The Roles of the Anthraquinone Parietin in the Tolerance to Desiccation of the Lichen Xanthoria parietina: Physiology and Anatomy of the Pale and Bright-Orange Thalli
Abstract
1. Introduction
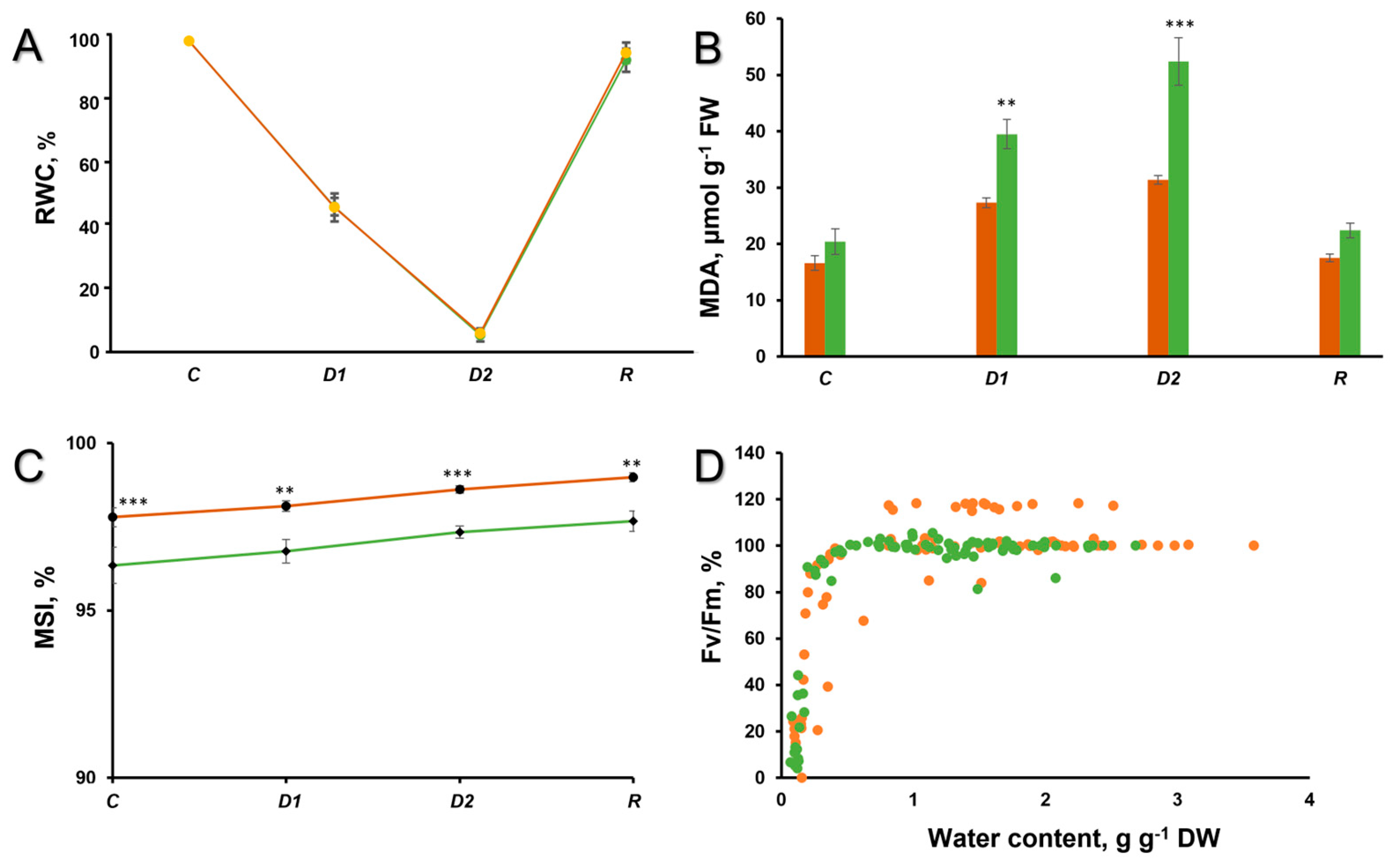
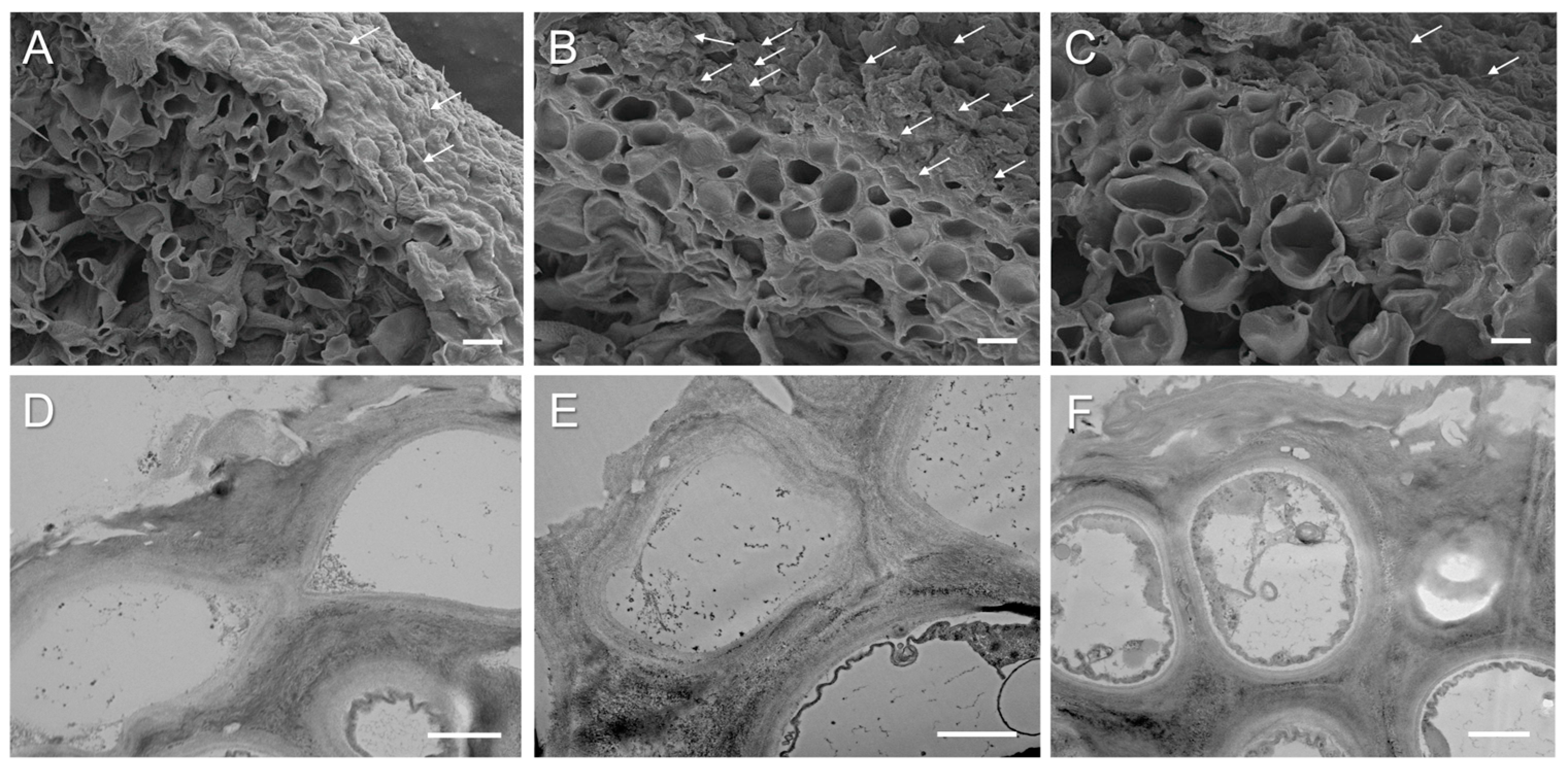
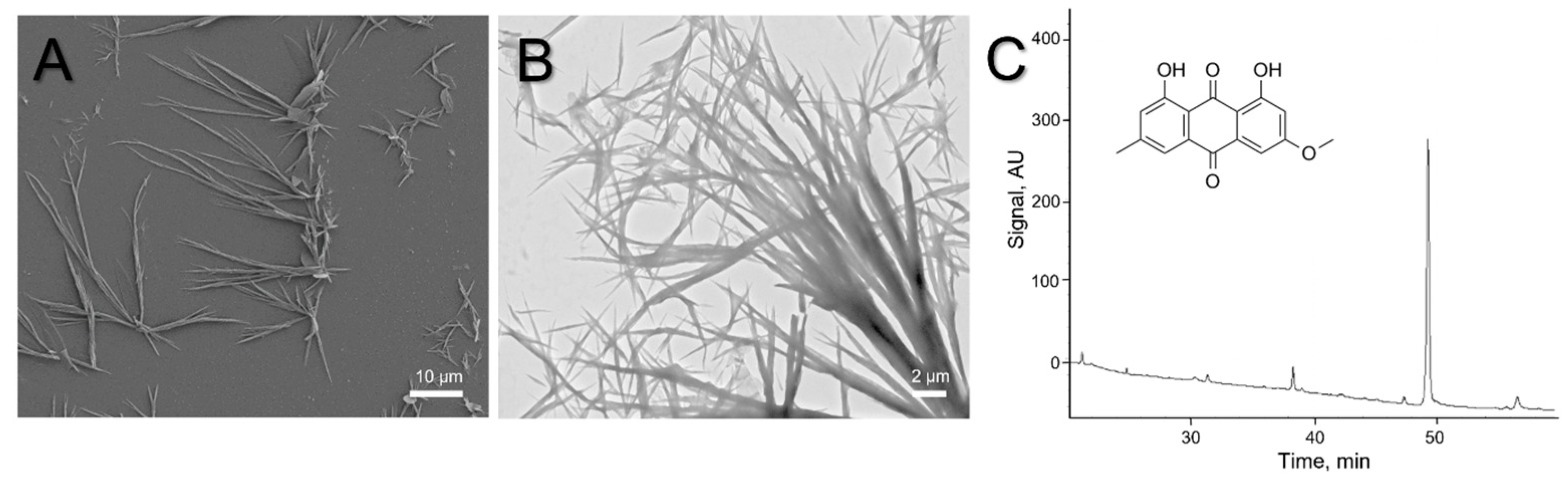
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Chlorophyll Fluorescence
3.3. Lipid Peroxidation and Membrane Stability Index
3.4. Light, Scanning (SEM), and Transmission Electron Microscopy (TEM)
3.5. High-Performance Liquid Chromatography
3.6. Thermogravimetry/Differential Scanning Calorimetry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honegger, R. Functional aspects of the lichen symbiosis. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 553–578. [Google Scholar] [CrossRef]
- Sanders, W.B. Composite lichen thalli of Stricta sp. from Brazil with morphologically similar lobes containing either chlorobiont or a cyanobiont layer. Symbiosis 2001, 31, 47–55. [Google Scholar]
- Henskens, F.L.; Green, T.G.A.; Wilkins, A. Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Ann. Bot. 2012, 110, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.P.; Hochman, A.; Nash, T.H. Desiccation tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Grube, M. Die Hard: Lichens. In Symbioses and Stress. Cellular Origin, Life in Extreme Habitats and Astrobiology, 17th ed.; Seckbach, J., Grube, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 509–523. [Google Scholar]
- Meeßen, J.; Sánchez, F.J.; Brandt, A.; Balzer, E.M.; de la Torre, R.; Sancho, L.G.; de Vera, J.P.; Ott, S. Extremotolerance and resistance of lichens: Comparative studies on five species used in astrobiological research I. Morphological and anatomical characteristics. Orig. Life Evol. Biosph. 2013, 43, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Meeßen, J.; Sánchez, F.J.; Sadowsky, A.; de la Torre, R.; Ott, S.; de Vera, J.P. Extremotolerance and resistance of lichens: Comparative studies on five species used in astrobiological research II. Secondary Lichen Compounds. Orig. Life Evol. Biosph. 2013, 43, 501–526. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C. J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005, 3, 273–287. [Google Scholar] [CrossRef]
