Abstract
Nowadays, owing to the new technological and industrial requirements for equipment, such as flexibility or multifunctionally, the development of all-solid-state supercapacitors and Li-ion batteries has become a goal for researchers. For these purposes, the composite material approach has been widely proposed due to the promising features of woven carbon fiber as a substrate material for this type of material. Carbon fiber displays excellent mechanical properties, flexibility, and high electrical conductivity, allowing it to act as a substrate and a collector at the same time. However, carbon fiber’s energy-storage capability is limited. Several coatings have been proposed for this, with nanostructured transition metal oxides being one of the most popular due to their high theoretical capacity and surface area. In this overview, the main techniques used to achieve these coatings—such as solvothermal synthesis, MOF-derived obtention, and electrochemical deposition—are summarized, as well as the main strategies for alleviating the low electrical conductivity of transition metal oxides, which is the main drawback of these materials.
1. Introduction
In recent years, energy-storage devices have become an essential part of our technology. In this way, the markets for both batteries and supercapacitors have continued to grow [1,2]. In the case of the battery market, valued in 2020 at USD 12.9 billion, it is expected to grow by 15.8% in terms of its compound annual growth rate (CAGR) until at least 2030 [3]. Despite the fact that there are several kinds of batteries within this market, not only are Li-ion batteries the most popular ones, but they are also expected to increase the most in market volume in absolute terms [3]. On the other hand, supercapacitors are said to be the great revolution in the energy-storage field. This market, valued at USD 4.46 billion in 2023, is expected to continue expanding by a 14.1% CAGR until 2030 [4]. However, the new central role of both markets has been to meet not only the increased demand but also the technical requirements of these devices. New supercapacitors and batteries, with increasingly higher power and energy densities, are needed to meet the requirements of technological advancements [5]. In fact, not only are electrochemical performance requirements on the rise, but so is the development of energy-storage devices with new functionalities, such as flexibility or the ability to act as a structural component. Regarding flexible energy-storage devices, the growing demand is driven by the significant expansion of the flexible electronic device market in recent years via smartphones and tablets [6]. Flexibility has been extended to new devices, especially in the field of monitoring biomedical signals, thanks to their easy wearability [7,8]. On the other hand, structural energy-storage devices are particularly interesting in terms of improving the performance of new electric vehicles through the combination of lightness and good mechanical behavior [9].
In this way, the electric vehicle market is presented as one of the largest consumers of these types of energy-storage devices, being estimated at EUR 518.000 million in 2023, and is expected to witness an annual growth rate of 14.5% from 2024 to 2030 [10]. Currently, electric vehicles have emerged as a major alternative to traditional gasoline or diesel combustion vehicles, being widely promoted both politically and industrially to curb the high levels of pollution in big cities [11] and the depletion of oil reserves [12,13]. However, electric vehicles have not yet fully surpassed combustion vehicles due, among other things, to their lower performance, especially in terms of autonomy, and higher weight (around 33% higher) [14]. To mitigate this problem, one of the most efficient alternatives is a reduction in the total vehicle mass, allowing the same amount of energy to propel a vehicle for a larger number of kilometers. Here is where the concept of multifunctionality gains importance, developing structures that, in addition to having sufficient structural properties for the desired applications, can also store energy. In this way, the total or partial need for conventional batteries or supercapacitors could be reduced, decreasing the vehicle’s final weight and thus increasing its autonomy [15].
The development of multifunctional materials that can also store energy while presenting good structural properties has been primarily focused on the development of composite materials [15]. The popularity of composite materials for such applications is mainly due to the suitability of carbon fiber as a mechanical reinforcement and as a substrate for electrodes. Its excellent structural properties, such as high elastic modulus and high tensile strength, make carbon fiber fabric an excellent candidate for the development of structural composite materials [16]. Additionally, its high electrical conductivity also allows people to use it as a current collector for application in supercapacitors and batteries [17]. On the other hand, the small diameter of the carbon fibers also enables the development of flexible composite materials [16,18]. In this way, multifunctional composite materials, developed for energy-storage purposes, are commonly based on carbon fiber electrodes; separators made of insulating fabric, usually fiberglass; and a polymeric matrix that acts as an electrolyte [19,20,21], as shown in the schematics in Figure 1a,b.
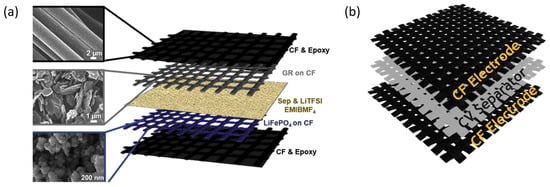
Figure 1.
(a) A reported structural battery scheme [21]. Copyright Elsevier, 2020. (b) Multifunctional material for energy-storage-layers scheme.
However, the development of this type of material faces two main challenges. The first one is the development of solid electrolytes, where high ionic mobility is a requirement for both supercapacitor and battery electrolyte development. However, as the stiffness increases, being necessary to obtain structural composites, the ionic mobility in the polymeric matrix decreases [15,22,23]. The second challenge is the functionalization of carbon fiber to act as a functional electrode [24]. Despite the fact that unmodified carbon fiber has been reported on several occasions to be a structural lithium-ion battery anode [22], its performance can be significantly increased through coating with other materials [24]. These coatings are necessary in the case of the development of cathodes [25,26,27,28] or supercapacitor electrodes [29,30,31].
