Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search for Publications for Publications with Data on Toxicological Aspects, Safety Assessment, and Green Toxicology of Gold Nanoparticles
2.2. Keywords and Selection of Scientific Data
2.3. Classification of Results
2.4. Presentation of the Results
- Toxicological Aspects of AuNPs: This section thoroughly examines AuNPs’ interactions with biological systems, covering their toxicological in vitro and in vivo impacts. It includes insights into cellular uptake, biodistribution, and toxicity mechanisms, such as oxidative and non-oxidative stress pathways. The analysis extends to the effects of AuNPs on immune cells, normal human cell lines, and organ-specific toxicity, offering a comprehensive view of their biological interactions and potential health risks.
- Safety Assessment of Gold Nanoparticles in Cosmetic Products: We assess the implications of using AuNPs in cosmetics and their safety. This segment also delves into regulatory frameworks and safety standards governing the use of nanoparticles in personal care products in the EU.
- Green Toxicology of Gold Nanoparticles: Dedicated to the environmental aspect, this section explores the integration of green chemistry principles into the synthesis and lifecycle of AuNPs. It focuses on reducing the environmental impact through sustainable practices, including biodegradability and recyclability, highlighting the importance of environmental stewardship in developing and applying nanotechnologies.
2.5. Uncertainties or Limitations of Review
3. Toxicological Aspects of Gold Nanoparticles
3.1. In Vitro Toxicology Studies on AuNPs
3.2. In Vivo Toxicology Studies of AuNPs
3.3. Toxicity of AuNPs against Immune Cells
3.4. Toxicity of AuNPs against Normal Human Cell Lines
3.4.1. Nervous System
3.4.2. Digestive System
3.4.3. Respiratory System
3.4.4. Cardio-Vascular System
3.4.5. Urinary System
3.4.6. Sensory Organs
3.4.7. Reproductive System
3.4.8. Human Cell Lines’ Toxicity Evaluation
3.5. Unfavourable Effects of AuNPs
3.6. Organ Toxicity of AuNPs
3.7. Size and Shape Impact of AuNPs on Toxicity
3.8. Toxicity Mechanisms
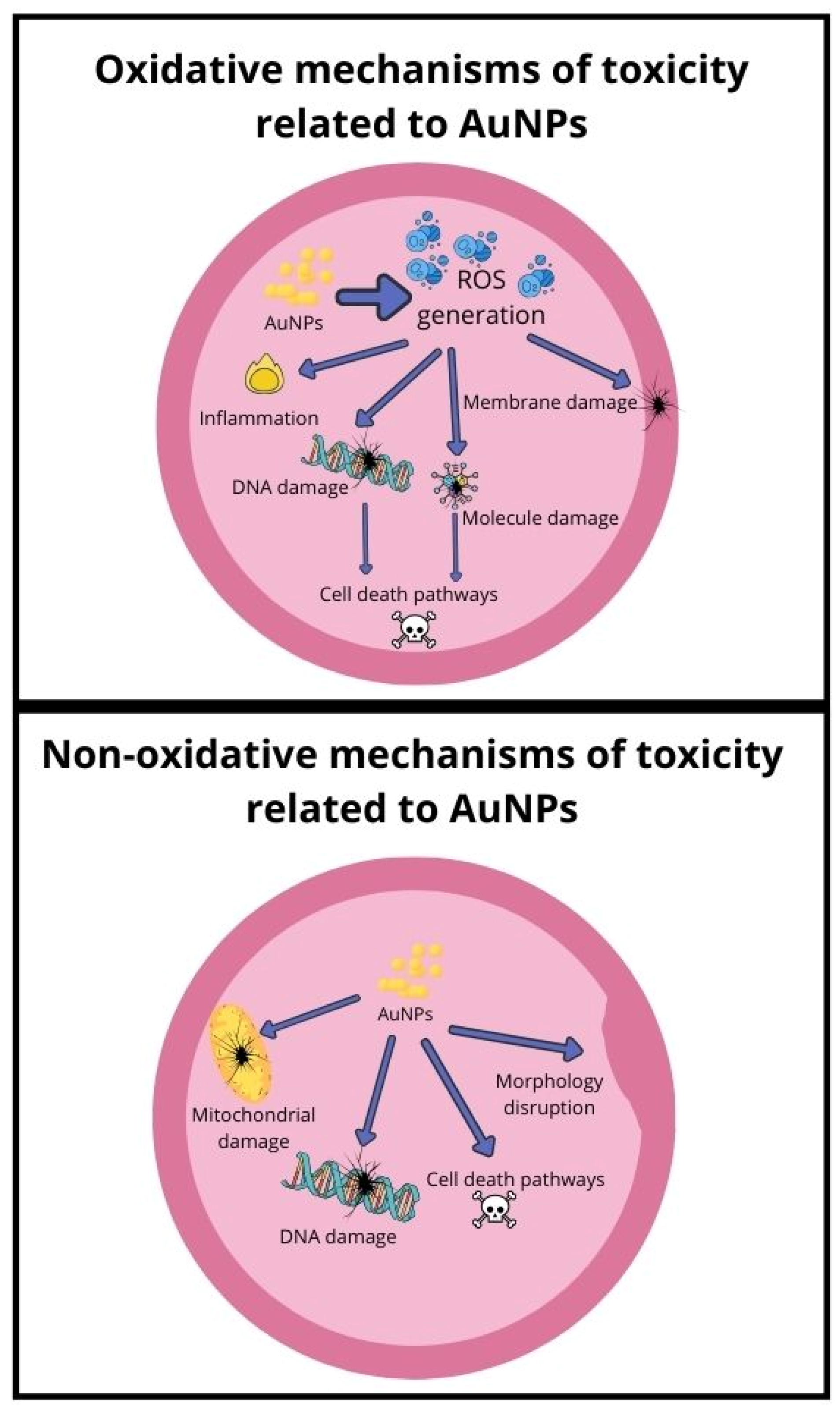
3.8.1. Mechanism Related to Oxidative Stress
3.8.2. Mechanism Related to Non-Oxidative Stress
4. Safety Assessment of Gold Nanoparticles in Cosmetic Products
5. Green Toxicology of Gold Nanoparticles
5.1. Green Synthesis of Gold Nanoparticles
5.2. Biotemplates Used for the Green Synthesis of Gold Nanoparticles
5.3. Applications of Green Synthesised Gold Nanoparticles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNPs | gold nanoparticles |
| GS | green synthesis |
| ROS | reactive oxygen species |
| GSH | glutathione |
| HESCs | human embryonic stem cells |
| CNS | central nervous system |
| PLT | platelet count |
| MPV | mean platelet volume |
| PCT | plateletcrit value, |
| PDW | platelet distribution width |
| PEG | polyethylene glycol |
| WBC | white blood cells |
| RBC | red blood cells |
References
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 Nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858. [Google Scholar] [CrossRef]
- Yen, H.-J.; Hsu, S.; Tsai, C.-L. Cytotoxicity and Immunological Response of Gold and Silver Nanoparticles of Different Sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Zaikova, T.; Hutchison, J.E.; Tanguay, R.L. Gold Nanoparticles Disrupt Zebrafish Eye Development and Pigmentation. Toxicol. Sci. 2013, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer Coated Gold Nanoparticles for Delivery Applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Král, P. Nanoparticles Self-Assembly within Lipid Bilayers. ACS Omega 2018, 3, 10631–10637. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.K.; Jittiwat, J.; Manikandan, J.; Ong, C.-N.; Yu, L.E.; Ong, W.-Y. Biodistribution of Gold Nanoparticles and Gene Expression Changes in the Liver and Spleen after Intravenous Administration in Rats. Biomaterials 2010, 31, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.-S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.-H. Size-Dependent Tissue Kinetics of PEG-Coated Gold Nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Wright, D.W. Size-Dependent Cellular Uptake of DNA Functionalized Gold Nanoparticles. Small 2016, 12, 5592–5600. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Carney, R.P.; Ikuma, K.; Stellacci, F.; Lau, B.L.T. Effects of Surface Compositional and Structural Heterogeneity on Nanoparticle–Protein Interactions: Different Protein Configurations. ACS Nano 2014, 8, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huo, S.; Mizuhara, T.; Das, R.; Lee, Y.-W.; Hou, S.; Moyano, D.F.; Duncan, B.; Liang, X.-J.; Rotello, V.M. The Interplay of Size and Surface Functionality on the Cellular Uptake of Sub-10 Nm Gold Nanoparticles. ACS Nano 2015, 9, 9986–9993. [Google Scholar] [CrossRef] [PubMed]
- Van Pomeren, M.; Peijnenburg, W.J.G.M.; Vlieg, R.C.; Van Noort, S.J.T.; Vijver, M.G. The Biodistribution and Immuno-Responses of Differently Shaped Non-Modified Gold Particles in Zebrafish Embryos. Nanotoxicology 2019, 13, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Tatovic, D.; McAteer, M.A.; Barry, J.; Barrientos, A.; Rodríguez Terradillos, K.; Perera, I.; Kochba, E.; Levin, Y.; Dul, M.; Coulman, S.A.; et al. Safety of the Use of Gold Nanoparticles Conjugated with Proinsulin Peptide and Administered by Hollow Microneedles as an Immunotherapy in Type 1 Diabetes. Immunother. Adv. 2022, 2, ltac002. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.F.; Hassabou, N.F. CD24-Gold Nanocomposite as Promising and Sensitive Biomarker for Cancer Stem Cells in Salivary Gland Tumors. Nanomed. Nanotechnol. Biol. Med. 2022, 46, 102598. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular Accumulation of Gold Nanoparticles Leads to Inhibition of Macropinocytosis to Reduce the Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 40493. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, G.; Galeone, A.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Ranking the in Vivo Toxicity of Nanomaterials in Drosophila Melanogaster. J. Nanoparticle Res. 2013, 15, 1936. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Mani, V.; Lou, B.-S.; Devasenathipathy, R.; Hou, Y.-S.; Elangovan, A. Green Synthesized Gold Nanoparticles Decorated Graphene Oxide for Sensitive Determination of Chloramphenicol in Milk, Powdered Milk, Honey and Eye Drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Maertens, A. Green Toxicology. ALTEX 2014, 31, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, W. A Tale of Two Databases: The Use of Web of Science and Scopus in Academic Papers. Scientometrics 2020, 123, 321–335. [Google Scholar] [CrossRef]
- Martín-Martín, A.; Thelwall, M.; Orduna-Malea, E.; Delgado López-Cózar, E. Google Scholar, Microsoft Academic, Scopus, Dimensions, Web of Science, and OpenCitations’ COCI: A Multidisciplinary Comparison of Coverage via Citations. Scientometrics 2021, 126, 871–906. [Google Scholar] [CrossRef] [PubMed]
- Gusenbauer, M.; Haddaway, N.R. Which Academic Search Systems Are Suitable for Systematic Reviews or Meta-Analyses? Evaluating Retrieval Qualities of Google Scholar, PubMed, and 26 Other Resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef]
- Babineau, J. Product Review: Covidence (Systematic Review Software). J. Can. Health Libr. Assoc. 2014, 35, 68. [Google Scholar] [CrossRef]
- Search—Consensus: AI Search Engine for Research. Available online: https://consensus.app/search/ (accessed on 14 February 2024).
