Genus Sambucus: Exploring Its Potential as a Functional Food Ingredient with Neuroprotective Properties Mediated by Antioxidant and Anti-Inflammatory Mechanisms
Abstract
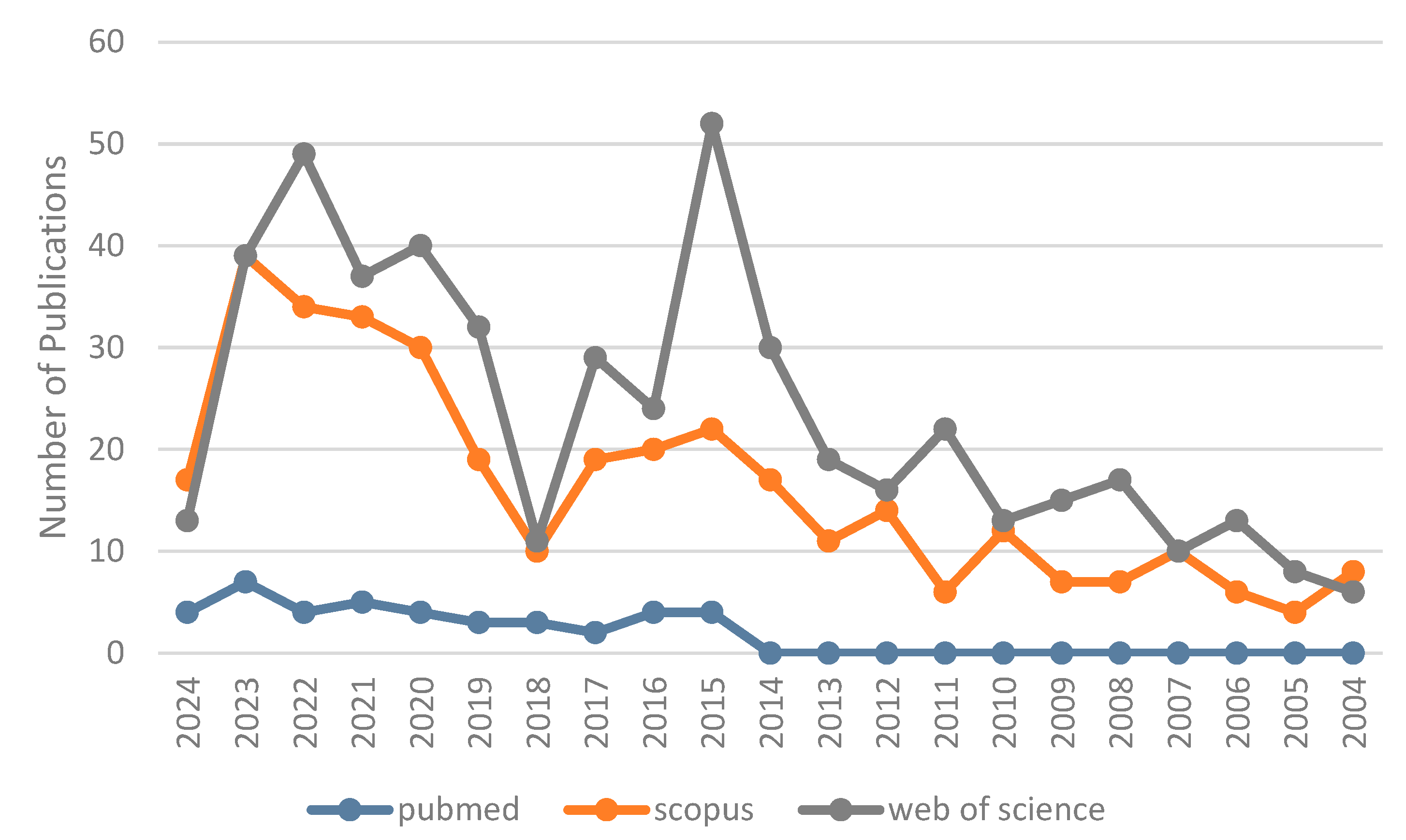
:1. Introduction
2. Study Design
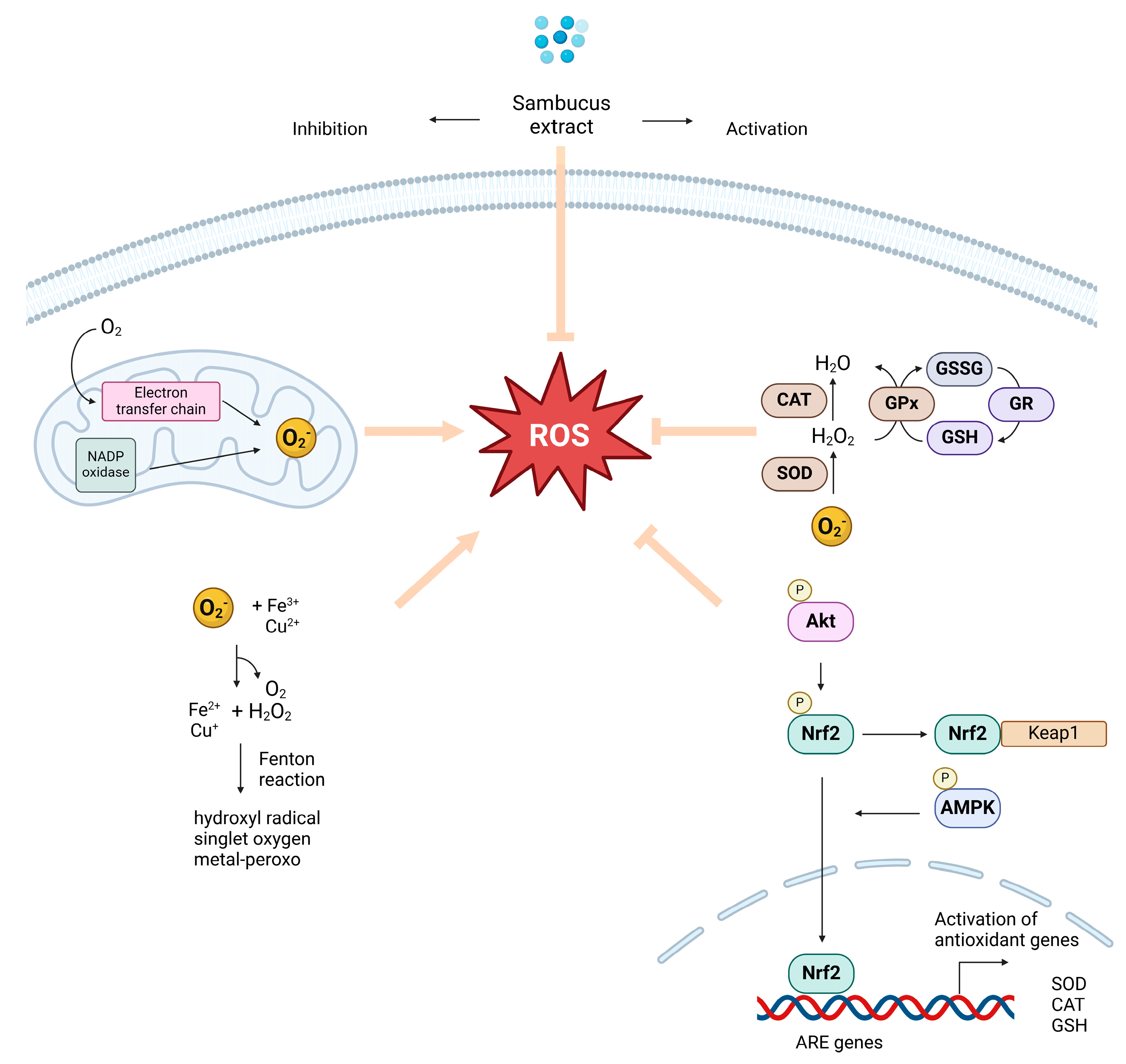
3. Oxidative Stress, Free Radicals, and Antioxidant Compounds Derived from Sambucus Plants: Implications for Neuroprotection
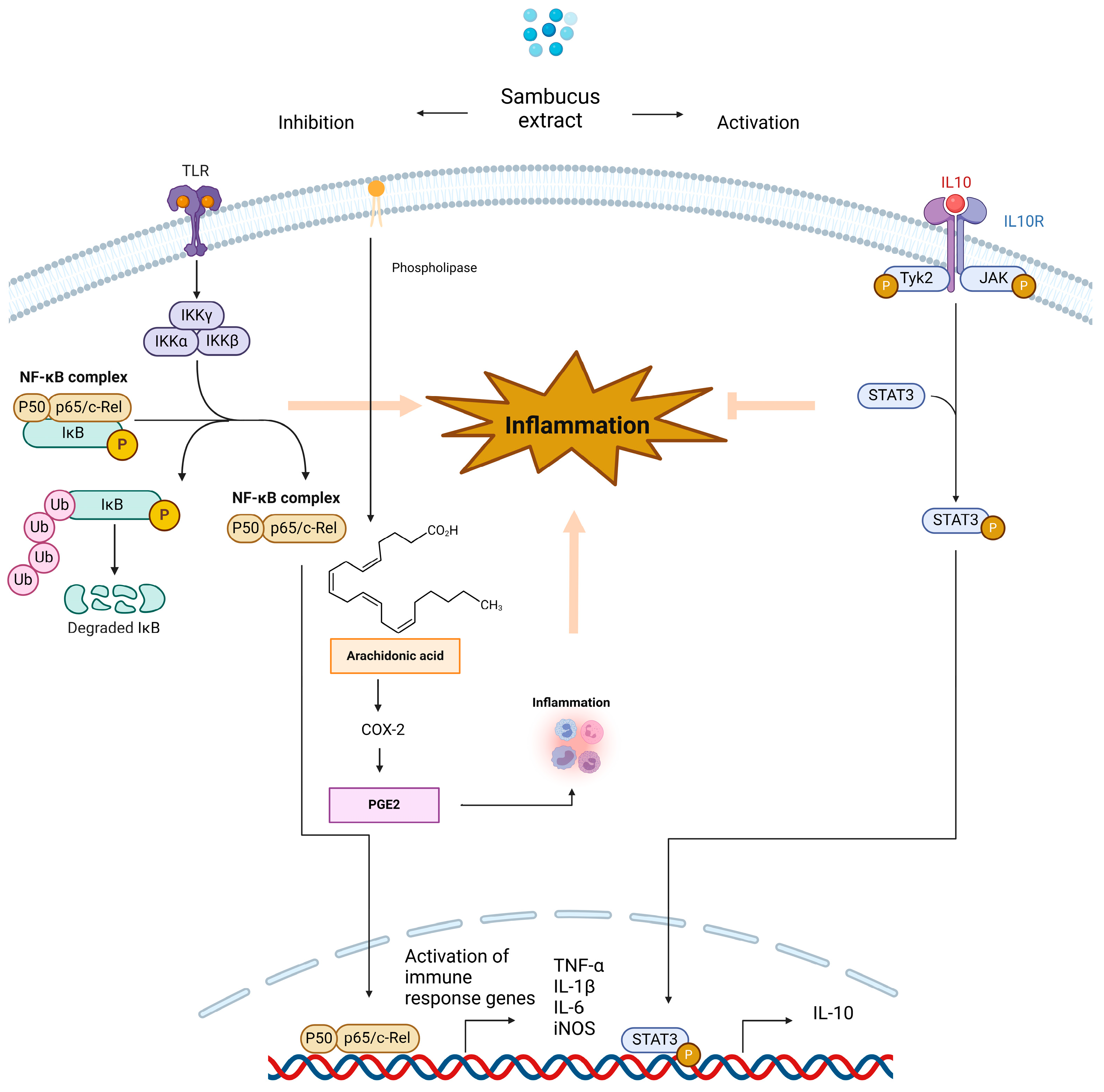
4. Inflammation, Inflammatory Mediators, and Anti-Inflammatory Compounds Derived from Sambucus Plants: Implications for Neuroprotection
5. Aging, Age-Related Neurodegeneration, and Anti-Aging Compounds Derived from Sambucus Plants
6. Neuroregenerative Activity of Bioactive Compounds Derived from Sambucus Plants
7. Neuroprotective Activity of Bioactive Compounds Derived from Sambucus Plants
8. Sambucus as a Functional Ingredient for Food
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waswa, E.N.; Mutinda, E.S.; Mkala, E.M.; Katumo, D.M.; Oulo, M.A.; Odago, W.O.; Amenu, S.G.; Ding, S.X.; Hu, G.W. Understanding the Taxonomic Complexes and Species Delimitation within Sambucus L. (Viburnaceae). Diversity 2022, 14, 906. [Google Scholar] [CrossRef]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef]
- Acharya, J.; Mukherjee, A. An account of Viburnum L. In the eastern Himalayan region. Acta Bot. Hung. 2014, 56, 253–262. [Google Scholar] [CrossRef]
- Amini, E.; Nasrollahi, F.; Sattarian, A.; Isazadeh-Araei, M.; Habibi, M. Morphological and anatomical study of the genus Sambucus L. (Adoxaceae) in Iran. Mod. Phytomorphol. 2021, 15, 23–29. [Google Scholar]
- Šiško, M.; Ivanuš, A.; Ivančič, A. Determination of Sambucus Interspecific Hybrid Structure using Molecular Markers. Agric. Sci. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Miraj, S. Chemical composition and pharmacological effects of Sambucus nigra. Der Pharma Chem. 2016, 8, 231–234. [Google Scholar]
- Shokrzadeh, M.; Saeedi Saravi, S.S. The chemistry, pharmacology and clinical properties of Sambucus ebulus: A review. J. Med. Plants Res. 2010, 4, 095–103. [Google Scholar]
- Jabbari, M.; Hashempur, M.H.; Razavi, S.Z.E.; Shahraki, H.R.; Kamalinejad, M.; Emtiazy, M. Efficacy and short-term safety of topical Dwarf Elder (Sambucus ebulus L.) versus diclofenac for knee osteoarthritis: A randomized, double-blind, active-controlled trial. J. Ethnopharmacol. 2016, 188, 80–86. [Google Scholar] [CrossRef]