The challenges in developing solid-state energy-storage systems, along with the trade-off between stiffness and ionic conductivity, necessitate the definition of new parameters to evaluate the multifunctionality of the materials being developed. In 2011, O’Brien et al. [32] defined multifunctional efficiency ( as the sum of the ratios between the specific energy () and the elastic modulus () of the multifunctional material with the specific energy ( and the elastic modulus ( of a conventional energy-storage system and a conventional structural material, respectively (Equation (1)). This definition was later expanded in 2015 by Snyder et al. [33], redefining these ratios to incorporate the shear modulus and specific power (Equations (2) and (3)).
Thus, a multifunctional composite material must satisfy to be of real interest to the industry. However, actually, the most advanced multifunctional materials have barely achieved multifunctional efficiencies of 0.5 [22].
2. TMOs for Energy-Storage Applications
As will be more extensively discussed in the next sections, TMOs have been widely reported for Li-ion battery anode and supercapacitor electrode applications. Nevertheless, the requirements for these energy-storage components, the TMO advantages and disadvantages for these purposes, and the strategies to address the drawbacks should be clarified in order to develop a comprehensive state of the art.
2.1. TMOs as Supercapacitor Electrodes
Supercapacitors are energy-storage devices in which the primary mechanism of energy storage is electrostatic interactions. Supercapacitors consist of three main components: two electrodes connected by an electrical circuit, and a dielectric medium between them called electrolyte. During charging cycles, an applied potential polarizes the electrodes, causing charges within the electrolyte to align on the surface of the electrodes, forming an electric double-layer capacitor (EDLC) through electrostatic interactions [34]. This arrangement is schematically illustrated in Figure 2a. The charge stored in this EDLC increases linearly with the potential. Moreover, the addition of a new charge element to the EDLC involves additional electrical work due to previously accumulated elements having the same charge sign [35]. On the other hand, during discharge, the external potential ceases, and electric current flows from the negatively charged electrode to the positive one, while charges in the electrolyte homogenize through diffusion. As a result, the potential between electrodes decreases linearly as the charge storage decreases. Because of this linear dependence of the accumulated or released charge on potential, the concept of capacitance emerges. Capacitance () is defined as the change in charge () with respect to the change in potential ()—in other words, the slope of this linear relationship. Thus, considering the mass or volume of material (), the specific capacitance () is defined as Equation (4) [36]:
In an EDLC supercapacitor, the relationship between charge and potential mainly depends on the area of the electric double layer () and its thickness (). Taking into account that the thickness may not be constant and the permittivity of the double layer () and vacuum (), the capacitance of the electrode is defined as Equation (5) [37]:
Moreover, it is also necessary to quantify the energy that a device can accumulate or release, hence the concept of specific energy or energy density (), which, considering the linear relationship between the charge and the potential, is given by [36] (Equation (6)):
On a technical level, it is not only important to consider the energy stored by the device, but it is also necessary to consider the power that can be supplied, that is, how quickly it can release that energy. Thus, the specific power or power density (), considering the discharge time (), can be defined as Equation (7) [38]:
The specific power of supercapacitors is particularly high compared to other energy-storage systems such as batteries or fuel cells, as can be seen in the Ragone plot shown in Figure 2b. This is because the energy-storage mechanism is based on electrostatic interactions, weak interactions without charge transfer, that allow rapid desorption of ions from the electric double layer. However, this same weakness of the bonds results in these devices having lower stored energy compared to their aforementioned competitors [39,40].
Nevertheless, there is another mechanism by which a supercapacitor can store energy, known as pseudocapacitance, characteristic of pseudocapacitors. Pseudocapacitance involves faradaic reactions between the polarized electrode surface and ions in the electrolyte with partial charge transfer. Since these reactions take place at a specific potential, there is an increase in the accumulated charge at that potential. As a result, the relationship between accumulated charge and potential is not linear. Figure 2c shows the galvanostatic charge–discharge and cyclic voltammetry tests for comparison between EDLC supercapacitors and pseudocapacitors. In galvanostatic tests, the accumulation of charge at a specific potential due to pseudocapacitive reactions results in a plateau in the curve at that potential, while in cyclic voltammetry tests, these reactions appear as peaks [41]. This additional energy-storage mechanism provides additional capacitance that depends on the number of exchanged electrons () in the reaction and can be theoretically calculated as Equation (8) [42], where is Faraday’s constant, is the molar mass of the material, and is the potential window.
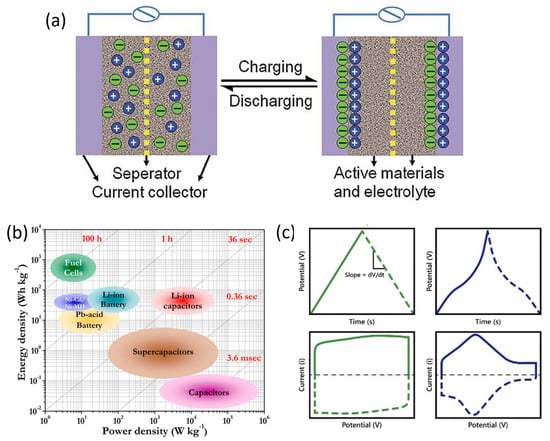
Figure 2.
(a) Supercapacitor charge−discharge mechanism [43]. Copyright Elsevier, 2013. (b) Ragone plot. Reproduced from [44]. (c) Typical GCD (up) and CV (down) curves of EDLC supercapacitors (green) and pseudocapacitors (blue). Reproduced from [45].