- Zhang, Q.; Neitzel, A. Choosing the Right Tool for the Job: Screening Tools for Systematic Reviews in Education. J. Res. Educ. Eff. 2023, 1–27. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing Complex 3D Tissue Physiology in Vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Pongsuchart, M.; Danladkaew, C.; Khomvarn, T.; Sereemaspun, A. Effect of Glutathione-Stabilized Gold Nanoparticles in 3T3 Fibroblast Cell. In Proceedings of the International Conference on Clean and Green Energy IPCBEE, Hong Kong, China, 5–7 January 2012. [Google Scholar]
- Cho, T.J.; MacCuspie, R.I.; Gigault, J.; Gorham, J.M.; Elliott, J.T.; Hackley, V.A. Highly Stable Positively Charged Dendron-Encapsulated Gold Nanoparticles. Langmuir 2014, 30, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Jeong, S.; Jang, S.H.; Park, J.; Park, J.H.; Ock, K.S.; Lee, S.Y.; Joo, S.-W. In Vitro Toxicity of Serum Protein-Adsorbed Citrate-Reduced Gold Nanoparticles in Human Lung Adenocarcinoma Cells. Toxicol. Vitr. 2012, 26, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell Selective Response to Gold Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bachand, G.D.; Allen, A.; Bachand, M.; Achyuthan, K.E.; Seagrave, J.C.; Brozik, S.M. Cytotoxicity and Inflammation in Human Alveolar Epithelial Cells Following Exposure to Occupational Levels of Gold and Silver Nanoparticles. J. Nanoparticle Res. 2012, 14, 1212. [Google Scholar] [CrossRef]
- Chuang, S.-M.; Lee, Y.-H.; Liang, R.-Y.; Roam, G.-D.; Zeng, Z.-M.; Tu, H.-F.; Wang, S.-K.; Chueh, P.J. Extensive Evaluations of the Cytotoxic Effects of Gold Nanoparticles. Biochim. Biophys. Acta 2013, 1830, 4960–4973. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, C.D.; Lapuente, J.D.; Porredon, C.; Ramos-López, D.; Sendra, J.; Borrás, M. In Vitro Safety Toxicology Data for Evaluation of Gold Nanoparticles–Chronic Cytotoxicity, Genotoxicity and Uptake. J. Nanosci. Nanotechnol. 2012, 12, 6185–6191. [Google Scholar] [CrossRef] [PubMed]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-Dependent Toxicity and Cell Interaction Mechanisms of Gold Nanoparticles on Mouse Fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic Effects of Gold Nanoparticles: A Multiparametric Study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Aueviriyavit, S.; Phummiratch, D.; Maniratanachote, R. Mechanistic Study on the Biological Effects of Silver and Gold Nanoparticles in Caco-2 Cells—Induction of the Nrf2/HO-1 Pathway by High Concentrations of Silver Nanoparticles. Toxicol. Lett. 2014, 224, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Vetten, M.A.; Tlotleng, N.; Tanner Rascher, D.; Skepu, A.; Keter, F.K.; Boodhia, K.; Koekemoer, L.-A.; Andraos, C.; Tshikhudo, R.; Gulumian, M. Label-Free in Vitro Toxicity and Uptake Assessment of Citrate Stabilised Gold Nanoparticles in Three Cell Lines. Part. Fibre Toxicol. 2013, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.-T.; Li, J.J.; Gurung, R.L.; Hande, M.P.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Toxicological Profile of Small Airway Epithelial Cells Exposed to Gold Nanoparticles. Exp. Biol. Med. 2013, 238, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, R.; Hutz, R.J. Gold Nanoparticles Enter Rat Ovarian Granulosa Cells and Subcellular Organelles, and Alter In Vitro Estrogen Accumulation. J. Reprod. Dev. 2009, 55, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface Charge of Gold Nanoparticles Mediates Mechanism of Toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Leifert, A.; Pan, Y.; Kinkeldey, A.; Schiefer, F.; Setzler, J.; Scheel, O.; Lichtenbeld, H.; Schmid, G.; Wenzel, W.; Jahnen-Dechent, W.; et al. Differential hERG Ion Channel Activity of Ultrasmall Gold Nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 8004–8009. [Google Scholar] [CrossRef] [PubMed]
- Sabella, S.; Brunetti, V.; Vecchio, G.; Galeone, A.; Maiorano, G.; Cingolani, R.; Pompa, P.P. Toxicity of Citrate-Capped AuNPs: An in Vitro and in Vivo Assessment. J. Nanoparticle Res. 2011, 13, 6821–6835. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of Cell Culture Media on the Dynamic Formation of Protein-Nanoparticle Complexes and Influence on the Cellular Response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Salado, J.; Insausti, M.; Lezama, L.; Gil de Muro, I.; Moros, M.; Pelaz, B.; Grazu, V.; de la Fuente, J.M.; Rojo, T. Functionalized Fe₃O₄@Au Superparamagnetic Nanoparticles: In Vitro Bioactivity. Nanotechnology 2012, 23, 315102. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Pujari, G.; Semwal, M.K.; Sarma, A.; Avasthi, D.K. In Vitro Studies on Radiosensitization Effect of Glucose Capped Gold Nanoparticles in Photon and Ion Irradiation of HeLa Cells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 301, 7–11. [Google Scholar] [CrossRef]
- Mallick, S.; Sun, I.-C.; Kim, K.; Yi, D.K. Silica Coated Gold Nanorods for Imaging and Photo-Thermal Therapy of Cancer Cells. J. Nanosci. Nanotechnol. 2013, 13, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Paino, I.M.M.; Marangoni, V.S.; Oliveira, R.d.C.S.d.; Antunes, L.M.G.; Zucolotto, V. Cyto and Genotoxicity of Gold Nanoparticles in Human Hepatocellular Carcinoma and Peripheral Blood Mononuclear Cells. Toxicol. Lett. 2012, 215, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold Nanoparticles: Distribution, Bioaccumulation and Toxicity. In Vitro and in Vivo Studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Oxidative Stress Contributes to Gold Nanoparticle-Induced Cytotoxicity in Human Tumor Cells. Toxicol. Mech. Methods 2014, 24, 161–172. [Google Scholar] [CrossRef]
- Gao, W.; Xu, K.; Ji, L.; Tang, B. Effect of Gold Nanoparticles on Glutathione Depletion-Induced Hydrogen Peroxide Generation and Apoptosis in HL7702 Cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Westermann, M.; Glei, M. In Vitro Uptake and Toxicity Studies of Metal Nanoparticles and Metal Oxide Nanoparticles in Human HT29 Cells. Arch. Toxicol. 2017, 91, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, T.; Colognato, R.; Nelissen, I.; Favilli, F.; Casals, E.; Ooms, D.; Leppens, H.; Ponti, J.; Stritzinger, R.; Puntes, V.; et al. The Suitability of Different Cellular in Vitro Immunotoxicity and Genotoxicity Methods for the Analysis of Nanoparticle-Induced Events. Nanotoxicology 2010, 4, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Terzuoli, G.; Renieri, T.; Iacoponi, F.; Castellini, C.; Giordano, C.; Collodel, G. In Vitro Effect of Gold and Silver Nanoparticles on Human Spermatozoa. Andrologia 2013, 45, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S.; Yum, Y.-N.; Kim, J.-H.; Song, H.-A.; Jeong, J.-Y.; Lim, Y.-T.; Chung, B.-H.; Park, S.-N. Induction of DNA Damage in L5178Y Cells Treated with Gold Nanoparticle. Biomol. Ther. 2009, 17, 92–97. [Google Scholar] [CrossRef]