- Ciocoiu, M.; Mirón, A.; Mares, L.; Tutunaru, D.; Pohaci, C.; Groza, M.; Badescu, M. The effects of Sambucus nigra polyphenols on oxidative stress and metabolic disorders in experimental diabetes mellitus. J. Physiol. Biochem. 2009, 65, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Zivković, J.; Tadić, V.; Arsić, I. Skin protection against solar UV radiation by natural plant products: Extracts from elder fruit (Sambucus nigra L.). In Proceedings of the Fifth International Conference on Radiation and Applications in Various Fields of Research, RAD Conference Proceedings, Budva, Muntenegru, 12–16 June 2017; Volume 2, pp. 231–236. [Google Scholar]
- Bahiense, J.B.; Marques, F.M.; Figueira, M.M.; Vargas, T.S.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharm. Biol. 2017, 55, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra extracts–natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Rodino, S.; Butu, A.; Petrache, P.; Butu, M.; Dinu-Pîrvu, C.; Cornea, C.P. Evaluation of the antimicrobial and antioxidant activity of sambucus ebulus extract. Farmacia 2015, 63, 751–754. [Google Scholar]
- Haș, I.M.; Teleky, B.E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Basile, B.; Mataffo, A.; Yousefi, S.; Salami, S.A.; Perrone, A.; Martinelli, F. Cultivation, Phytochemistry, Health Claims, and Genetic Diversity of Sambucus nigra, a Versatile Plant with Many Beneficial Properties. Horticulturae 2023, 9, 488. [Google Scholar] [CrossRef]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Ağalar, H.; Demirci, B.; Demirci, F.; Kırımer, N. The Volatile Compounds of the Elderflowers Extract and the Essential Oil. Rec. Nat. Prod. 2017, 5, 491–496. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Najda, A.; Sałata, A.; Krajewska, A. Bioactive compounds and antioxidant properties of black elderberry (Sambucus nigra L.). Acta Sci. Pol. Hortorum Cultus 2022, 21, 143–156. [Google Scholar]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Gleńsk, M.; Czapińska, E.; Woźniak, M.; Ceremuga, I.; Włodarczyk, M.; Terlecki, G.; Ziółkowski, P.; Seweryn, E. Triterpenoid Acids as Important Antiproliferative Constituents of European Elderberry Fruits. Nutr. Cancer. 2017, 69, 643–651. [Google Scholar] [CrossRef]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Bahorun, T.; Jen, L.S. Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat. Res./Rev. Mutat. Res. 2003, 544, 03–215. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-Derived Neuroprotective Agents: Cutting the Cycle of Cell Death through Multiple Mechanisms. Evid.-Based Complement. Altern. Med. 2017, 2017, 3574012. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Trush, M. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Nikolova, K.; Buhalova, D.; Andonova, V.; Gugleva, V.; Petkova, N.; Yotkovska, I.; Ivanova, N. Antioxidant Activity and Chemical Characteristics of Sambucus nigra L. Blossom from Different Regions in Bulgaria. Horticulturae 2022, 8, 309. [Google Scholar] [CrossRef]
