Combined Proteomic and Metabolomic Analysis Reveals Comprehensive Regulation of Somatostatin DNA Vaccine in Goats
Abstract
1. Introduction
2. Results
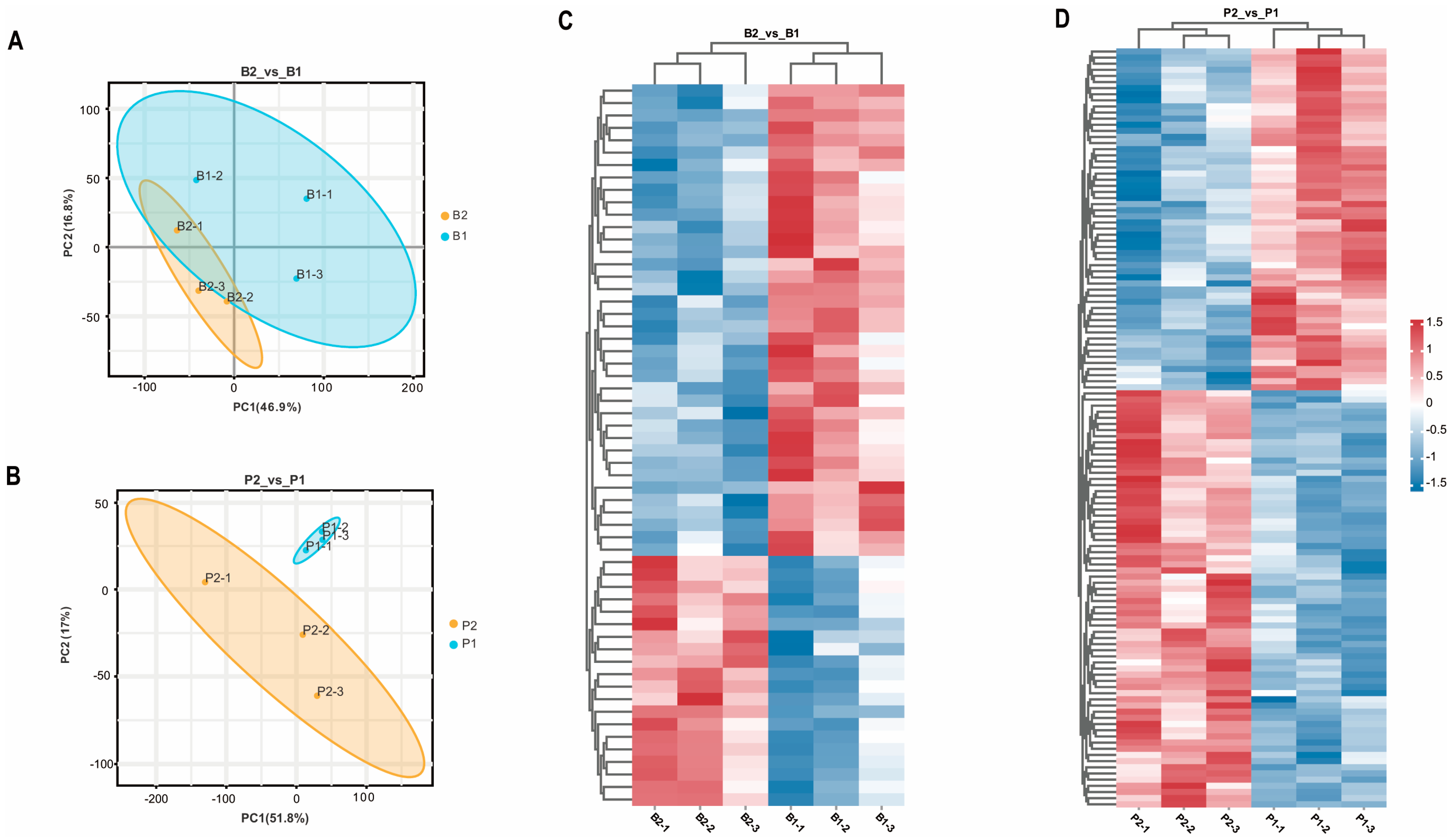
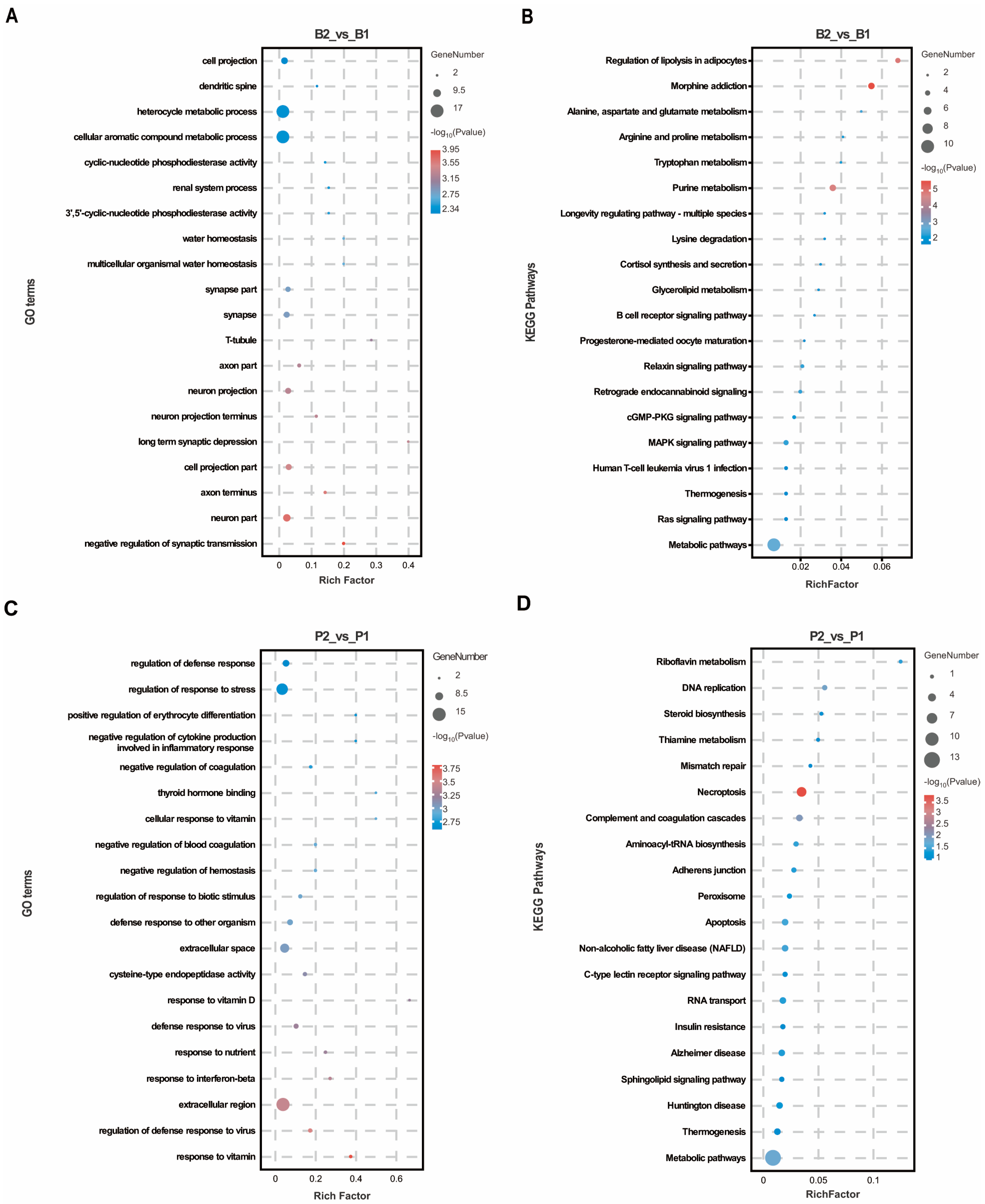
2.1. Effect of SS Vaccine Treatment on the Hypothalamic–Pituitary Proteome of Wethers
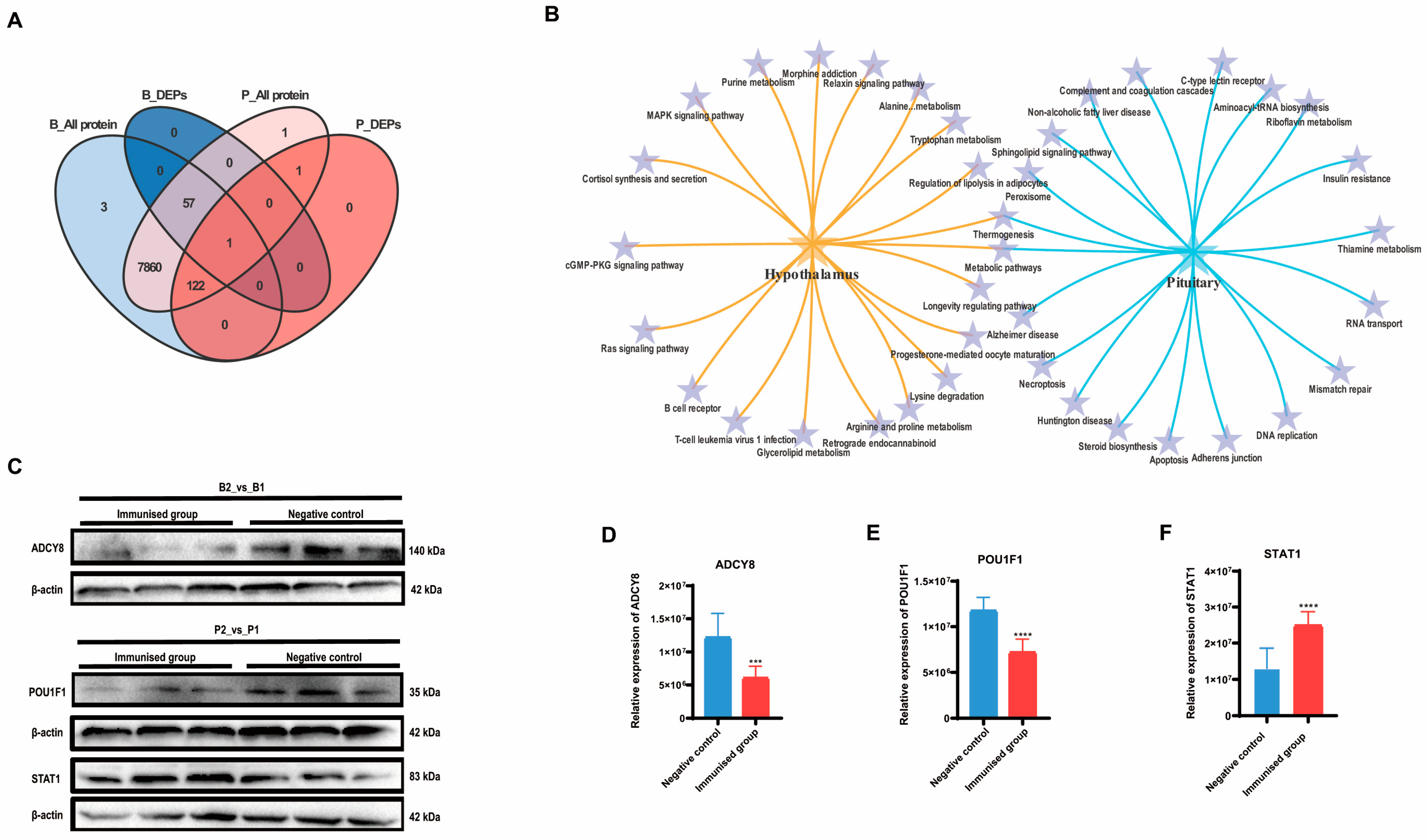
2.2. Integrated Metabolomics and Proteomics Analysis of Regulatory Networks in SS Vaccine-Treated Wethers
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Animals and Immunization
4.3. Proteomic Analysis
4.4. Metabolomic Analysis
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todini, L. Thyroid hormones in small ruminants: Effects of endogenous environmental and nutritional factors. Animal 2007, 1, 997–1008. [Google Scholar] [CrossRef]
- Devesa, J. The Complex World of Regulation of Pituitary Growth Hormone Secretion: The Role of Ghrelin, Klotho, and Nesfatins in It. Front. Endocrinol. 2021, 12, 636403. [Google Scholar] [CrossRef]
- Han, Y.; Na, R.; Jiang, X.; Wu, J.; Han, Y.; Zeng, Y.; Guangxin, E.; Liang, A.; Yang, L.; Zhao, Y.; et al. Effect of a novel somatostatin-14 DNA vaccine fused to tPA signal peptide and CpG adjuvant on goat lactation and milk composition. Small Rumin. Res. 2020, 187, 106107. [Google Scholar] [CrossRef]
- Meng, J.Z.; Li, Q.Y.; Xiao, L.L.; Liu, W.C.; Gao, Z.J.; Gong, L.; Lan, X.Y.; Wang, S.L. Immunization against inhibin DNA vaccine as an alternative therapeutic for improving follicle development and reproductive performance in beef cattle. Front. Endocrinol. 2024, 14, 1275022. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Zeng, F.M.; Jiang, X.P.; Liu, G.Q.; Kasimu, H.; Ling, S.; Girmay, S. Effect of Kisspeptin-54 immunization on performance, carcass characteristics, meat quality and safety of Yiling goats. Meat Sci. 2020, 166, 108139. [Google Scholar] [CrossRef] [PubMed]