- Jain, S.; Coulter, J.A.; Butterworth, K.T.; Hounsell, A.R.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Gold Nanoparticle Cellular Uptake, Toxicity and Radiosensitisation in Hypoxic Conditions. Radiother. Oncol. 2014, 110, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Liaw, J.-W.; Kao, Y.-C.; Huang, M.-Y.; Lee, C.-Y.; Rau, L.-R.; Huang, C.-Y.; Wei, K.-C.; Ye, T.-C. Internalized Gold Nanoparticles Do Not Affect the Osteogenesis and Apoptosis of MG63 Osteoblast-Like Cells: A Quantitative, In Vitro Study. PLoS ONE 2013, 8, e76545. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Debnath, N.; Mitra, S.; Datta, A.; Goswami, A. Comparative Analysis of Stability and Toxicity Profile of Three Differently Capped Gold Nanoparticles for Biomedical Usage. Biometals 2012, 25, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and Oxidative Stress Associated with Gold Nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Chueh, P.J.; Liang, R.-Y.; Lee, Y.-H.; Zeng, Z.-M.; Chuang, S.-M. Differential Cytotoxic Effects of Gold Nanoparticles in Different Mammalian Cell Lines. J. Hazard. Mater. 2014, 264, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.K.; Al-Ayed, M.S.; Alhomida, A.S. Effect of Gold Nanoparticles on Glutathione and Malondialdehyde Levels in Liver, Lung and Heart of Rats. Saudi J. Biol. Sci. 2012, 19, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Raji, V.; Kumar, J.; Rejiya, C.S.; Vibin, M.; John, A.; Abraham, A. Synthesis, Characterisation and Biocompatibility of Surface-Functionalised Gold Nanoparticles. J. Exp. Nanosci. 2012, 7, 174–188. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Mali, V.; Bodhankar, S.; Pokharkar, V. In Vitro Cytotoxicity and in Vivo Sub-Acute Oral Toxicity Assessment of Porphyran Reduced Gold Nanoparticles. Toxicol. Environ. Chem. 2012, 94, 1357–1367. [Google Scholar] [CrossRef]
- Piryazev, A.P.; Azizova, O.A.; Aseichev, A.V.; Dudnik, L.B.; Sergienko, V.I. Effect of Gold Nanoparticles on Production of Reactive Oxygen Species by Human Peripheral Blood Leukocytes Stimulated with Opsonized Zymosan. Bull. Exp. Biol. Med. 2013, 156, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Whaley Bishnoi, S. Comparative Toxicity Study of Ag, Au, and Ag–Au Bimetallic Nanoparticles on Daphnia Magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef]
- Downs, T.R.; Crosby, M.E.; Hu, T.; Kumar, S.; Sullivan, A.; Sarlo, K.; Reeder, B.; Lynch, M.; Wagner, M.; Mills, T.; et al. Silica Nanoparticles Administered at the Maximum Tolerated Dose Induce Genotoxic Effects through an Inflammatory Reaction While Gold Nanoparticles Do Not. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.K.; Alhomida, A.S.; Al-Ayed, M.S. Effects of Naked Gold Nanoparticles on Proinflammatory Cytokines mRNA Expression in Rat Liver and Kidney. BioMed Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, H.; Wu, J.; Meng, Z.; Xing, R.; Tian, A.; Tian, X.; Guo, L.; Zhang, Y.; Nie, G.; et al. No Overt Structural or Functional Changes Associated with PEG-Coated Gold Nanoparticles Accumulation with Acute Exposure in the Mouse Heart. Toxicol. Lett. 2013, 222, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.A.; Hassan, S.A.; Knittel, L.L.; Balbo, A.; Aronova, M.A.; Brown, P.H.; Schuck, P.; Leapman, R.D. Biointeractions of Ultrasmall Glutathione-Coated Gold Nanoparticles: Effect of Small Size Variations. Nanoscale 2016, 8, 6577–6588. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, X.; K Hammer, B.; Du, S.; Chen, Y. Nanoparticles Inhibit DNA Replication by Binding to DNA: Modeling and Experimental Validation. ACS Nano 2013, 7, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Lanry Yung, L.-Y. Gold Nanoparticles Induce Oxidative Damage in Lung Fibroblasts In Vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Orsiere, T.; De Meo, M.; Thill, A.; Zeyons, O.; Proux, O.; Masion, A.; Chaurand, P.; Spalla, O.; et al. CeO2 Nanoparticles Induce DNA Damage towards Human Dermal Fibroblasts in Vitro. Nanotoxicology 2009, 3, 161–171. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; Puntes, V. Instability of Cationic Gold Nanoparticle Bioconjugates: The Role of Citrate Ions. J. Am. Chem. Soc. 2009, 131, 13320–13327. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, N.J.; Abdelhalim, M.A.K.; El-Ansary, A.K.; Alhomida, A.S.; Ong, W.Y. Identification of Potential Biomarkers of Gold Nanoparticle Toxicity in Rat Brains. J. Neuroinflamm. 2012, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Freese, C.; Uboldi, C.; Gibson, M.I.; Unger, R.E.; Weksler, B.B.; Romero, I.A.; Couraud, P.-O.; Kirkpatrick, C.J. Uptake and Cytotoxicity of Citrate-Coated Gold Nanospheres: Comparative Studies on Human Endothelial and Epithelial Cells. Part. Fibre Toxicol. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Ganesan, S. In Vitro Cytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents. J. Nanomater. 2012, 2012, 734398. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Joshi, P.; Shanker, V.; Ansari, Z.A.; Singh, S.P.; Chakrabarti, P. Contrasting Effect of Gold Nanoparticles and Nanorods with Different Surface Modifications on the Structure and Activity of Bovine Serum Albumin. Langmuir 2011, 27, 7722–7731. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gu, X.; Zhang, K.; Ding, Y.; Wei, X.; Zhang, X.; Zhao, Y. Gold Nanoparticles Trigger Apoptosis and Necrosis in Lung Cancer Cells with Low Intracellular Glutathione. J. Nanopart. Res. 2013, 15, 1745. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Wang, H.; Li, L.; Tan, L.; Fu, C.; Nie, G.; Chen, D.; Tang, F. Impact of PEGylation on the Biological Effects and Light Heat Conversion Efficiency of Gold Nanoshells on Silica Nanorattles. Biomaterials 2013, 34, 6967–6975. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, D.; Taylor, U.; Rehbock, C.; Jakobi, J.; Klein, S.; Kues, W.A.; Barcikowski, S.; Rath, D. Reprotoxicity of Gold, Silver, and Gold–Silver Alloy Nanoparticles on Mammalian Gametes. Analyst 2014, 139, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Young, K.L.; Scott, A.W.; Hao, L.; Mirkin, S.E.; Liu, G.; Mirkin, C.A. Hollow Spherical Nucleic Acids for Intracellular Gene Regulation Based upon Biocompatible Silica Shells. Nano Lett. 2012, 12, 3867–3871. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Lee, Y.S.; Seo, S.H.; Kim, Y.S.; Choi, E.M. Gold Nanoparticles Attenuates Antimycin A-Induced Mitochondrial Dysfunction in MC3T3-E1 Osteoblastic Cells. Biol. Trace Elem. Res. 2013, 153, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Kazemi, B. Triglyceride-Coated Nanoparticles: Skin Toxicity and Effect of UV/IR Irradiation on Them. Toxicol. Vitr. 2013, 27, 1847–1854. [Google Scholar] [CrossRef]
- Chaicherd, S.; Killingsworth, M.C.; Pissuwan, D. Toxicity of Gold Nanoparticles in a Commercial Dietary Supplement Drink on Connective Tissue Fibroblast Cells. SN Appl. Sci. 2019, 1, 336. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, H.-Y.; Wu, D.; Wang, Y.-Y.; Chang, J.-H.; Zhai, Z.-B.; Meng, A.-M.; Liu, P.-X.; Zhang, L.-A.; Fan, F.-Y. Toxicologic Effects of Gold Nanoparticles in Vivo by Different Administration Routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.C.; Chan, W.C. Nanotoxicity: The Growing Need for in Vivo Study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the Role of Surface Charges in Cellular Adsorption versus Internalization by Selectively Removing Gold Nanoparticles on the Cell Surface with a I2/KI Etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.-Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute Toxicity and Pharmacokinetics of 13 Nm-Sized PEG-Coated Gold Nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Morsy, E.A.; Hussien, A.M.; Ibrahim, M.A.; Farroh, K.Y. The Effect of Different Concentrations of Gold Nanoparticles on Growth Performance, Toxicopathological and Immunological Parameters of Broiler Chickens. Biosci. Rep. 2020, 40, BSR20194296. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Lee, W.-M.; Shin, Y.-J.; Yoon, S.-J.; Kim, S.W.; Kwak, J.I.; An, Y.-J. Derivation of Guideline Values for Gold (III) Ion Toxicity Limits to Protect Aquatic Ecosystems. Water Res. 2014, 48, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, G.; Galeone, A.; Brunetti, V.; Maiorano, G.; Rizzello, L.; Sabella, S.; Cingolani, R.; Pompa, P.P. Mutagenic Effects of Gold Nanoparticles Induce Aberrant Phenotypes in Drosophila Melanogaster. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pompa, P.P.; Vecchio, G.; Galeone, A.; Brunetti, V.; Sabella, S.; Maiorano, G.; Falqui, A.; Bertoni, G.; Cingolani, R. In Vivo Toxicity Assessment of Gold Nanoparticles in Drosophila Melanogaster. Nano Res. 2011, 4, 405–413. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Hong, M.-Y.; Onischuk, A.A.; Chiou, J.C.; Sorokina, I.V.; Tolstikova, T.; Steve Huang, G. Control of In Vivo Transport and Toxicity of Nanoparticles by Tea Melanin. J. Nanomater. 2012, 2012, 746960. [Google Scholar] [CrossRef]