- Palomino, O.; García-Aguilar, A.; González, A.; Guillén, C.; Benito, M.; Goya, L. Biological actions and molecular mechanisms of sambucus Nigra L. In neurodegeneration: A cell culture approach. Molecules 2021, 26, 4829. [Google Scholar] [CrossRef]
- Franceschelli, S.; Lanuti, P.; Ferrone, A.; Gatta, D.M.P.; Speranza, L.; Pesce, M.; Grilli, A.; Cacciatore, I.; Ricciotti, E.; Di Stefano, A.; et al. Modulation of apoptotic cell death and neuroprotective effects of glutathione—L-Dopa codrug against H2O2-induced cellular toxicity. Antioxidants 2019, 8, 319. [Google Scholar] [CrossRef]
- Franzoni, F.; Scarfò, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002, 33, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chen, C.M. The role of oxidative stress in Parkinson’s disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Neves, D.; Valentão, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, J.M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- May, N.; de Sousa Alves Neri, J.L.; Clunas, H.; Shi, J.; Parkes, E.; Dongol, A.; Wang, Z.; Jimenez Naranjo, C.; Yu, Y.; Huang, X.F.; et al. Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients 2023, 15, 3912. [Google Scholar] [CrossRef]
- Simonyi, A.; Chen, Z.; Jiang, J.; Zong, Y.; Chuang, D.Y.; Gu, Z.; Lu, C.H.; Fritsche, K.L.; Greenlief, C.M.; Rottinghaus, G.E.; et al. Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sci. 2015, 128, 30–38. [Google Scholar] [CrossRef]
- Jiang, J.M.; Zong, Y.; Chuang, D.Y.; Lei, W.; Lu, C.H.; Gu, Z.; Fritsche, K.L.; Thomas, A.L.; Lubahn, D.B.; Simonyi, A.; et al. Effects of elderberry juice from different genotypes on oxidative and inflammatory responses in microglial cells. Acta Hortic. 2015, 1061, 281–288. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Timmers, M.; Ma, S.Y.; Thieme, A.; McCabe, G.P.; Vest, J.H.C.; Lila, M.A.; Rochet, J.C. Lumbee, traditional medicine: Neuroprotective activities of medicinal plants used to treat Parkinson’s disease-related symptoms. J. Ethnopharmacol. 2017, 206, 408–425. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kiss, A.L. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. 2022, 27, 1610136. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Butola, L.K.; Dhok, A.; Ambad, R.; Kanyal, D.; Jha, R.K. Roshan Kumar Jha Leukotrienes and Inflammation—A Review. Indian J. Forensic Med. Toxicol. 2021, 15, 295–301. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.Y. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Nakaso, K. Roles of Microglia in Neurodegenerative Diseases. Yonago Acta Med. 2024, 67, 1–8. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. Proc. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Khan, A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Rashid, S.; Alsaffar, R.M.; Kamal, M.A.; Rehman, M.U. Inflammation and Alzheimer’s Disease: Mechanisms and Therapeutic Implications by Natural Products. Mediat. Inflamm. 2021, 2021, 9982954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Wangensteen, H.; Barsett, H. Elderberry and elderflower extracts, phenolic compounds, and metabolites and their effect on complement, RAW 264.7 macrophages and dendritic cells. Int. J. Mol. Sci. 2017, 18, 584. [Google Scholar] [CrossRef] [PubMed]
- Santin, J.R.; Benvenutti, L.; Broering, M.F.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; de Souza, J.A.; Kopp, M.A.T.; de Souza, P.; da Silva, R.d.C.V.; et al. Sambucus nigra: A traditional medicine effective in reducing inflammation in mice. J. Ethnopharmacol. 2022, 283, 114736. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Rychlik, J.; Juzwa, W.; Myszka, K.; Dembczyński, R.; Białas, W. Anti-inflammatory effects of gastrointestinal digested Sambucus nigra L. fruit extract analysed in co-cultured intestinal epithelial cells and lipopolysaccharide-stimulated macrophages. J. Funct. Foods 2015, 19, 649–660. [Google Scholar] [CrossRef]
- Seymenska, D.; Teneva, D.; Nikolova, I.; Benbassat, N.; Denev, P. In Vivo Anti-Inflammatory and Antinociceptive Activities of Black Elder (Sambucus nigra L.) Fruit and Flower Extracts. Pharmaceuticals 2024, 17, 409. [Google Scholar] [CrossRef]
- Namakin, K.; Moghaddam, M.H.; Sadeghzadeh, S.; Mehranpour, M.; Vakili, K.; Fathi, M.; Golshan, A.; Bayat, A.H.; Tajik, A.H.; Eskandari, N.; et al. Elderberry diet improves gut-brain axis dysfunction, neuroinflammation, and cognitive impairment in the rat model of irritable bowel syndrome. Metab. Brain Dis. 2023, 38, 1555–1572. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Y.; Tang, S.; Chen, Q.; Zhang, M.; Ma, J.; Qin, J.; Yu, H. Anti-Aging Effects of Anthocyanin Extracts of Sambucus canadensis Caused by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 1528. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Ghosh, S.K.; Kumari, C. Adult neurogenesis in humans: A review of basic concepts, history, current research, and clinical implications. Innov. Clin. Neurosci. 2019, 16, 30–37. [Google Scholar] [PubMed]