During recent years, transition metal oxides (TMOs) have been extensively studied as materials for supercapacitor electrodes. The interest is driven by two fundamental reasons: the reactivity of these compounds to undergo pseudocapacitance reactions and their ease of synthesis in various nano-scale morphologies [42]. Both the presence of pseudocapacitance reactions and high values of surface area, resulting from the low dimensionality of these nanostructures, favor elevated specific capacitance values. However, the main drawback of TMOs for supercapacitor electrode applications is their low electrical conductivity [46,47,48]. This lack of electrical conductivity restricts the passage of electrons involved in chemical reactions, as well as the formation of the electric double layer. For this reason, several strategies to improve this aspect have been widely suggested [42,49]:
- Combination with high electrical conductivity materials. The combined use with other compounds that exhibit high electrical conductivity, such as carbon nanoplates, graphene oxide, reduced graphene oxide, carbon nanotubes [50,51], carbon black, MXenes, or conductive polymers [52,53].
- Sulfurization or selenization. These elements, which are softer Pearson bases than oxygen, polarize the metal bond less, reducing the band gap [54].
- Creation of oxygen vacancies. Oxygen vacancies lead to the presence of metal cations with a higher oxidation state within the crystalline structure, resulting in the appearance of discrete energy levels allowed in the band gap, facilitating the passage of electrons from the valence band to the conduction band [55,56].
- Doping with donor dopants. Doping with elements such as P, which promotes electrons to the conduction band [57].
- Preparation of multimetallic or multivalent TMOs. Similarly to the previous case, the presence of different cations leads to lower band gaps due to the greater richness of allowed energy levels [58,59].
2.2. TMOs as Li-Ion Batteries Anodes
Rechargeable batteries are energy-storage devices whose charge-storage mechanism is based on reversible faradaic reactions [35]. Li-ion batteries have been the most widely distributed and commercialized since they entered the market due to the small atomic radius of Li+ facilitating its diffusion. Moreover, the Li+/Li potential is quite low, −3.04 V versus a standard hydrogen electrode, resulting in a high potential that translates into high energy and power densities [60]. The energy density of batteries is higher than that achieved by supercapacitors [39], as can be observed in Figure 2b. This higher energy density is due to the storage through chemical reactions, which are more energetic than the electrostatic interactions characteristic of supercapacitors. However, this same difference also results in a lower power density due to the kinetic limitations of charge-transfer reactions [35].
Lithium-ion batteries consist of a negative electrode or anode, a positive electrode or cathode, and an electrolyte. As shown in Figure 3a, the Fermi level of the anode (or electrochemical potential of the anode) with Li intercalated () is higher than the electrochemical potential of the cathode material (), so initially, it is the cathode that contains lithium [61]. The difference between the two Fermi levels corresponds to the open-circuit voltage () of the battery by Equation (9), with being the electron charge [62]:
During discharge, Fermi levels tend to equalize, so a current flow from the anode to the cathode is accompanied by the deintercalation of Li+ from the anode. Moreover, the intercalation into the cathode and the corresponding diffusion through the electrolyte also tend to equalize. On the contrary, during charging, an external potential raises the cathode potential above the anode, promoting the reverse process, the Li+ intercalation in the anode and the deintercalation in the cathode [63]. A scheme of the process is given in Figure 3b. In ideal conditions, the open-circuit voltage would correspond to the potential required to charge the battery () and would be the potential exhibited during discharge (), following Equations (10) and (11). However, there is a polarization overpotential () that increases the former and decreases the latter [62].
The overpotential consists of three components [60]: the activation overpotential, the ohmic polarization overpotential, and the concentration polarization overpotential [64]. The first one, the activation overpotential, is related to the activation energy of charge-transfer reactions. The second one, the ohmic polarization overpotential, is due to the internal resistance of the electrodes. Finally, the concentration polarization overpotential is due to limitations in the transport of Li+ ions to the active sites of the electrode. On the other hand, considering the specific case of batteries, the energy-storage mechanism renders the magnitude defined as capacitance in the case of supercapacitors obsolete, due to charge storage occurring at a specific potential rather than varying linearly with it [35]. Thus, the specific capacity of a battery (Equation (12)) is defined as the magnitude that measures the charge provided by it (), i.e., the integral of the current provided between the start and end of discharge per unit mass or volume () [62,65]. Moreover, it is related to energy density that can be defined as Equation (13) since the charge () is released at constant potential ().
This release of charge at constant potential gives rise to very characteristic curves, both in cyclic voltammetry tests and galvanostatic charge–discharge tests. In the former, intense peaks appear at the potential of the reaction [45]. On the contrary, these peaks appear as plateaus in galvanostatic charge–discharge tests [45], since as was aforementioned, the charge is released or stored constantly at reaction potentials. In this way, typical CV and GCD curves are shown in Figure 3c. The charge and discharge curves consist of three regions [60], as can be seen in Figure 3d [64]. Taking the example of the discharge curve, first, there is an exponentially decreasing potential region due to the activation overpotential. Next, the plateau appears due to the electrochemical reaction, where the overpotential is mainly due to ohmic polarization. Finally, when there are no active sites left in the electrode, the concentration overpotential causes an exponential drop in potential (I·R drop). It has to be pointed out that these overpotentials vary with the current density of the process. At a higher current density, higher ohmic polarization overpotential occurs since, according to Ohm’s law, the potential (overpotential in this case) is proportional to the current intensity. The concentration overpotential is also increased, as the kinetics of ion diffusion through the electrolyte limits the intercalation process. Thus, both the potential of the cell and its capacity decrease as the current density of the charge–discharge process increases. In addition, this drop is more dramatic as the internal resistance of the electrodes increases, as can be deduced from Ohm’s law. This performance drop is an important point for industry, so wide ranges of current density are desirable for technological applications. For this reason, low electrical resistivity is an essential condition for Li-ion battery electrode materials.