- Sadauskas, E.; Jacobsen, N.R.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Kreyling, W.; Wallin, H. Biodistribution of Gold Nanoparticles in Mouse Lung Following Intratracheal Instillation. Chem. Cent. J. 2009, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Rattanapinyopituk, K.; Shimada, A.; Morita, T.; Sakurai, M.; Asano, A.; Hasegawa, T.; Inoue, K.; Takano, H. Demonstration of the Clathrin- and Caveolin-Mediated Endocytosis at the Maternal–Fetal Barrier in Mouse Placenta after Intravenous Administration of Gold Nanoparticles. J. Vet. Med. Sci. 2014, 76, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Mandal, S.; Garrovo, C.; Astolfo, A.; Bonifacio, A.; Latawiec, D.; Menk, R.H.; Arfelli, F.; Huewel, S.; Legname, G.; et al. Functionalized Gold Nanoparticles: A Detailed in Vivo Multimodal Microscopic Brain Distribution Study. Nanoscale 2010, 2, 2826–2834. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.-M.; Zhang, L.-A.; Fan, F.-Y. Size-Dependent in Vivo Toxicity of PEG-Coated Gold Nanoparticles. Int. J. Nanomed. 2011, 6, 2071–2081. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Fan, F.-Y.; Fan, S.-J. In Vivo Renal Clearance, Biodistribution, Toxicity of Gold Nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, T.H.L.; Nguyen, T.T.; Fort, E.; Nguyen, T.P.; Hoang, T.M.N.; Nguyen, T.Q.; Tran, H.N. Capping and in Vivo Toxicity Studies of Gold Nanoparticles. Adv. Nat. Sci Nanosci. Nanotechnol. 2012, 3, 015002. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer Cells Are Central in the Removal of Nanoparticles from the Organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-Modified Gold Nanoparticles following Intratracheal Instillation and Intravenous Injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Z.; Ma, J.; Wang, X.; Zhang, Y.; Wang, W.; Yuan, Z. The Systematic Evaluation of Size-Dependent Toxicity and Multi-Time Biodistribution of Gold Nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yahyaei, B.; Nouri, M.; Bakherad, S.; Hassani, M.; Pourali, P. Effects of Biologically Produced Gold Nanoparticles: Toxicity Assessment in Different Rat Organs after Intraperitoneal Injection. AMB Express 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and Toxicity of Gold Nanoparticles after Repeated Administration in Mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Ji, Y.; Liu, J.; Cheng, X.; Guo, H.; Zhang, W.; Wu, X.; Xu, H. Using Gold Nanorods Core/Silver Shell Nanostructures as Model Material to Probe Biodistribution and Toxic Effects of Silver Nanoparticles in Mice. Nanotoxicology 2014, 8, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Kreyling, W.G.; Simon, U. Toxic Effects and Biodistribution of Ultrasmall Gold Nanoparticles. Arch. Toxicol. 2017, 91, 3011–3037. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Brandão, A.; Soares, M.E.; Morais, T.; Duarte, J.A.; Pereira, L.; Soares, L.; Neves, C.; Pereira, E.; Bastos, M.d.L.; et al. Short- and Long-Term Distribution and Toxicity of Gold Nanoparticles in the Rat after a Single-Dose Intravenous Administration. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle Size-Dependent and Surface Charge-Dependent Biodistribution of Gold Nanoparticles after Intravenous Administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Abdelmottaleb Moussa, S.A. The Gold Nanoparticle Size and Exposure Duration Effect on the Liver and Kidney Function of Rats: In Vivo. Saudi J. Biol. Sci. 2013, 20, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Monteiro-Riviere, N.A.; Kannan, R.; Riviere, J.E. A Computational Framework for Interspecies Pharmacokinetics, Exposure and Toxicity Assessment of Gold Nanoparticles. Nanomedicine 2016, 11, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of Gold Nanoparticles in Biomedical and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Ambwani, S.; Kakade Datta, P.; Kandpal, D.; Arora, S.; Ambwani, T.K. Cytotoxic Effects of Gold Nanoparticles Exposure Employing in Vitro Animal Cell Culture System as Part of Nanobiosafety. AIP Conf. Proc. 2016, 1724, 020091. [Google Scholar] [CrossRef]
- Simpson, C.A.; Salleng, K.J.; Cliffel, D.E.; Feldheim, D.L. In Vivo Toxicity, Biodistribution, and Clearance of Glutathione-Coated Gold Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.A., Jr.; Gupta, S.; Koshkina, N.; Corr, S.J.; Zhang, S.; Curley, S.A.; Han, G. Gold Nanoparticles Stabilized with MPEG-Grafted Poly(l-Lysine): In Vitro and in Vivo Evaluation of a Potential Theranostic Agent. Bioconjug. Chem. 2015, 26, 39–50. [Google Scholar] [CrossRef]
- Bozich, J.S.; Lohse, S.E.; Torelli, M.D.; Murphy, C.J.; Hamers, R.J.; Klaper, R.D. Surface Chemistry, Charge and Ligand Type Impact the Toxicity of Gold Nanoparticles to Daphnia magna. Environ. Sci. Nano 2014, 1, 260–270. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular Uptake and Cytotoxicity of Gold Nanorods: Molecular Origin of Cytotoxicity and Surface Effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of Anchoring Ligands and Particle Size on the Colloidal Stability and In Vivo Biodistribution of Polyethylene Glycol-Coated Gold Nanoparticles in Tumor-Xenografted Mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid–PEG Conjugates Sterically Stabilize and Reduce the Toxicity of Phytantriol-Based Lyotropic Liquid Crystalline Nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, S.; Franco, G.; Regoli, M.; Bracciali, M.; Morandi, V.; Sgaragli, G.; Bertelli, E.; Valoti, M. Gold Nanoparticles Uptake and Cytotoxicity Assessed on Rat Liver Precision-Cut Slices. Toxicol. Sci. 2012, 128, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Long, W.; Shen, X.; Wu, D.; Song, S.-S.; Sun, Y.-M.; Liu, P.-X.; Fan, S.; Fan, F.; et al. Sex Differences in the Toxicity of Polyethylene Glycol-Coated Gold Nanoparticles in Mice. Int. J. Nanomed. 2013, 8, 2409–2419. [Google Scholar] [CrossRef]
- Botha, T.L.; James, T.E.; Wepener, V. Comparative Aquatic Toxicity of Gold Nanoparticles and Ionic Gold Using a Species Sensitivity Distribution Approach. J. Nanomater. 2015, 2015, 986902. [Google Scholar] [CrossRef]
- Dey, A.K.; Gonon, A.; Pécheur, E.-I.; Pezet, M.; Villiers, C.; Marche, P.N. Impact of Gold Nanoparticles on the Functions of Macrophages and Dendritic Cells. Cells 2021, 10, 96. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Wang, F.; Sun, C.; Wang, L.; Wang, J.; Sun, F. Enhancement of Lipopolysaccharide-Induced Nitric Oxide and Interleukin-6 Production by PEGylated Gold Nanoparticles in RAW264.7 Cells. Nanoscale 2012, 4, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, C.; Rothen-Rutishauser, B.; Mühlfeld, C.; Schmid, O.; Ferron, G.A.; Maier, K.L.; Gehr, P.; Lenz, A.-G. Effects and Uptake of Gold Nanoparticles Deposited at the Air–Liquid Interface of a Human Epithelial Airway Model. Toxicol. Appl. Pharmacol. 2010, 242, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Sánchez-Tilló, E.; Pujals, S.; Farrera, C.; López, C.; Giralt, E.; Celada, A.; Lloberas, J.; Puntes, V. Homogeneous Conjugation of Peptides onto Gold Nanoparticles Enhances Macrophage Response. ACS Nano 2009, 3, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Chen, H.; Chen, Q.; Wang, J.; Ho, H.P.; Wong, C.K.; Kong, S.K. CTAB-Coated Gold Nanorods Elicit Allergic Response through Degranulation and Cell Death in Human Basophils. Nanoscale 2012, 4, 4447–4449. [Google Scholar] [CrossRef] [PubMed]