- Enciu, A.M.; Nicolescu, M.I.; Manole, C.G.; Mureşanu, D.F.; Popescu, L.M.; Popescu, B.O. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Tyler, S.E.B.; Tyler, L.D.K. Pathways to healing: Plants with therapeutic potential for neurodegenerative diseases. IBRO Neurosci. Reports 2023, 14, 210–234. [Google Scholar] [CrossRef]
- Haratizadeh, S.; Ebrahim Zadeh, M.A.; Nazm Bojnordi, M.; Ghasemi Hamidabadi, H.; Abdanipour, A.; Akhtari, J. Effect of sambucus ebulus extract on neural stem cell prolifration under oxidative stress condition. Sci. J. Kurdistan Univ. Med. Sci. 2018, 23, 26–35. [Google Scholar]
- Liu, S.P.; Hsu, C.Y.; Fu, R.H.; Huang, Y.C.; Chen, S.Y.; Lin, S.Z.; Shyu, W.C. Sambucus williamsii induced embryonic stem cells differentiated into neurons. Biomedicine 2015, 5, 3. [Google Scholar] [CrossRef]
- Suh, W.S.; Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Iridoid Glycosides from the Twigs of Sambucus williamsii var. coreana and Their Biological Activities. J. Nat. Prod. 2017, 80, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhshi, H.; Moghaddam, M.H.; Sani, M.; Parvardeh, S.; Boroujeni, M.E.; Vakili, K.; Fathi, M.; Azimi, H.; Mehranpour, M.; Abdollahifar, M.A.; et al. The elderberry diet protection against intrahippocampal Aβ-induced memory dysfunction; the abrogated apoptosis and neuroinflammation. Toxicol. Res. 2023, 12, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhshi, H.; Irani, S.; Aliaghaei, A. Elderberry Diet Improves Memory Function and Prevents Cell Death in Rat Models of Alzheimer’s Disease Induced by Amyloid Beta Injection. J. Otorhinolaryngol. Facial Plast. Surg. 2022, 8, 1–6. [Google Scholar]
- Mendes, D.; Peixoto, F.; Oliveira, M.M.; Andrade, P.B.; Videira, R.A. Brain Effects of SC-Nanophytosomes on a Rotenone-Induced Rat Model of Parkinson’s Disease—A Proof of Concept for a Mitochondria-Targeted Therapy. Int. J. Mol. Sci. 2022, 23, 12699. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.H.; Bayat, A.H.; Eskandari, N.; Abdollahifar, M.A.; Fotouhi, F.; Forouzannia, A.; Rafiei, R.; Hatari, S.; Seraj, A.; Shahidi, A.M.E.J.; et al. Elderberry diet ameliorates motor function and prevents oxidative stress-induced cell death in rat models of Huntington disease. Brain Res. 2021, 1762, 147444. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.F.; Musich, M.; Costa, A.N.; Gonzales, J.; Gonzales, H.; Ferguson, B.J.; Kille, B.; Thomas, A.L.; Wei, X.; Liu, P.; et al. Feasibility and Preliminary Efficacy of American Elderberry Juice for Improving Cognition and Inflammation in Patients with Mild Cognitive Impairment. Int. J. Mol. Sci. 2024, 25, 4352. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Muhire, É.; Annabi, B. Antiproliferative efficacy of elderberries and elderflowers (Sambucus canadensis) on glioma and brain endothelial cells under normoxic and hypoxic conditions. J. Funct. Foods 2018, 40, 164–179. [Google Scholar] [CrossRef]
- Fathi, H.; Ebrahimzadeh, M.A.; Ziar, A.; Mohammadi, H. Oxidative damage induced by retching; antiemetic and neuroprotective role of Sambucus ebulus L. Cell Biol. Toxicol. 2015, 31, 231–239. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Patinha, S.; Rudnitskaya, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M. Sustainable Valorization of Sambucus nigra L. Berries: From Crop Biodiversity to Nutritional Value of Juice and Pomace. Foods 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Veloso, M.I.; Coelho, E.; Trabulo, O.; Coimbra, M.A. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Appl. Sci. 2022, 12, 9463. [Google Scholar] [CrossRef]
- Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin digestibility and stability of elderberry antioxidants to heat treatment in vitro. Molecules 2017, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Csorba, V.; Tóth, M.; László, A.M.; Kardos, L.; Kovács, S. Cultivar and year effects on the chemical composition of elderberry (Sambucus nigra L.) fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 770–782. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Appenteng, M.K.; Krueger, R.; Johnson, M.C.; Ingold, H.; Bell, R.; Thomas, A.L.; Greenlief, C.M. Cyanogenic glycoside analysis in American elderberry. Molecules 2021, 26, 1384. [Google Scholar] [CrossRef] [PubMed]
- Baeza, R.; Sánchez, V.; Salierno, G.; Molinari, F.; López, P.; Chirife, J. Storage stability of anthocyanins in freeze-dried elderberry pulp using low proportions of encapsulating agents. Food Sci. Technol. Int. 2021, 27, 135–144. [Google Scholar] [CrossRef]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789048186617. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Myracle, A.D. Development and evaluation of kefir products made with aronia or elderberry juice: Sensory and phytochemical characteristics. Int. Food Res. J. 2018, 25, 1373–1383. [Google Scholar]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.M.L.P.V.O.