- Arosio, M.; Ronchi, C.L.; Gebbia, C.; Cappiello, V.; Beck-Peccoz, P.; Peracchi, M. Stimulatory Effects of Ghrelin on Circulating Somatostatin and Pancreatic Polypeptide Levels. J. Clin. Endocrinol. Metab. 2003, 88, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Peng, X.L.; Li, K.; Zhao, Y.H.T.; Jiang, X.P.; Guang-Xin, E.; Zhao, Y.J.; Yea, J.H.; Xu, L.; Zhao, Q.T.; et al. Evaluation of the Fusion Type CpG Adjuvant for the Enhancement of Somatostatin DNA Vaccine in Ram Lambs. Pak. J. Zool. 2019, 51, 413–419. [Google Scholar] [CrossRef]
- Eigler, T.; Ben-Shlomo, A. Somatostatin system: Molecular mechanisms regulating anterior pituitary hormones. J. Mol. Endocrinol. 2014, 53, R1–R19. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Catalano, R.; Mangili, F.; Locatelli, M.; Lania, A.G.; Arosio, M.; Spada, A.; Mantovani, G. A novel pathway activated by somatostatin receptor type 2 (SST2): Inhibition of pituitary tumor cell migration and invasion through cytoskeleton protein recruitment. Int. J. Cancer 2018, 142, 1842–1852. [Google Scholar] [CrossRef]
- Parandeh, F.; Amisten, S.; Verma, G.; Mohammed Al-Amily, I.; Dunér, P.; Salehi, A. Inhibitory effect of UDP-glucose on cAMP generation and insulin secretion. J. Biol. Chem. 2020, 295, 15245–15252. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Vitali, E.; Treppiedi, D.; Lania, A.G.; Mantovani, G. Filamin A in somatostatin and dopamine receptor regulation in pituitary and the role of cAMP/PKA dependent phosphorylation. Horm. Metab. Res. 2014, 46, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Rhie, D.J.; Yi, S.Y.; Hahn, S.J.; Sim, S.S.; Jo, Y.H.; Kim, M.S. Somatostatin potentiates voltage-dependent K+ and Ca2+ channel expression induced by nerve growth factor in PC12 cells. Dev. Brain Res. 1999, 112, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yuan, T.; Qi, R.; He, J.; Fu, Y.; Niu, D.; Li, W. Effect of immunization with a recombinant cholera toxin B subunit/somatostatin fusion protein on immune response and growth hormone levels in mice. Biotechnol. Lett. 2012, 34, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liang, A.; Han, L.; Guo, A.; Jiang, X.; Yang, L. Efficacy and safety of an oral somatostatin DNA vaccine without antibiotic resistance gene in promoting growth of piglets. Scand. J. Immunol. 2014, 79, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zu, Z.; Riaz, H.; Dan, X.; Yu, X.; Liu, S.; Guo, A.; Wen, Y.; Liang, A.; Yang, L. Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice. Animals 2022, 12, 1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H.B. Ca2+-stimulated ADCY1 and ADCY8 regulate distinct aspects of synaptic and cognitive flexibility. Front. Cell. Neurosci. 2023, 17, 1215255. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, A.; Liu, N.A.; Melmed, S. Somatostatin and dopamine receptor regulation of pituitary somatotroph adenomas. Pituitary 2017, 20, 93–99. [Google Scholar] [CrossRef]
- Varco-Merth, B.; Rotwein, P. Differential effects of STAT proteins on growth hormone-mediated IGF-I gene expression. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E847–E855. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Jensen, E.A.; Young, J.A.; Mathes, S.C.; List, E.O.; Carroll, R.K.; Kuhn, J.; Onusko, M.; Kopchick, J.J.; Murphy, E.R.; Berryman, D.E. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: A new frontier for microbial endocrinology. Growth Horm. IGF Res. 2020, 53–54, 101333. [Google Scholar] [CrossRef]
- Terry, N.A.; Ngaba, L.V.; Wilkins, B.J.; Pi, D.; Gheewala, N.; Kaestner, K.H. Lipid malabsorption from altered hormonal signaling changes early gut microbial responses. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G580–G591. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yu, Y.Y.; Cluette-Brown, J.; Martin, C.R.; Claud, E.C. Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci. Rep. 2018, 8, 5443. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yasui, M.; Sano, M.; Fukuwatari, T. Fluorometric Determination of 2-Oxoadipic Acid, a Common Metabolite of Tryptophan and Lysine, by High-Performance Liquid Chromatography with Pre-Chemical Derivatization. Biosci. Biotechnol. Biochem. 2011, 75, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Tang, X.; Fan, C.; Wang, L.; Shen, W.; Ren, S.E.; Zhang, L.; Zhang, Y. Gastrointestinal Tract and Dietary Fiber Driven Alterations of Gut Microbiota and Metabolites in Durco x Bamei Crossbred Pigs. Front. Nutr. 2022, 8, 806646. [Google Scholar] [CrossRef]
- Aguilera-Romero, A.; Gehin, C.; Riezman, H. Sphingolipid homeostasis in the web of metabolic routes. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Markaki, I.; Bergstrom, S.; Tsitsi, P.; Remnestal, J.; Manberg, A.; Hertz, E.; Paslawski, W.; Sorjonen, K.; Uhlen, M.; Mangone, G.; et al. Cerebrospinal Fluid Levels of Kininogen-1 Indicate Early Cognitive Impairment in Parkinson’s Disease. Mov. Disord. 2020, 35, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, S.Y.; Bae, Y.S. Functional roles of sphingolipids in immunity and their implication in disease. Exp. Mol. Med. 2023, 55, 1110–1130. [Google Scholar] [CrossRef]