- Basile, A.; Rigano, D.; Loppi, S.; Di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; De Ruberto, F.; Molinari, A.M.; et al. Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin. Int. J. Mol. Sci. 2015, 16, 7861–7875. [Google Scholar] [CrossRef] [PubMed]
- Łaska, G.; Kiercul, S.; Piotrowska-Niczyporuk, A.; Jacob, M.; Pasco, D. Secondary metabolites isolated from Xanthoria parietina (L.) Th. Fr. lichen and their biological activity. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Choi, G.J.; Lee, S.W.; Jang, K.S.; Kim, J.S.; Cho, K.Y.; Kim, J.C. Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews. Crop Protect. 2004, 23, 1215–1221. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Gutberlet, B.; Preis, E.; Abdelsalam, A.M.; Abu Dayyih, A.; Abdelkader, A.; Bakowsky, U. Parietin cyclodextrin-inclusion complex as an effective formulation for bacterial photoinactivation. Pharmaceutics 2022, 14, 357. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar]
- Pang, M.J.; Yang, Z.; Zhang, X.L.; Liu, Z.F.; Fan, J.; Zhang, H.Y. Physcion, a naturally occurring anthraquinone derivative, induces apoptosis and autophagy in human nasopharyngeal carcinoma. Acta Pharmacol. Sin. 2016, 37, 1623–1640. [Google Scholar] [CrossRef]
- Honegger, R.; Peter, M.; Scherrer, S. Drought induced structural alterations at the mycobiont-photobiont interface in a range of foliose macrolichens. Protoplasma 1996, 190, 221–232. [Google Scholar] [CrossRef]
- Fortuna, L.; Tretiach, M. Effects of site-specific climatic conditions on the radial growth of the lichen biomonitor Xanthoria parietina. Environ. Sci. Pollut. Res. 2018, 25, 34017–34026. [Google Scholar] [CrossRef]
- Lindblom, L.; Ekman, S. New evidence corroborates population differentiation in Xanthoria parietina. Lichenologist 2007, 39, 259–271. [Google Scholar] [CrossRef]