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef] [PubMed]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophysics 2019, 33, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in embedding techniques of anthocyanins: Improving stability, bioactivity and bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Šojić, B.; Đurović, S.; Radojković, M. Elderberry (Sambucus nigra L.) juice as a novel functional product rich in health-promoting compounds. RSC Adv. 2020, 10, 44805–44814. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef] [PubMed]
- Mratinic, E.; Fotiric, M. Selection of black elderberry (Sambucus nigra L.) and evaluation of its fruits usability as biologically valuable food. Genetika 2007, 39, 305–314. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Légrádi, F.; Abrankó, L.; Stéger-Máté, M. Effects of food processing technology on valuable compounds in elderberry (Sambucus nigra L.) varieties. Acta Biol. Szeged. 2014, 58, 45–48. [Google Scholar]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Edible Films Prepared with Different Biopolymers, Containing Polyphenols Extracted from Elderberry (Sambucus nigra L.), to Protect Food Products and to Improve Food Functionality. Food Bioprocess Technol. 2020, 13, 1742–1754. [Google Scholar] [CrossRef]
- Bratu, M.M.; Doroftei, E.; Negreanu-Pirjol, T.; Hostina, C.; Porta, S. Determination of antioxidant activity and toxicity of Sambucus nigra fruit extract using alternative methods. Food Technol. Biotechnol. 2012, 50, 177–182. [Google Scholar]
- Banach, M.; Khaidakov, B.; Korewo, D.; Węsierska, M.; Cyplik, W.; Kujawa, J.; Ahrné, L.M.; Kujawski, W. The chemical and cytotoxic properties of sambucus Nigra extracts—A natural food colorant. Sustainability 2021, 13, 12702. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nogueira, A.; Rocha, C.M.R.; Wilson, C.P.; Teixeira, J.A.; Botelho, C. Sambucus nigra flower and berry extracts for food and therapeutic applications: Effect of gastrointestinal digestion on in vitro and in vivo bioactivity and toxicity. Food Funct. 2022, 13, 6762–6776. [Google Scholar] [CrossRef]

| Species | Animal Model | Sambucus Treatment | Dose of the Sambucus Extract | Duration of Treatment | Neuroprotective Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|