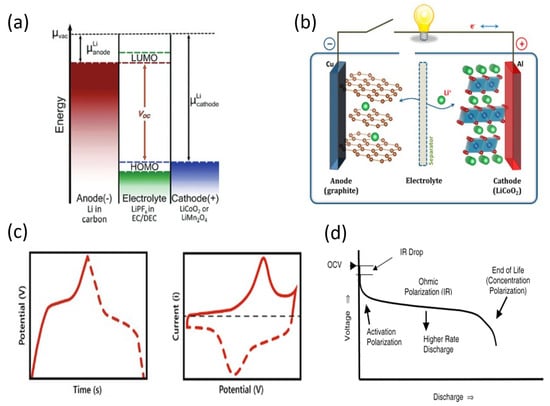
Figure 3.
(a) Energy levels of Li−ion battery components. Reprinted with permission from [66]. Copyright 2013, American Chemical Society. (b) Li−ion battery scheme. Reprinted with permission from [62]. Copyright 2013, American Chemical Society. (c) Typical GCD (left) and CV (right) curves of Li−ion batteries [45]. (d) Voltage evolution during a discharge. Reprinted with permission from [64]. Copyright 2004, American Chemical Society.
In conclusion, TMOs are promising materials for Li-ion battery anodes due to their high specific capacity values compared to carbon-based anodes, the most commonly used in conventional batteries [67]. These high-capacity values arise from the lithiation/delithiation mechanism of these materials, which is conversion-based. This mechanism involves reversible reactions with lithium within the crystalline structure, leading to the formation of new species, unlike the intercalation/deintercalation process typical of carbon-based anodes [46,67].
However, the main drawback of TMOs for battery applications is their low level of electrical conductivity, which limits their rate capability, i.e., their performance at high current densities [46,47,48]. Thus, strategies explained in the above section in order to improve the electrical conductivity of TMOs are also widely proposed for the development of TMOs for Li-ion battery anodes, as the combined use with other compounds that exhibit high electrical conductivity, sulfurization or selenization, creation of oxygen vacancies, or the development of multimetallic or multivalent TMOs. Additionally, TMOs for battery applications face a significant challenge: the decrease in capacity over charge–discharge cycles. This decrease is due to the volume expansion of the crystalline structure during lithiation–delithiation processes, leading to material fracture and a loss of electrical contact [61]. In this way, nanostructured and porous TMOs help to mitigate this issue alleviating the stress on the crystalline structure during the volume expansion [67].
3. Nanostructured TMOs on Carbon Fiber for Energy-Storage Applications: Obtention Methods
After introducing the requirements for TMOs for supercapacitor electrodes and Li-ion battery anode applications, along with the main reported strategies to overcome their drawbacks, this section will discuss the primary methods for developing TMO coatings on carbon fiber for these applications. In addition to reviewing the state of the art, a comprehensive discussion will be provided.
3.1. Solvothermal Synthesis
Solvothermal, or hydrothermal if the solvent is water, synthesis is the most common route to achieve TMOs [68]. This technique consists of the reaction of a precursor solution under temperature and pressure conditions above the solvent boiling point, generally heating the solution inside a reactor Teflon-lined stainless-steel autoclave, as shown in Figure 4a [69]. The main advantage of this method is the control of the size and shape of the products by varying self-assembly conditions: temperature, time, solvent, or auxiliary reagents. High temperature and pressure favor nucleation, and these factors have a great influence on the size of the final product. On the other hand, the reaction time plays an important role in the size and shape. For example, Ostwald ripening is a commonly proposed model for TMO growth [70]. In that model, smaller spheres redissolve and redeposit on bigger ones, increasing the medium size over time. Moreover, time can promote morphological evolution, for example, from nanosheet to nanoflowers [71], or from spheres to elongated twin-spheres [72].
The shape of the resulting product usually follows the Bravais–Friedel–Donnay–Harker (BFDH) model. The main point in that model is that the growth of the crystal follows a given direction by the surface anisotropy. The system tends to minimize the surface energy of the crystal. Then, the most energetic crystal planes exhibit a higher growth rate and smaller interlayer distances, whereas the less energetic crystal planes show greater stability, lower growth rates, and higher interlayer distances. Thus, Equation (14) defines the growth rate (Rhkl) in a given direction as being inversely proportional to the perpendicular atomic layer spacing (dhkl) [73].
Because of the different physicochemical properties of each solvent increasing the possibility of forming hydrogen bonds or a hydrophobic effect, some crystal faces are favored, promoting different morphologies. Additionally, some solvents, like ethylene glycol (EG) or diethylene glycol (DEG), with more than one functional group, can act as a cross-linker, giving rise to different shapes [74,75], making it possible to control the shape by modulating the ratio between two different solvents [76]. Moreover, because the interaction between the crystal and the solvent depends on the physicochemical properties of the solvent and the crystal surface, the pH also plays a relevant role in the promotion of the growth of certain faces instead of others [74]. In some cases, the use of auxiliary reagents, such as urea, NH4F, or HMTA, influences the shape and size too, varying the growth rate of some crystal faces [73,74]. For example, urea decomposes according to the following reactions [74]:
On the one hand, precipitates with metal cations in the form of carbonates, which posteriorly can be calcinated in the air to obtain final TMO [77,78]. On the other hand, creates a reductor atmosphere that avoids metal cation oxidation [79]. In addition, the resulting from urea or HMTA decomposition can act as hydroxyls anions source, which essentially results in some TMO formation [80,81,82]. Other auxiliary reagents are surfactants such as CTAB, which avoids agglomeration, allowing the formation of nanostructured crystals [83].