- Beck, A.; Mayr, C. Nitrogen and carbon isotope variability in the green-algal lichen Xanthoria parietina and their implications on mycobiont-photobiont interactions. Ecol. Evol. 2012, 2, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; McEvoy, M. Seasonal changes in solar radiation drive acclimation of the sun-screening compound parietin in the lichen Xanthoria parietina. Basic Appl. Ecol. 2005, 6, 75–82. [Google Scholar] [CrossRef]
- Armstrong, R.; Bradwell, T. Growth of crustose lichens: A review. Geogr. Ann. A 2010, 92, 3–17. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Bradwell, T. Growth of foliose lichens: A review. Symbiosis 2011, 53, 1–16. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Pellegrini, E.; Candotto Carniel, F.; Incerti, G.; Lorenzini, G.; Nali, C.; Tretiach, M. Ozone and desiccation tolerance in chlorolichens are intimately connected: A case study based on two species with different ecology. Environ. Sci. Pollut. Res. 2018, 25, 8089–8103. [Google Scholar] [CrossRef]
- Cecconi, E.; Fortuna, L.; Benesperi, R.; Bianchi, E.; Brunialti, G.; Contardo, T.; Di Nuzzo, L.; Frati, L.; Monaci, F.; Munzi, S.; et al. New interpretative scales for lichen bioaccumulation data: The Italian proposal. Atmosphere 2019, 10, 136. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y. Secondary lichen compounds as protection against excess solar radiation and herbivores. Prog. Botan. 2012, 73, 283–304. [Google Scholar]
- Kalinowski, R.; Bačkor, M.; Pawlik-Skowrońska, B. Parietin in the tolerant lichen Xanthoria parietina (L.) Th. Fr. increases protection of Trebouxia photobionts from cadmium excess. Ecol. Indicat. 2015, 58, 132–138. [Google Scholar] [CrossRef]
- Vannini, A.; Paoli, L.; Ceccarelli, S.; Sorbo, S.; Basile, A.; Carginale, V.; Nali, C.; Lorenzini, G.; Pica, M.; Loppi, S. Physiological and ultrastructural effects of acute ozone fumigation in the lichen Xanthoria parietina: The role of parietin and hydration state. Environ. Sci. Pollut. Res. 2018, 25, 8104–8112. [Google Scholar] [CrossRef] [PubMed]
- Souza-Egipsy, V.; Valladares, F.; Ascaso, C. Water distribution in foliose lichen species: Interactions between method of hydration, lichen substances and thallus anatomy. Ann. Botan. 2000, 86, 595–601. [Google Scholar] [CrossRef][Green Version]
