 5 ppm AZT solution (50 mL) | R2 | k, min−1 | R2 | k, min−1 | R2 | k, min−1 |
| 1 mL 30% H2O2 | 0.9848 | 0.0070 | 0.9599 | 0.0325 | 0.9208 | 0.0673 |
| 2 mL 30% H2O2 | 0.9908 | 0.0073 | 0.9755 | 0.0281 | 0.9451 | 0.0787 |
| 3 mL 30% H2O2 | 0.9576 | 0.0067 | 0.9220 | 0.0210 | 0.8717 | 0.0844 |
| No. | Catalyst | Process Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|
| 1. | 10% Cu2O/TiO2 nanotubes, 1.5 g/L | Visible light irradiation, AZT 100 μg/mL, pH 7 | 100% in 1.5 h | [11] |
| 2. | PAC/Fe/Ag/Zn, 0.04 g/L | UV irradiation, pH 9, AZT 10 mg/L, pseudo-first-order kinetic | 99% in 2 h | [13] |
| 3. | Ag@Bi4O5I2/SPION@calcium alginate | Visible light (300 W Xe lamp), AZT 10 mg/L, 0.3 mg/mL | 98% in 1.5 h | [16] |
| 4. | K2S2O8 5.0 to 80.0 mg/L | Simulated solar irradiation 30 min (1.5 KW xenon lamp, 290–800 nm), 50.0 mL solution, AZT 1.0 mg/L, pH 5 | 70% in 2 h | [17] |
| 5. | SrTiO3, 30 mg | UV irradiation, AZT 20 mg/L, pH 12, pseudo-first-order reaction kinetics | 99% in 4 h | [18] |
| 6. | (a) MoS2 (b) MoS2-GO | Visible light irradiation, AZT 100 mg/L | (a) 75% in 3 h (b) 87% in 3 h | [19] |
| 7. | TiO2 P25, 227 ng/L (UV), 250 ng/L (solar) | UV irradiation 55 Wm−2, Solar 33 W m−2, domestic wastewater | 52% (UV), 87% (solar) | [20] |
| 8. | GO@Fe3O4/ZnO/SnO2, 1 g/L, | UV-C Irradiation, AZT 30 mg/L, pH 3 | 90% in 2 h | [21] |
| 9. | ZrO2/Ag@TiO2 | Visible light irradiation, AZT 20 mg/L (50 mL solution) | 90% in 8 h | [23] |
| 10. | La-TiO2/active carbon | UV irradiation, pH 4, AZT 10 ppm | 96% in 1.5 h | [25] |
| 11. | Fe (III)-oxalate | UV irradiation, AZT 10 mg/L, pH 4, | 83% in 2 h | [26] |
| 12. | 35% H2O2, 482.0 ppm as oxidant | Simulated sunlight irradiation 500 W/m2, AZT 1 ppm (50 mL solution), pH 9 | 100% in 2 h | [27] |
| 13. | No catalyst, only UV irradiation, | Xenon arc lamp (power 500 watts, UV power: 765 W/m2, Wavelength 300–800 nm), AZT 1.0 μg/L, pH 7, Temp. 40 °C | 90% in 7 days | [30] |
| 14. | 7.5 mg/L FeSO4 + 27.5 mg/L H2O2 | Simulated solar irradiation, UV power 50 mW/cm2, Wavelength 290–800 nm, AZT 3 mg/L, pH 3 | 92% in 0.5 h | [31] |
| 15. | Gd3+ doped BiVO4, catalyst 2 g/L | UV-LED irradiation, Wavelength 370 nm, power 4.65 mW/cm2, domestic wastewater | 63% in 3 h | [32] |
| 16. | No catalyst, only UV irradiation, | UV irradiation power 163 mW/cm2, AZT 110 mg/L, pH 7. Temp. 65 °C | 73% in 0.8 h | [33] |
| 17. | Photo-Fenton catalyst, Fe2+, 20 mg/L, H2O2 50 mg/L | Solar UV power 2.65 mW/cm2, Reactor volume 2 × 15 L, AZT 25 ng/L, pH 7 | 24% in 3 h | [34] |
| 18. | 3 mL of 30% H2O2 solution | UV irradiation intensity 50 mW/cm2, AZT 1.0 ppm (50 mL solution), pH 9.0, zero-order reaction kinetics | 100% in 1 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouli, N.I. Photodegradation of a Broad-Spectrum Antibiotic Azithromycin Using H2O2 under Ultraviolet Irradiation. Int. J. Mol. Sci. 2024, 25, 6702. https://doi.org/10.3390/ijms25126702
Zouli NI. Photodegradation of a Broad-Spectrum Antibiotic Azithromycin Using H2O2 under Ultraviolet Irradiation. International Journal of Molecular Sciences. 2024; 25(12):6702. https://doi.org/10.3390/ijms25126702
Chicago/Turabian StyleZouli, Nasser Ibrahim. 2024. "Photodegradation of a Broad-Spectrum Antibiotic Azithromycin Using H2O2 under Ultraviolet Irradiation" International Journal of Molecular Sciences 25, no. 12: 6702. https://doi.org/10.3390/ijms25126702
APA StyleZouli, N. I. (2024). Photodegradation of a Broad-Spectrum Antibiotic Azithromycin Using H2O2 under Ultraviolet Irradiation. International Journal of Molecular Sciences, 25(12), 6702. https://doi.org/10.3390/ijms25126702