After the hydrothermal synthesis, thermal treatment in a furnace is a common procedure in order to obtain the final product from other precipitates, to change the morphology, or to increase the crystallinity. These treatments can take place in air, in an inert atmosphere, or involve two steps in different atmospheres [84,85,86,87,88].
Carbon fiber woven is a common substrate for solvothermally obtained TMOs. Several TMOs, such as RuO2 [89], MnO2 [90,91], Fe2O3 [92,93], NiO [94], CuO [95,96], ZnO [97,98], TiO2 [87,99], Co3O4 [100], or other spinel structures [88,101,102], have been successfully synthesized on carbon fiber woven through a solvothermal or hydrothermal way for supercapacitors or Li-ion batteries. Some of these coatings are shown in Figure 4b,c. In Figure 4b, TiO2 arrays synthesized by Wang and coworkers for a Li-ion anode are presented. They used tetrabutyl titanate as a Ti source and submitted the solution to 180 °C for 12 h. The obtained material displayed a remaining capacity of 188 mAh/g after 500 cycles at a current rate of 0.2 C. On the other hand, Figure 4c shows CuO nanoflowers obtained by Xu and coworkers for supercapacitor electrode applications. In this work, the mass loading of CuO was controlled by the time reaction, reaching the optimum after 12 h of synthesis, when the displayed specific capacitance was 839.9 F/g at a scan rate of 0.1 mV/s, while the bare carbon fiber fabric only exhibited 0.3 F/g at the same conditions. However, there are several works that synthesize these TMOs or even others over other carbon fiber substrates, like carbon fiber paper or carbon fiber mats for energy-storage applications [103,104,105,106,107,108,109,110]. However, these substrates do not exhibit enough mechanical resistance to be used in structural applications, making it necessary to use continuous carbon fiber to achieve this purpose. In order to synthesize TMOs on woven carbon fiber, the carbon fiber is usually functionalized to favor the nucleation on it. There are various methods, such as O2-plasma treatment [85] or an autopolymerization coating of dopamine followed by subsequent carbonization [92]. However, the most common surface treatment consists of the partial oxidation of the surface with an acid treatment such as HNO3 [89,92,111], HCl [86], or H2SO4/HNO3 mixture in 3:1 proportion in volume [90,94]. These treatments oxidize the carbon fiber surface, generating hydroxyl, carbonyl, and carboxyl groups [112], which can lead to the nucleation of metal cations through electrostatic interactions. However, the more oxidative the treatment is, the more carbon fiber can be deteriorated, decreasing its mechanical properties [112]. Following acid treatment, the fibers must be washed and dried. Another complementary strategy involves seeding metal cations onto the carbon fibers. This process includes repeatedly dipping the fiber into a metal cation solution and subsequently drying it. Typically, a metal cation solution is prepared with a salt of the metal cation whose leaving group is easily evaporated during the drying, for example, acetate. This method creates nucleation points on the carbon fiber, which can lead to the subsequent growth of the TMO during the hydrothermal step [81,113,114].
As it was mentioned in previous sections, electrical conductivity is relevant to achieving a functional Li-ion battery or a supercapacitor. Many efforts have been made in order to improve the TMO’s electrical conductivity and, of course, TMOs on carbon fiber are not an exception. A developed strategy is the doping of TMOs with other compounds that display higher electrical conductivity. Among these compounds, the most usually used are carbon nanostructures such as CNTs [115], graphene [113], rGO [116,117], or carbon dots glucose-derived [118]. In this context, Xie and coworkers demonstrated that graphene@Co3O4 coatings performed significantly better as Li-ion anodes than Co3O4 alone. Specifically, the graphene@Co3O4 coatings exhibited a specific capacity of 391 mAh/g after 300 cycles at 100 mA/g, compared to only 216 mAh/g for Co3O4 under the same conditions [113].
These compounds or their precursors can be incorporated into the hydrothermal synthesis with other reagents or attached posteriorly through dipping or soaking into a solution containing these compounds. Also, it can be part of a hierarchical structure, which will be discussed later. MXenes, relatively new 2D materials that show exceptional electrical conductivity, have also been reported for this purpose [92]. Another group of materials that can improve the TMO’s electrical conductivity are conductive polymers such as polyaniline (PANI) [119], polypyrrole (PPy) [120,121], or poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS) [102]. For instance, Song and coworkers enhanced the specific capacitance of a positive supercapacitor electrode by 24% at a current density of 1 A/g by spraying a polymer onto carbon fiber coated with hydrothermally obtained CoFe2O4 [102]. Using these materials, electrons can move along these polymers due to their conjugated systems of π bonds, achieving electrical conductivities on the order of 105 S/cm [122]. The main technique used to coat the CF/TMO with this kind of polymer is electrodeposition. In this process, an electrical current is applied between the substrate and another electrode into a monomer solution to allow polymerization over the substrate, obtaining thin layers over the CF/TMO. However, simpler techniques, such as spraying or immersing, have also been reported [96,114]. Another reported strategy consists of doping with boron through the addition of H3BO3 in the synthesis step [123]. Besides, other heavier chalcogenides, such as sulfides or selenides, have been synthesized over carbon cloth for these applications, such as MoS2 [124], CoS2 [125], Ni3S2 [126], Ni3S4 [127], NiCo2S4 [128], CoNi2S4 [129], and CoFe2Se4 [130].