- Lorenz, C.; Bianchi, E.; Alberini, A.; Poggiali, G.; Benespe, R.; Papini, A.; Brucato, J.R. UV photo-degradation of the secondary lichen substance parietin: A multi-spectroscopic analysis in astrobiology perspective. Life Sci. Space Res. 2024, 41, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen metabolites: An overview of some secondary metabolites and their biological potential. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 175–209. [Google Scholar]
- Solhaug, K.A.; Gauslaa, Y.; Nybakken, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Ustvedt, E.M. Is parietin a UV-B or a bluelight screening pigment in the lichen Xanthoria parietina? Photochem. Photobiol. 2003, 2, 424–432. [Google Scholar]
- Ndhlovu, N.T.; Minibayeva, F.; Beckett, R.P. A role for secondary metabolites in desiccation tolerance in lichens. Microbiol. Res. 2024, 15, 225–235. [Google Scholar] [CrossRef]
- Kosanić, M.; Monojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Evaluation of the antioxidant capacities and cytotoxic effects of ten parmeliaceae lichen species. Evid.-Based Compl. Alter. Med. 2016, 2016, 3169751. [Google Scholar]
- Hidalgo, M.E.; Fernández, E.; Quilhot, W.; Lissi, E.A. Antioxidant capacity of depsides and depsidones. Phytochemistry 1994, 37, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Gauslaa, Y. Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ. 2004, 27, 167–176. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I.; Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 11–123. [Google Scholar]
- Pichler, G.; Grube, M.; Muggia, L.; Carniel, C.F.; Kranner, I. How to build a lichen: From metabolite release to symbiotic interplay. New Phytol. 2023, 238, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Baumann, K.; Emrich, D.; Schermer, M.; Eckhardt, K.U.; Jandl, G.; Lakatos, M. The dark side of orange: Multiorganismic continuum dynamics within a lichen of the Atacama Desert. Mycologia 2024, 116, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Mugas, M.L.; Calvo, G.; Marioni, J.; Céspedes, M.; Martinez, F.; Sáenz, D.; Casas, A. Photodynamic therapy of tumour cells mediated by the natural anthraquinone parietin and blue light. J. Photochem. Photobiol. B Biol. 2021, 214, 112089. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of hemicellulose, cellulose, and lignin content in different types of biomasses by thermogravimetric analysis and pseudocomponent kinetic model (TGA-PKM method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y. Acetone rinsing-a method for testing ecological and physiological roles of secondary compounds in living lichens. Symbiosis 2001, 30, 301–315. [Google Scholar]
- Solhaug, K.A.; Larsson, P.; Gauslaa, Y. Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 2010, 231, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.C.F.; Nagy, Z.; Csintalan, Z.; Takacs, Z. Water-content components in bryophytes: Analysis of pressure–volume relationships. J. Exp. Bot. 1998, 49, 1845–1854. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, N.R. Changes in Lipid Peroxidation and Lipolytic and Free-Radical Scavenging Enzyme Activities during Aging and Sprouting of Potato (Solanum tuberosum) Seed-Tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.E.; Zammit, R.; Scott, P.; Nyamongo, D.O.; Daws, M.I.; Kranner, I. Glutathione half-cell reduction potential as a seed viability marker of the potential oilseed crop Vernonia galamensis. Indust. Crop. Prod. 2010, 32, 687–691. [Google Scholar] [CrossRef]
- Daminova, A.G.; Rogov, A.M.; Rassabina, A.E.; Beckett, R.P.; Minibayeva, F.V. Effect of melanization on thallus microstructure in the lichen Lobaria pulmonaria. J. Fungi 2022, 8, 791. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Le Pogam, P.; Herbette, G.; Boustie, J. Analysis of Lichen Metabolites, a Variety of Approaches. In Recent Advances in Lichenology; Upreti, D., Divakar, P., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 229–261. [Google Scholar]

| Thallus | Treatment | ES Yield, mg g−1 DM | Parietin Content, mg g−1 DM |
|---|---|---|---|
| Pale | C | 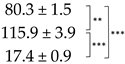 | 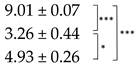 |
| D2 | |||
| R | |||