| Sambucus nigra | Rat model of Alzheimer’s disease | Elderberry-enriched diet | 2% | 8 weeks | Improved memory and learning functions Alleviated astrogliosis and astrocyte reactivity Reduced apoptosis and neuronal degeneration Preserved spatial distribution of hippocampal neurons | Antioxidant Anti-inflammatory (decreased TNF-α and IL-1β) Anti-apoptotic (decreased caspase-3) | [83] |
| Sambucus nigra | Rat model of Alzheimer’s disease | Elderberry-enriched diet | 2% | 8 weeks | Improved spatial memory, learning, and long-term memory Prevented neuronal degeneration in the hippocampus Increased neuronal density and decreased number of degenerated neurons | Not specified | [84] |
| Sambucus nigra | Rat model of Parkinson’s disease | Elderberry-enriched diet: SC-Nanophytosomes (elderberry anthocyanins and marine algae polar membrane lipids) | 2.5 mg/mL | 3 weeks | Improved motor symptoms Normalized α-synuclein levels Restored antioxidant enzyme activity in brain regions | Attenuated mitochondrial dysfunction Enhanced respiratory control rate Increased activity of individual respiratory complexes Improved fatty acid profile of membrane phospholipids | [85] |
| Sambucus nigra | Rat model of Huntington’s disease | Elderberry diet | 2% | 8 weeks | Improved motor coordination, locomotion and muscle activity Prevented striatal volume reduction and neuronal loss Attenuated microglial activation and neuroinflammation Reduced apoptotic marker caspase-3 and pro-inflammatory cytokine TNF-α Decreased reactive oxygen species and enhanced glutathione content in the striatum | Anti-inflammatory Anti-apoptotic Antioxidant | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merecz-Sadowska, A.; Sitarek, P.; Zajdel, K.; Sztandera, W.; Zajdel, R. Genus Sambucus: Exploring Its Potential as a Functional Food Ingredient with Neuroprotective Properties Mediated by Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2024, 25, 7843. https://doi.org/10.3390/ijms25147843
Merecz-Sadowska A, Sitarek P, Zajdel K, Sztandera W, Zajdel R. Genus Sambucus: Exploring Its Potential as a Functional Food Ingredient with Neuroprotective Properties Mediated by Antioxidant and Anti-Inflammatory Mechanisms. International Journal of Molecular Sciences. 2024; 25(14):7843. https://doi.org/10.3390/ijms25147843
Chicago/Turabian StyleMerecz-Sadowska, Anna, Przemysław Sitarek, Karolina Zajdel, Wiktoria Sztandera, and Radosław Zajdel. 2024. "Genus Sambucus: Exploring Its Potential as a Functional Food Ingredient with Neuroprotective Properties Mediated by Antioxidant and Anti-Inflammatory Mechanisms" International Journal of Molecular Sciences 25, no. 14: 7843. https://doi.org/10.3390/ijms25147843