In order to achieve larger capacities or better conductivities, hierarchical structures on carbon fiber that involve TMOs are also being studied. These structures pursue taking advantage of synergetic effects between more than one component. Some works generate TMO nanoforms and, over these nanoforms, synthesize another thinner coating of another component, which presents higher energy-storage capability. In this way, the first coating is used to increase the specific area, and the second one is used to improve the capacity or capacitance. To achieve these structures, double solvothermal synthesis has been successfully reported [131,132]. However, solvothermal synthesis can be combined with other synthesis methods, like electrochemical deposition (ECD) [97,133]. On the other hand, in some hierarchical structures, some nanostructures apport not only surface area or energy-storage capability but also electrical conductivity. Similarly, Li and coworkers grew CNTs on carbon fiber via chemical vapor deposition, followed by the synthesis of MnO2 or Fe2O3 on the surface of the CNTs [115]. These materials demonstrated specific capacitances of 367.44 F/g at 2 mA/g and 361.8 F/g at 3 mA/g, respectively, as supercapacitor electrodes.
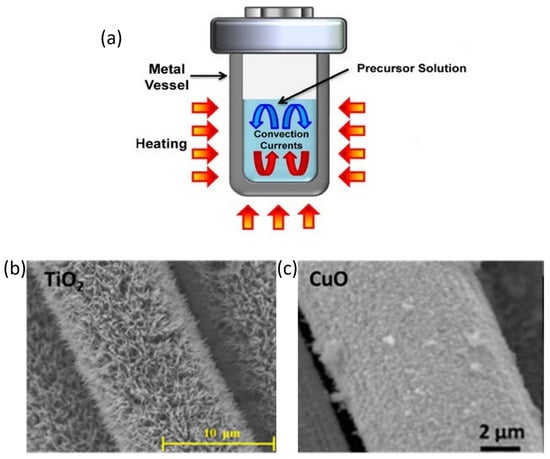
Figure 4.
(a) Teflon-lined stainless-steel autoclave [65]. Copyright John Wiley and Sons, 2013. (b) Different TMOs obtained over carbon fiber: TiO2 [99] (Copyright Elsevier, 2016) and (c) CuO [96] (Copyright Elsevier, 2016).
Finally, some works related to TMO coatings on carbon fiber obtained using the solvothermal method for a supercapacitor electrode and Li-ion battery anode applications are summarized in Table 1 and Table 2, respectively.

Table 1.
TMOs on carbon fiber for supercapacitor electrode applications.

Table 2.
TMOs on carbon fiber for Li-ion battery anode applications.
3.2. MOF-Derived TMOs
In recent years, metal–organic frameworks (MOFs) have been widely studied for different purposes [149,150,151]. These materials consist of crystals compounded by metallic centers linked among them by organic ligands, which exhibit high porosity. Due to their large porosity, which gives rise to a large surface area, MOFs are an interesting material for developing electrodes for energy-storage applications. This surface area results in an increment in the number of electroactive sites and the reactivity [152,153]. Moreover, porosity improves Li+ accommodation, improving cyclic performance and ionic diffusion in the case of batteries [18]. However, the electrical conductivity and chemical stability of pristine MOFs are low. Thus, MOFs could be carbonized to achieve higher conductivity values and chemical stability [153,154,155,156,157,158]. During carbonization, metal centers are reduced to M0 nanoclusters into a carbon and nitrogen skeleton. Thus, electrical conductivity and chemical stability improve, but some possible electrochemical reactions between metal centers and the electrolyte can disappear, which can decrease the capacitance or capacity. On the other hand, porosity and surface area are reduced due to the structure collapse during carbonization. Another option is to use MOFs as a template to obtain TMOs through oxidation [54,67,152,159,160]; in this case, a nanostructured TMO is obtained. Because of their high porosity, MOF-derived TMOs displayed higher performance as both a supercapacitor electrode and Li-ion battery anode in comparison with bare TMOs. In the case of supercapacitors, the large surface area provides higher specific capacitance values, while, in the case of Li-ion batteries, this large surface reduces Li+-diffusion pathways, resulting in a specific capacity increment. Not only that, but cycling stability also increases due to the easier Li+ accommodation inside the porous structure [160,161].
Although other MOF coatings have been reported as TMO precursors on carbon fiber [162,163], the most commonly used are zeolitic imidazole frameworks ZIF-8, ZIF-67, and ZIF-L. These MOFs are composed of Zn2+, in the case of ZIF-8 and ZIF-L, or Co2+, in the case of ZIF-67 and ZIF-L, as a metallic center and 2-methylimidazole as a ligand. The structures of different ZIFs are shown in Figure 5a. As it occurs in solvothermal synthesis, the first step in order to synthesize ZIFs on carbon fiber is to generate hydroxyl, carboxyl, and carbonyl groups on the fiber surface, which act as nucleation points [164,165,166]. Alternatively, another reported strategy to achieve a correct adhesion between ZIFs and carbon fiber is immersing the carbon fiber into a dopamine hydrochloride solution, followed by polymerization into Tris-Cl buffer and subsequent carbonization in order to generate an N-doped super-hydrophilic layer on the carbon fiber surface, which can lead to MOF growth [167].