- Trickler, W.J.; Lantz-McPeak, S.M.; Robinson, B.L.; Paule, M.G.; Slikker, W.; Biris, A.S.; Schlager, J.J.; Hussain, S.M.; Kanungo, J.; Gonzalez, C.; et al. Porcine Brain Microvessel Endothelial Cells Show Pro-Inflammatory Response to the Size and Composition of Metallic Nanoparticles. Drug Metab. Rev. 2014, 46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Senut, M.; Zhang, Y.; Liu, F.; Sen, A.; Ruden, D.M.; Mao, G. Size-Dependent Toxicity of Gold Nanoparticles on Human Embryonic Stem Cells and Their Neural Derivatives. Small 2016, 12, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.-H.; Park, K.; Yi, J.; Ryu, D.-Y. Oxidative Stress-Dependent Toxicity of Silver Nanoparticles in Human Hepatoma Cells. Toxicol. Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver Nanoparticles Induce Cytotoxicity by a Trojan-Horse Type Mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver Nanoparticles (AgNPs) Cause Degeneration of Cytoskeleton and Disrupt Synaptic Machinery of Cultured Cortical Neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Muralikrishnan, S.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Nanoparticle-Induced Pulmonary Toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K. Exposure to Gold Nanoparticles Produces Cardiac Tissue Damage That Depends on the Size and Duration of Exposure. Lipids Health Dis. 2011, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, A.; Li, Z. Reversible Cardiac Hypertrophy Induced by PEG-Coated Gold Nanoparticles in Mice. Sci. Rep. 2016, 6, 20203. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.K.; Deda, D.K.; Rodrigues, S.F.D.P.; Drewes, C.C.; Bolonheis, S.M.; Kiyohara, P.K.; Toledo, S.P.D.; Colli, W.; Araki, K.; Farsky, S.H.P. In Vivo and In Vitro Toxicity and Anti-Inflammatory Properties of Gold Nanoparticle Bioconjugates to the Vascular System. Toxicol. Sci. 2014, 142, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; Peixoto De Almeida, M.; Araújo, A.M.; Bastos, M.D.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, X.; Wang, Q.; Zou, H.; Xie, Y.; Liu, H.; Zhou, Y.; Liu, P.; Dai, H. Differential Toxicity Mechanism of Gold Nanoparticles in HK-2 Renal Proximal Tubular Cells and 786-0 Carcinoma Cells. Nanomedicine 2020, 15, 1079–1096. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Akila, S.; Nadar, M.S.A.M.; Narendhirakannan, R.T.; Chatterjee, S. Biosynthesized Vitis Vinifera Seed Gold Nanoparticles Induce Apoptotic Cell Death in A431 Skin Cancer Cells. RSC Adv. 2016, 6, 82205–82218. [Google Scholar] [CrossRef]
- Wiwanitkit, V.; Sereemaspun, A.; Rojanathanes, R. Effect of Gold Nanoparticles on Spermatozoa: The First World Report. Fertil. Steril. 2009, 91, e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, J.; Zhang, Y.; Rong, H.; Lu, W.; Jiang, L. Effects of Aggregation and the Surface Properties of Gold Nanoparticles on Cytotoxicity and Cell Growth. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Johansson, F.; Klefbohm, B.; Johansson, U.E. Correction: Gold- and Silver Nanoparticles Affect the Growth Characteristics of Human Embryonic Neural Precursor Cells. PLoS ONE 2013, 8, e58211. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Kuhn, H.; Brandau, W.; Esche, H.; Schmid, G. Cellular Uptake and Toxicity of Au55 Clusters. Small 2005, 1, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Datta, P.; Patra, H.K.; Dasgupta, A.K.; Gomes, A. In Vivo Interaction of Gold Nanoparticles after Acute and Chronic Exposures in Experimental Animal Models. J. Nanosci. Nanotechnol. 2013, 13, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K.; Jarrar, B.M. Renal Tissue Alterations Were Size-Dependent with Smaller Ones Induced More Effects and Related with Time Exposure of Gold Nanoparticles. Lipids Health Dis. 2011, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-P.; Lai, C.-S.; Hung, C.-J.; Dhaiveegan, P.; Tsai, M.-L.; Chiu, C.-L.; Fang, J.-M. Subchronic Oral Toxicity Evaluation of Gold Nanoparticles in Male and Female Mice. Heliyon 2021, 7, e06577. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Kim, K.-W.; Kim, M.H.; Yu, Y.S. Intravenously Administered Gold Nanoparticles Pass through the Blood-Retinal Barrier Depending on the Particle Size, and Induce No Retinal Toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, 1801451. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum Sized Gold Nanoclusters with Atomic Precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Khalili Fard, J.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in Vitro and in Vivo Toxicity of Gold Nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Ozcicek, I.; Aysit, N.; Cakici, C.; Aydeger, A. The Effects of Surface Functionality and Size of Gold Nanoparticles on Neuronal Toxicity, Apoptosis, ROS Production and Cellular/Suborgan Biodistribution. Mater. Sci. Eng. C 2021, 128, 112308. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Kuracka, L.; Zitnanova, I.; Scsukova, S.; Kollar, J.; Konarikova, K.; Laubertova, L. Assessment of the Potential Health Risk of Gold Nanoparticles Used in Nanomedicine. Oxidative Med. Cell. Longev. 2022, 2022, e4685642. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Díaz, E.; Valcárcel, M. Toxicity of Gold Nanoparticles. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 207–254. ISBN 978-0-444-63285-2. [Google Scholar]
- Ji, J.; Sun, J.; Zhang, Y.; Sun, X. Cell-Based Metabolomics Approach for Anticipating and Investigating Cytotoxicity of Gold Nanorods. Foods 2022, 11, 3569. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconjugate Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.E.; Hendi, A.A. Gold Nanoparticles Induce Apoptosis in MCF-7 Human Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2012, 13, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, X.; Lin, H.; Chen, H.; Huang, D.; Li, D.; Shan, H.; Gao, J. Gold Nanoparticles Impair Autophagy Flux through Shape-Dependent Endocytosis and Lysosomal Dysfunction. J. Mater. Chem. B 2018, 6, 8127–8136. [Google Scholar] [CrossRef] [PubMed]
- Bucchianico, S.D.; Fabbrizi, M.R.; Cirillo, S.; Uboldi, C.; Gilliland, D.; Valsami-Jones, E.; Migliore, L. Aneuploidogenic Effects and DNA Oxidation Induced in Vitro by Differently Sized Gold Nanoparticles. Int. J. Nanomed. 2014, 9, 2191–2204. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.; Londero, E.; Ferreira, G.K.; Rezin, G.T.; Zanoni, E.T.; De Souza Notoya, F.; Leffa, D.D.; Damiani, A.P.; Daumann, F.; Rohr, P.; et al. Gold Nanoparticles Induce DNA Damage in the Blood and Liver of Rats. J. Nanoparticle Res. 2014, 16, 2727. [Google Scholar] [CrossRef]