- Hanson, A.; Poudyal, D.; Hagemeister, A.; Reindl, K.M.; Sheridan, M.A. The ERK and PI3K signaling pathways mediate inhibition of insulin-like growth factor-1 receptor mRNA expression by somatostatin. Mol. Cell. Endocrinol. 2010, 315, 57–62. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.; Zhang, L.; Guo, J.; Fang, S.; E, G.; Zeng, Y.; Huang, Y.; Han, Y. Combined Proteomic and Metabolomic Analysis Reveals Comprehensive Regulation of Somatostatin DNA Vaccine in Goats. Int. J. Mol. Sci. 2024, 25, 6888. https://doi.org/10.3390/ijms25136888
Qin G, Zhang L, Guo J, Fang S, E G, Zeng Y, Huang Y, Han Y. Combined Proteomic and Metabolomic Analysis Reveals Comprehensive Regulation of Somatostatin DNA Vaccine in Goats. International Journal of Molecular Sciences. 2024; 25(13):6888. https://doi.org/10.3390/ijms25136888
Chicago/Turabian StyleQin, Ge, Li Zhang, Jiaxue Guo, Shiyong Fang, Guangxin E, Yan Zeng, Yongfu Huang, and Yanguo Han. 2024. "Combined Proteomic and Metabolomic Analysis Reveals Comprehensive Regulation of Somatostatin DNA Vaccine in Goats" International Journal of Molecular Sciences 25, no. 13: 6888. https://doi.org/10.3390/ijms25136888
APA StyleQin, G., Zhang, L., Guo, J., Fang, S., E, G., Zeng, Y., Huang, Y., & Han, Y. (2024). Combined Proteomic and Metabolomic Analysis Reveals Comprehensive Regulation of Somatostatin DNA Vaccine in Goats. International Journal of Molecular Sciences, 25(13), 6888. https://doi.org/10.3390/ijms25136888