Both ZIF-8/ZIF-67 or ZIF-L are usually obtained on carbon fiber via coprecipitation of the reactants. However, the obtention of one or the other depends on two main parameters: the solvent and the metal/ligand ratio [168]. In the case of ZIF-L, the plane (0 1 0) energy surface is larger than the (1 0 0) and (0 0 1) plane energy surface, giving rise to layers linked by hydrogen bonds between them, resulting in laminar morphologies, while the ZIF-67 and ZIF-8 structure builds 3D morphologies. Water as a solvent favors ZIF-L obtention due to the fact that, in this case, the hydrogen of the amine group of 2-mIM tends to form hydrogen bonds that link layers. In other solvents like methanol, this hydrogen tends to dissociate; then, the nitrogen attaches to other M2+, making 3D morphologies. On the other hand, lower metal/ligand ratios favor ZIF-8 or ZIF-67 obtention due to large concentrations of 2-mIM shifting the dynamic equilibrium and bringing on dehydrogenation. On the contrary, higher metal/ligand ratios favor ZIF-L obtention. Moreover, it is important to control concentration and synthesis time in order to achieve a homogeneous coating.
Next, after MOF synthesis, the samples are submitted to a heat treatment in an oxidative atmosphere in order to obtain the TMO material. In this step, the temperature is the most relevant parameter to take into account, especially in the case of batteries, since it determines the pore volume of the nanosheets. If the temperature is too high, the structure collapses, and volume expansion owing to the lithiation–delithiation process induces mechanical stress into nanosheets, reducing the capacity as the number of cycles increases. This phenomenon was demonstrated by Fu and coworkers for MOF-derived Co3O4 coatings on carbon fiber [169]. In their work, samples calcinated at 450 °C, 500 °C, and 550 °C exhibited around 5 mAh/g in the initial cycles at 0.5 mA/cm2 as Li-ion anodes. However, after 100 cycles, the sample calcinated at 500 °C retained 3.10 mAh/g, while samples calcinated at 450 °C and 500 °C only exhibited 2.30 and 2.05 mAh/g, respectively. However, the optimum temperature reported to obtain the TMO could be detrimental to the mechanical properties of carbon fiber.
The obtained TMOS are Co3O4 from ZIF-67 and Co-ZIF-L and ZnO from Zn-ZIF-L and ZIF-8. Co3O4 is one of the most attractive TMOs for energy-storage applications due to its high specific capacity [54]. The crystalline structure of this material is available in Figure 5b. However, other isostructural TMOs in which cobalt occupying tetrahedral sites are substituted by other metals such as Zn, Cu, Ni, or Mn are interesting in order to reduce toxicity and environmental impact [170,171,172,173]. One example of these materials is shown in Figure 5c, where Liu and coworkers synthesized MOF-derived Ni-doped cobalt–cobalt nitride on carbon fiber surface for supercapacitor electrode applications, demonstrating a specific capacitance of 361.93 C/g at a current density of 2 mA/cm2 [174]. Another example is the Co3O4 and ZnCo2O4 MOF-derived coatings developed by Lim and coworkers. The bimetallic sample exhibited a specific capacity of 40.74 mAh/g at 2 mA/cm2, while the monometallic one displayed 12.73 mAh/g under the same test conditions [175]. Not only that, but mixed cobaltite spinels also have displayed better capacities due to the presence of different metals, which favors more chemical reactions, better electrical conductivity, and charge mobility as a result of polaron hopping processes [170,171,172]. The difference in electronegativity and volume between the two metals favors polaron-assisted Li+ or OH− diffusion [175]. Mixed cobaltite spinels can be obtained from ZIFs in two main ways. One is by coprecipitation of Co2+ and the other metal cation in the corresponding stoichiometric proportion [164], and the other is by the substitution of cations immersing Co-MOF in a solution that contains the other cation [174,176,177].
As has been aforementioned, the main disadvantage of TMOs is their poor electrical conductivity, so different strategies have been reported to increase MOF-derived TMO’s conductivity. One possibility is doping with phosphorus atoms, which act as electron donors, through phosphorylation in an Ar atmosphere with NaH2PO2 as a P source. In this way, P-Co3O4 synthesized on carbon fiber by Liu and coworkers displayed a specific capacitance as a supercapacitor electrode, which is 1.52 times greater than that of a Co3O4 reference sample [166]. Another strategy consists of a partial reduction of TMO with NaBH4 to create oxygen vacancies, which not only improve electrical conductivity but also enhance electron and ion transfer between the electrode and the electrolyte [167]. In this way, Dai and coworkers improved the specific capacity of MOF-derived Co3O4 as a supercapacitor electrode by 2.8 times [167]. Nevertheless, the most common method to obtain these oxygen vacancies is to submit the MOF to an annealing step into an inert atmosphere previously to the necessary calcination to obtain the TMO material. Moreover, ZIFs can be functionalized as chalcogenides in order to reach higher electrical conductivity [167,175,178]. Se and S are softer Pearson’s bases than O, giving rise to less polarized links that favor electron movement. Despite this conductivity improvement, the cycling stability of these compounds is poorer than the corresponding oxides due to a larger expansion volume during lithiation. The obtention of metal sulfurs is usually carried out with thioacetamide (TAA) [179,180,181,182,183] or sulfur powder [184,185] as a sulfidation agent and subsequent thermal treatment, while to produce selenides, selenium powder is the most common selenium source [186,187]. Some works have developed MOF-derived chalcogenides on woven carbon fiber, i.e., Zhang and coworkers synthesized ZIF-derived CoO, CoS, CoSe, and CoTe on carbon fiber cloth, resulting in CoS, which exhibited larger capacitance (3576.0 mF/cm2 at a current density of 5 mA/cm2) as a supercapacitor electrode [188]. Moreover, Yang and coworkers developed a NiCo-alloy sulfide on a carbon fiber surface from Co(2-mIM) MOF for LIBs, obtaining 213 mAh/g at a current density of 1 A/g [189]. To conclude this section, Table 3 and Table 4 summarize MOF-derived TMOs obtained on carbon fiber for a supercapacitor electrode and Li-ion battery anode applications, respectively.