- Huwaidi, A.; Kumari, B.; Robert, G.; Guérin, B.; Sanche, L.; Wagner, J.R. Profiling DNA Damage Induced by the Irradiation of DNA with Gold Nanoparticles. J. Phys. Chem. Lett. 2021, 12, 9947–9954. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, H.; Aleanizy, F.; Alqahtani, F.; Bukhari, A.; Soliman, S.; Sau, S.; Iyer, A. Visualizing the 4D Impact of Gold Nanoparticles on DNA. Int. J. Mol. Sci. 2024, 25, 542. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directorate General for Health and Food Safety. In Opinion on Gold (Nano)—Colloidal Gold (Nano), Gold Thioethylamino Hyaluronic Acid (Nano) and Acetyl Heptapeptide-9, Colloidal Gold (Nano); Ed. Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Kulkarni, N.; Muddapur, U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the Methodologies Used in the Synthesis Gold Nanoparticles by Chemical Reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of Gold Nanoparticles: A Green Approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Green and Energy-Efficient Methods for the Production of Metallic Nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2354–2376. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.; Poinern, G. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, G.A.; Pescador-Rojas, J.A.; Ortega-Lopez, J.; Salazar, J.S.; Balderas-López, J.A. Thermal Diffusivity Measurement of Spherical Gold Nanofluids of Different Sizes/Concentrations. Nanoscale Res. Lett. 2012, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.M.S.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Zeiri, Y.; Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Reducing Agents. Int. J. Nanomed. 2014, 2014, 4007–4021. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe Vera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, A.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juárez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum Chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Gurav, D.D.; Jabgunde, A.M.; Kale, S.; Pardesi, K.; Shinde, V.; Bellare, J.; et al. Gnidia Glauca Flower Extract Mediated Synthesis of Gold Nanoparticles and Evaluation of Its Chemocatalytic Potential. J. Nanobiotechnol. 2012, 10, 17. [Google Scholar] [CrossRef]
- Nayan, V.; Onteru, S.K.; Singh, D. Mangifera Indica Flower Extract Mediated Biogenic Green Gold Nanoparticles: Efficient Nanocatalyst for Reduction of 4-nitrophenol. Environ. Prog. Sustain. Energy 2018, 37, 283–294. [Google Scholar] [CrossRef]
- Cardoso-Avila, P.E.; Patakfalvi, R.; Rodríguez-Pedroza, C.; Aparicio-Fernández, X.; Loza-Cornejo, S.; Villa-Cruz, V.; Martínez-Cano, E. One-Pot Green Synthesis of Gold and Silver Nanoparticles Using Rosa canina L. Extract. RSC Adv. 2021, 11, 14624–14631. [Google Scholar] [CrossRef] [PubMed]
- Moosavy, M.-H.; De La Guardia, M.; Mokhtarzadeh, A.; Khatibi, S.A.; Hosseinzadeh, N.; Hajipour, N. Green Synthesis, Characterization, and Biological Evaluation of Gold and Silver Nanoparticles Using Mentha spicata Essential Oil. Sci. Rep. 2023, 13, 7230. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare Mediated Green Synthesis of Biocompatible Gold Nanoparticles Simultaneously Possessing Plasmonic, Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Khan, H.; Liaqat, W.; Zeb, M.A. Phytochemical Screening, Green Synthesis of Gold Nanoparticles, and Antibacterial Activity Using Seeds Extract of Ricinus communis L. Microsc. Res. Tech. 2022, 85, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Pal, U.; Gomez, L.M.; Agarwal, V. Size Controlled Green Synthesis of Gold Nanoparticles Using Coffea arabica Seed Extract and Their Catalytic Performance in 4-Nitrophenol Reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. KONA Powder Part. J. 2020, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yust, B.G.; Rao, N.Z.; Schwarzmann, E.T.; Peoples, M.H. Quantification of Spent Coffee Ground Extracts by Roast and Brew Method, and Their Utility in a Green Synthesis of Gold and Silver Nanoparticles. Molecules 2022, 27, 5124. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Gupta, R.K. Nanotechnology and Potential of Microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Lim, K.; Macazo, F.C.; Scholes, C.; Chen, H.; Sumampong, K.; Minteer, S.D. Elucidating the Mechanism behind the Bionanomanufacturing of Gold Nanoparticles Using Bacillus subtilis. ACS Appl. Bio Mater. 2020, 3, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- Correa-Llantén, D.N.; Muñoz-Ibacache, S.A.; Castro, M.E.; Muñoz, P.A.; Blamey, J.M. Gold Nanoparticles Synthesized by Geobacillus Sp. Strain ID17 a Thermophilic Bacterium Isolated from Deception Island, Antarctica. Microb. Cell Factories 2013, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of Discrete Spherical Gold Nanoparticles Using the Metal-Reducing Bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green Synthesis and Characterization of Gold Nanoparticles Using Endophytic Fungi Fusarium solani and Its In-Vitro Anticancer and Biomedical Applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different Active Biomolecules Involved in Biosynthesis of Gold Nanoparticles by Three Fungus Species. J. Biomed Nanotechnol. 2011, 7, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-Mediated Biosynthesis of Inorganic Nanomaterials as a Promising Route in Nanobiotechnology—A Review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Hassaan, M. Green Synthesis of Ag and Au Nanoparticles from Micro and Macro Algae—Review. Int. J. Atmos. Ocean. Sci. 2018, 2, 10. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Nageswara Rao, G. Green Synthesis of Gold Nanoparticles Using Marine Algae and Evaluation of Their Catalytic Activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Namvar, F.; Azizi, S.; Ahmad, M.B.; Shameli, K.; Mohamad, R.; Mahdavi, M.; Tahir, P.M. Green Synthesis and Characterization of Gold Nanoparticles Using the Marine Macroalgae Sargassum muticum. Res. Chem. Intermed. 2015, 41, 5723–5730. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green Synthesis of Gold Nanoparticles Using Brown Algae Cystoseira baccata: Its Activity in Colon Cancer Cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Characterization of Biosynthesized Gold Nanoparticles from Aqueous Extract of Chlorella vulgaris and Their Anti-Pathogenic Properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef]
- Schwartz-Duval, A.S.; Sokolov, K.V. Prospecting Cellular Gold Nanoparticle Biomineralization as a Viable Alternative to Prefabricated Gold Nanoparticles. Adv. Sci. 2022, 9, 2105957. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Duval, A.S.; Konopka, C.J.; Moitra, P.; Daza, E.A.; Srivastava, I.; Johnson, E.V.; Kampert, T.L.; Fayn, S.; Haran, A.; Dobrucki, L.W.; et al. Intratumoral Generation of Photothermal Gold Nanoparticles through a Vectorized Biomineralization of Ionic Gold. Nat. Commun. 2020, 11, 4530. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A Review of the Green Syntheses and Anti-Microbial Applications of Gold Nanoparticles. Green Chem. Lett. Rev. 2017, 10, 216–227. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.H. Green Synthesis of Silver and Gold Nanoparticles and Their Potential Applications as Therapeutics in Cancer Therapy; a Review. Inorg. Chem. Commun. 2022, 143, 109610. [Google Scholar] [CrossRef]
- Varna, M.; Xuan, H.V.; Fort, E. Gold Nanoparticles in Cardiovascular Imaging. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef] [PubMed]
- Jannathul Firdhouse, M.; Lalitha, P. Biogenic Green Synthesis of Gold Nanoparticles and Their Applications—A Review of Promising Properties. Inorg. Chem. Commun. 2022, 143, 109800. [Google Scholar] [CrossRef]
- Noah, N. Green Synthesis: Characterization and Application of Silver and Gold Nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–135. ISBN 978-0-08-102579-6. [Google Scholar]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Ambrosi, A.; Airò, F.; Merkoçi, A. Enhanced Gold Nanoparticle Based ELISA for a Breast Cancer Biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Abhijith, K.S.; Thakur, M.S. Application of Green Synthesis of Gold Nanoparticles for Sensitive Detection of Aflatoxin B1 Based on Metal Enhanced Fluorescence. Anal. Methods 2012, 4, 4250. [Google Scholar] [CrossRef]
- Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612. [Google Scholar] [CrossRef]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1896. [Google Scholar] [CrossRef] [PubMed]
| Organism | Effects | Particle | References |
|---|---|---|---|
| 3T3 cells | Produce more reactive oxygen species than plain AuNPs | Monodispersed AuNPs of diameter 15 ± 1 nm | [33] |
| A549 and Vero cells | No toxicity | Citrate- and MUA-Coated Nanospheres of 13 and 60 nm and MUA-Coated Gold Nanostars of 60 nm | [34] |
| A549 cells | Intrinsic and extrinsic apoptotic pathways reflected in cell damage | AuNPsof diameter approximately 17 nm, coated with serum proteins | [35] |
| A549 cells | Cytotoxicity by substantial changes in nuclear morphology and nuclear condensation. Assumed circular shape because of the induced stress | AuNPs with an average dynamic diameter of 33 nm | [36] |
| A549 cells | An inflammatory response | BioPureTM silver and gold nanoparticles with a diameter between 20 and 60 nm in a concentration of 1 mg/mL | [37] |
| AGS, A549, NIH3T3, PK-15, and Vero cells | Suppression of growth of cells in a dose-dependent manner by delay of cell cycle and induction of apoptosis | AuNPs of three sizes: (10 nm × 39 nm, 10 nm × 41 nm, 10 nm × 45 nm) | [38] |
| Balb/3T3 cells | Oxidative stress reflected in DNA damage but with reduced cytotoxicity | Spherical AuNPs of 12 nm diameter, uncoated and coated with hyaluronic acid in a concentration of 10 mg/mL in PBS | [39] |
| Balb/3T3 cells | Cytotoxicity by disruption of actin cytoskeleton | Citrate-stabilized AuNPs of 5 and 15 nm diameter in concentrations of 2, 10, 20, 39.2, 58.8 g/mL | [40] |
| C17.2 and PC12 cells | Induced oxidative stress by cell viability and deformations of actin and tubulin | 4 nm diameter AuNPs in concentrations ranging from 10 to 200 nM. | [41] |
| Caco-2 cells | Did not produce acute cytotoxicity | AuNPsin concentration 0, 5, 10, 20, 40, 80, 125, 250, 500 or 1000 g/mL | [42] |
| CHO, BEAS-2B, and HEK293 cells | Exert higher toxicity | Citrate-stabilized AuNPs of diameter 14 nm (concentration 2.25 × 1012 nps/mL) and 20 nm (concentration 7.76 × 1011 nps/mL) | [43] |
| Epithelial cells of airways | Elevation of lipid peroxidase, DNA damage, and cytotoxicity | AuNPs of 20 nm diameter in concentration 1 nM/L | [44] |
| Granulose cells of the ovary | Induced an elevation in estrogen accumulation | 10 nm AuNPs in concentration 2.85 × 10 10/mL | [45] |
| HaCaT (Human keratinocyte cell line) | Cell death by apoptosis and necrosis | The average particle sizes are reported as follows: 1.8 ± 0.7 nm for neutral particles (MEEE), 1.6 ± 0.8 nm for positive particles (TMAT), and 1.8 ± 0.7 nm for negative particles (MES). | [46] |
| HEK293 cells | Modified gene expression and had no toxicity | Phosphine-stabilized and thiol-stabilized AuNPs of 1.4 nm diameter | [47] |
| HeLa and U937 cells | Cytotoxic | 15, 40 and 80 nm Citrate-capped AuNPs in various concentrations | [48,49] |
| HeLa cells | No indication of cytotoxicity | AuNPs of diameter ranging from 4.0 to 5.4 nm in different concentrations | [50] |
| HeLa cells | No toxicity effects | Silica-coated AuNRs of diameter ranging from 4 to 16 nm in concentration 1–400 µg/mL and glucose-capped AuNPs of diameter within 5–9 nm at concentration 5.5 µM/mL | [51,52] |
| HepG2 and PBMC cells | In vitro cytotoxicity and genotoxicity effects at low concentrations | AuNPs capped with either sodium citrate (average diameter of 18.2 ± 0.4 nm) or polyamidoamine dendrimers (average diameter of 10.9 ± 0.4 nm) Concentrations from 0.01 to 50.0 M | [53] |
| HepG2 cells | AuNPs do not change the concentration of inflammatory markers compared to the control. Indicated tails moment similar to those from the positive control exposed to hydrogen peroxide | Citrate-stabilFd AuNPs with 10, 30 or 60 nm of diameter size. The concentration of 10 ppb and 10 ppm | [54] |
| HL-60 and HepG2 cell lines | Cytotoxic effects associated with reduction in GSH and increase in ROS | AuNPs with diameters of 30, 50 and 90 nm in concentrations 1–25 mg/mL | [55] |
| HL7702 cells (Human liver cell lines) | Early decrease in cytosolic GSH, depolarisation of mitochondrial transmembrane potential, and apoptosis | AuNPs with a diameter of 8 nm and 37 nm | [56] |
| HT29 cells (Human colorectal adenocarcinoma) | Significant reduction in viability of cells. However, no genotoxic effects | AuNPs with a diameter of 31.99 ± 0.16 nm and a concentration of 9.8 µg/mL | [57] |
| Human cell lines | Little or no immunotoxic, cytotoxic, and genotoxic effects | 4.5 nm AuNPs in the concentration of 6.05 × 1013 nanoparticles/mL a | [58] |
| Human spermatozoa | Affects viability and motility | 50 nm sized AuNPswith concentrations 30, 60, 125, 250 and 500 µM | [59] |
| L5178Y cells | No damage to the DNA at 60 nm but damage at 100 nm | 4, 50, 100 and 200 nm sized AuNPs in concentrations of 0, 6.25, 12.5, 25, 50, 100 and 200 μg/mL | [60] |
| MDA-MB-231 cells (Breast cells) | Reduction in proliferation | 1.9 nm spherical AuNPs (Aurovist™) | [61] |
| MG63 cells | Low long-term toxicity | AuNPs of diameter 10 nm in concentrations of 1 and 10 ppm | [62] |
| MRC-5 cells | Slight hepatotoxic and nephrotoxic | AuNPs capped with GNPC and GNPBwith an average diameter size of 15–20 nm and concentrations 51, 128, 320, 800, 2000 and 5000 ppm | [63] |
| MRC-5 cells | High lipid peroxidation, upregulation of antioxidants, expressions of protein and gene of stress response | 20 nm diameter AuNPs in 1 nM concentration | [64] |
| Vero, MRC-5, and NIH/3T3 cells | Reduction in growth related to apoptosis and autophagy | Nano-rod structure with an average length of 10–40 nm with concentrations 0, 36, 72, 180, 360 and 720 ng/mL | [65] |
| Rat liver | Yield a great lipid peroxidation | AuNPs of diameter 10 nm. Doses of 50 µL of NP solution | [66] |
| Tumor ascites and normal peritoneal cells | No morphological changes and cell death | Functionalized AuNPs of diameter 4.5, 10 and 20 nm in concentrations 10, 25, 50 and 100 mM | [67] |
| Vero cells | No toxicological effects | Porphyran-reduced AuNPs with an average particle size of 14 ± 2 nm in concentrations 10, 50 and 100 µM | [68] |
| Organism | Effects | Particle | References |
|---|---|---|---|
| BALB/c mice | Apoptosis and inflammation of liver tissue | 13 nm PEG-Coated AuNPs with the average injected numbers of particles per mice: 1.76 × 1011, 8.8 × 1011, and 4.4 × 1012 for low, middle, and high doses, respectively. | [95,96] |
| Broiler chicken | Caused recognisable oxidative damage to blood, histopathological changes, up-regulation of IL-6, expression of Nrf2 gene, fragmentation of DNA, a significant decrease in antibody titer against avian influenza (AI) and Newcastle disease (ND) | Gold nanoparticles colloidal solution (25 ± 5 nm) | [97] |
| D. magna | LC50 was reported as 2 mg/l after 48 h | Nanoparticles with a diameter of approximately 15 nm | [70] |
| D. magna, T. arcticus | LC50 was reported as 0.64 mg/l after 48 h for D. magna and 14.4 mg/l after 96 h for T. arcticus | 0–10 mg/L concentration of Au3+ | [98] |
| Drosophila melanogaster | Caused transmissible mutagenic effects | Citrate-capped 15 nm AuNPs in the concentration of 100 pM | [99] |
| Drosophila melanogaster | Sharp decline in fertility and life span, presence of DNA fragments, and strong over-expression of stress proteins | Citrate-capped 15 nm AuNPs in six different concentrations (1.9, 3.8, 19, 38, 190, and 380 pmol/L) dispersed in food | [100] |