| Bright-Orange | C | 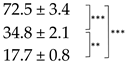 | 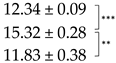 |
| D2 R |
| Thallus | Stages of Mass Loss | Residual Mass, % | |
|---|---|---|---|
| I | II | ||
| TG, %/DTG, °C | TG, %/DTG, °C | ||
| Pale | 5.9/83.4 | 61.6/312.5 | 32.5 |
| Bright-orange | 6.6/82.0 | 69.8/315.7 | 23.6 |
| Acetone-rinsed | 4.8/85.2 | 65.0/313.9 | 30.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daminova, A.G.; Leksin, I.Y.; Khabibrakhmanova, V.R.; Gurjanov, O.P.; Galeeva, E.I.; Trifonova, T.V.; Khamatgalimov, A.R.; Beckett, R.P.; Minibayeva, F.V. The Roles of the Anthraquinone Parietin in the Tolerance to Desiccation of the Lichen Xanthoria parietina: Physiology and Anatomy of the Pale and Bright-Orange Thalli. Int. J. Mol. Sci. 2024, 25, 7067. https://doi.org/10.3390/ijms25137067
Daminova AG, Leksin IY, Khabibrakhmanova VR, Gurjanov OP, Galeeva EI, Trifonova TV, Khamatgalimov AR, Beckett RP, Minibayeva FV. The Roles of the Anthraquinone Parietin in the Tolerance to Desiccation of the Lichen Xanthoria parietina: Physiology and Anatomy of the Pale and Bright-Orange Thalli. International Journal of Molecular Sciences. 2024; 25(13):7067. https://doi.org/10.3390/ijms25137067
Chicago/Turabian StyleDaminova, Amina G., Ilya Y. Leksin, Venera R. Khabibrakhmanova, Oleg P. Gurjanov, Ekaterina I. Galeeva, Tatyana V. Trifonova, Ayrat R. Khamatgalimov, Richard P. Beckett, and Farida V. Minibayeva. 2024. "The Roles of the Anthraquinone Parietin in the Tolerance to Desiccation of the Lichen Xanthoria parietina: Physiology and Anatomy of the Pale and Bright-Orange Thalli" International Journal of Molecular Sciences 25, no. 13: 7067. https://doi.org/10.3390/ijms25137067
APA StyleDaminova, A. G., Leksin, I. Y., Khabibrakhmanova, V. R., Gurjanov, O. P., Galeeva, E. I., Trifonova, T. V., Khamatgalimov, A. R., Beckett, R. P., & Minibayeva, F. V. (2024). The Roles of the Anthraquinone Parietin in the Tolerance to Desiccation of the Lichen Xanthoria parietina: Physiology and Anatomy of the Pale and Bright-Orange Thalli. International Journal of Molecular Sciences, 25(13), 7067. https://doi.org/10.3390/ijms25137067