| Female and male mice | Liver and kidney damage whose effects were sex-dependent. Damage to the neuronal system | Different diameters of AuNPs ranging from 3 to 100 nm | [101] |
| Female mice | Spherical AuNPs in live and macrophages | AuNPs of diameter 2, 4 and 100 nm in, respectively—15 × 1013 particles/mL, 9 × 1010/mL and the 100 nm 6 × 109/mL. | [102] |
| Fetal mouse organs | No indication of toxicity in the fetus and placenta | 20 and 50 nm AuNPs | [103] |
| Male CD1 mice | Accumulation at various parts of the brain | Protein and polyelectroylte coated AuNPs with a diameter of 15 ± 1 nm injected in the concentration of 144.5 nM | [104] |
| Male Wistar rats | AuNPs persist and accumulate in the spleen and liver | AuNPs of 20 nm diameter were injected at 15.1 µg/mL. | [11] |
| Male WU Wistar rats | Large particles of spherical AuNPs were observed in blood, spleen, and liver, while smaller particles were seen in the spleen, blood, thymus, lungs, liver, kidney, testis, heart, and brain | Gold nanoparticles have a 10, 50, 100 and 250 nm diameter. Injection concentration was respectively 77, 96, 89 and 108 µg/mL | [105] |
| ICR Mice | Lungs, kidney hemorrhage, lymphocytic infiltration, and inflammatory response | PEGylated 13 nm gold colloids | [87] |
| Mice | Liver damage | 5, 10, 30, and 60 nm PEG-coated AuNPS dosed 4000 µg/kg | [106] |
| Mice | Apoptosis and acute inflammation | 13 nm PEG-coated AuNPs. The mean quantities of particles injected per mouse were 1.76 × 1011, 8.8 × 1011, and 4.4 × 1012 for the low, medium, and high doses, respectively. | [96] |
| Mice | Affects kidney function and produces toxicity | GSH- and BSA-coated AuNCs with an average size of 2.1 nm. The injected concentration is up to 7550 µg/mL | [107] |
| Mice | Greatest toxicity and affecting organ index. Induced reduction in RBC, spleen index, and body weight | Citrate-capped AuNPs of diameter 13.5 nm in different concentrations varying from 137.5 to 2200 µg/kg | [93] |
| Mice | Produced no effect on normal growth | AuNPs capped with BSA and HSePEGeCOOH in a diameter of about 4 nm in various concentrations | [108] |
| BALB/C Mice | Caused loss of weight and appetite. However, smaller AuNPs did not produce any sickness | Naked colloidal AuNPs ranging in diameter from 3 to 100 nm injected intraperitoneally at a dose of 8 mg/kg/week | [5] |
| Mice (ddy) | AuNPs of all sizes were noticed in the spleen, liver, and lungs | AuNPs ranging in size from 15 to 200 nm administered in 1 g/kg intravenously | [109] |
| Pregnant C57BL/6 mice | Non-crossing of maternal-fetal barrier | 2 and 40 nm AuNPs injected intravenously and 40 nm intraperitoneally | [110] |
| Rats | Accumulation in the spleen and liver | PEG-coated AuNPsofdiameter ranging from 11 to 31 nm injected in various concentrations | [111] |
| Rats | ROS-induced cytotoxicity that is size-dependent | PEG-coated AuNPs in diameter ranging in size between 6.2 and 61.2 nm | [112] |
| Rats | Distribution of AuNPs was observed in the testis, liver, and kidney. However, there were no effects on the testis, whereas mild changes were noticed in the kidney and liver sections | AuNPs with an average size of 50 nm and various concentrations | [113] |
| Wistar rats | Traces of AuNPs in the kidney, spleen, liver, intestine, urine, and feces. Smaller NPs induced greater effects on DNA damage | AuNPs of 10, 30 or 60 nm diameter injected 0.4 mL/day | [54] |
| Wistar rats | Accumulate in neurons, liver, spleen, kidney, and cross the blood-brain barrier; no toxicity | 12.5 nm citrate-coated AuNPs in different doses—40, 200 and 400 µg/kg/day | [114] |
| Zebrafish embryo | Delay in the development of eyes and pigmentation | 1.3 nm AuNPs (functionalized with TMATeAuNPs)in concentrations ranging from 0.08 to 50 mg/L | [7] |
| System | Cell Line/Model | Nanoparticle Characterization | Effect | Reference |
|---|---|---|---|---|
| Nervous System | Porcine brain microvascular endothelial cells (pBMECs) | Diameter of 3 nm and 5 nm in concentration 15 µg/mL | No significant secretion of pro-inflammatory mediators (IL-1b, TNFα, PGE2) or negative effect on BBB integrity | [139] |
| Nervous System | human embryonic stem cells (hESCs) and neural derivatives | Diameter of 1.5 nm and 4 nm in six different concentrations ranging from 0.001 to 10 µg/mL | Pronounced neurotoxic effects, significant cell death, disruption of neural differentiation, altered DNA methylation patterns | [140] |
| Digestive System | Caco-2 cells (model for human intestinal epithelium) | Diameter less than 100 nm. Various concentrations of 0, 5, 10, 20, 40, 80, 125, 250, 500 or 1000 µg/mL | Internalisation without significant cytotoxic effects or oxidative stress | [42] |
| Digestive System | Model intestinal epithelial cell line | A diameter of 15 nm AuNPs dosed in the concentration of 50 ppm | Size-dependent absorption, accumulation, and cytotoxic effects, including mitochondrial dysfunction | [144] |
| Respiratory System | Human lung fibroblast cell line MRC-5 | Diameter of 1 nm. Cells treated with 1 nM concentration. | Induced oxidative stress, formation of autophagosomes, increased expression of autophagy-related proteins | [64] |
| Cardiovascular System | Human umbilical vein endothelial cells (HUVEC) | Diameter of approximately 20 nm Cells treated with about 1 × 1011 AuNPs. | No significant cytotoxicity, no lysis of human erythrocytes or apoptosis/necrosis of endothelial cells | [148] |
| Urinary System | Human kidney cells (HK-2) | Diameter of 13 nm and 60 nm. The concentrations ranging from 1 μM to 60 μM | 13 nm nanospheres (particularly coated with MUA) exhibit the highest toxicity, affecting mitochondrial function and inducing programmed cell death | [149] |
| Urinary System | HK-2 and 786-0 cells | Diameter of 5 nm and 200 nm. Cells treated in the concentrations of 1 and 10 μg/mL | Induced apoptosis and inhibited cell proliferation, differential effects on cellular parameters and signalling pathways | [150] |
| Sensory Organs | Keratinocyte cell line (HaCaT) and human epidermoid skin cancer cell line (A431) | AuNPs were synthesised with Vitis vinifera seed extract with a diameter ranging from 40 nm to 55 nm. Cells treated with 5, 10, 15, 20, 25 µM concentration | No cytotoxicity against HaCaT cells, cytotoxicity against cancer cells with increased reactive oxygen species and induction of apoptosis | [151] |
| Reproductive System | Human sperm | Diameter of 9 nm. Concentration of 44 ppm. | 25% of sperm lost mobility, evidence of nanoparticle penetration into sperm heads and tails | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. https://doi.org/10.3390/ijms25074057
Niżnik Ł, Noga M, Kobylarz D, Frydrych A, Krośniak A, Kapka-Skrzypczak L, Jurowski K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. International Journal of Molecular Sciences. 2024; 25(7):4057. https://doi.org/10.3390/ijms25074057
Chicago/Turabian StyleNiżnik, Łukasz, Maciej Noga, Damian Kobylarz, Adrian Frydrych, Alicja Krośniak, Lucyna Kapka-Skrzypczak, and Kamil Jurowski. 2024. "Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review" International Journal of Molecular Sciences 25, no. 7: 4057. https://doi.org/10.3390/ijms25074057
APA StyleNiżnik, Ł., Noga, M., Kobylarz, D., Frydrych, A., Krośniak, A., Kapka-Skrzypczak, L., & Jurowski, K. (2024). Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. International Journal of Molecular Sciences, 25(7), 4057. https://doi.org/10.3390/ijms25074057