Table 3.
MOF-derived TMOs obtained on carbon fiber for supercapacitor electrode applications.

Table 4.
MOF-derived TMOs obtained on carbon fiber for Li-ion battery anode applications.
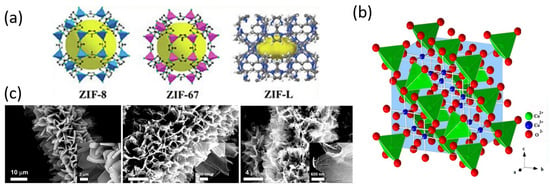
Figure 5.
(a) ZIF-8, ZIF-67, and ZIF-L structure [168]. Copyright Elsevier, 2021. (b) Co3O4 crystalline structure. (c) ZIF-L coating on carbon fiber, MOF-derived NiCo2O4, and Ni-doped cobalt–cobalt nitride on carbon fiber. Reproduced with permission from [174]. Copyright 2018, American Chemical Society.
3.3. Electrochemical Deposition (ECD)
Electrochemical deposition is another reported synthesis route for the obtention of TMOs on carbon fiber. In this technique, carbon fiber is placed as a working electrode in a two- or three-electrode system in which the electrolyte contains the corresponding metal cations, as shown in Figure 6a. A constant potential or a constant current applied promotes the metal cation’s migration to the working electrode and their subsequent oxidation, obtaining the coating. The TMO coating thickness and shape are influenced by the electrodeposition parameters, such as the constant potential or current, the electrodeposition time, and the electrolyte concentration. In addition, several cyclovoltammetry cycles can be applied instead of a constant potential or current to promote migration and oxidation [197].
MnO2 is the most reported TMO obtained by electrochemical deposition on carbon fiber due to its high theoretical capacitance, environmental friendliness, natural abundance, and low cost [198]. As previously discussed, increasing the electrical conductivity is essential to improve TMOs’ electrochemical performance for both supercapacitor and battery electrode applications. One strategy can be the modification of the carbon fiber surface. One example is the growth of MnO2 nanosheets over carbon fiber coated with AgCNT film [199], obtaining the film via simple immersion in AgCNT ink. Using this method, Ko and coworkers achieved a specific capacitance of 325 F/g at a 1 A/g current density [199]. SEM images of this material are shown in Figure 6b. Other studies have explored hierarchical structures compounded by TMOs and carbon nanocompounds. For instance, Sha and coworkers grew vertical graphenes (VG) on carbon fiber using PECVD to achieve synergetic effects between VG and MnO2, achieving an areal capacitance of 30.7 mF/cm2 [200]. Another reported strategy involves the polymerization of conductive polymers alongside TMOs, such as the simultaneous electropolymerization of PEDOT:PSS and the electrodeposition of MnO2. This approach, used by Wang and coworkers, resulted in an electrode with an areal capacitance of 386 mF/cm2 at a current density of 1 mA/cm2 [201].
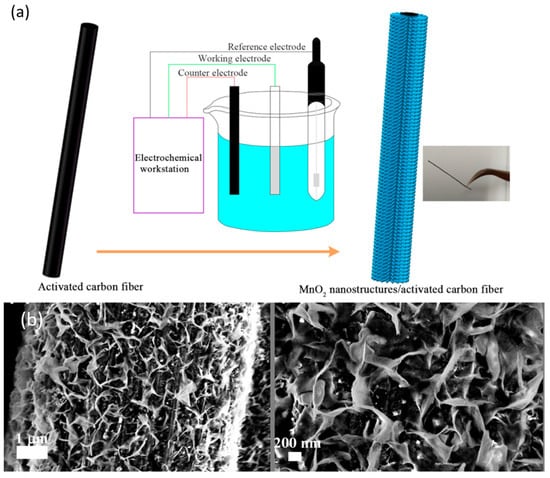
Figure 6.
(a) Three-electrode system for MnO2 electrochemical deposition over carbon fiber [202]. Copyright Elsevier, 2017. (b) MnO2 coating over carbon fiber. Reproduced with permission from [199]. Copyright 2019, American Chemical Society.
In addition to MnO2, other TMOs obtained by electrodeposition on carbon fiber substrates are Fe2O3 [201], V2O5 [203], Co3O4 [204], NiCo2O4 [205], and NiO [206]. However, only a few of these works involve a strategy to improve the electrical conductivity. Li and coworkers modified carbon fiber with Ni via electrodeposition previous to NiCo2O4 electrodeposition [207]. Additionally, a hierarchical structure in which NiO coats CNT grown on carbon fiber paper via CVD was proposed. In this work, the proposed electrode material by Zhang and coworkers demonstrated a specific capacitance of 1317 F/g at 1.2 A/g [206]. On the other hand, conductor polymers like PPy have been reported to improve conductivity in this kind of coating [205]. Moreover, other transition metal chalcogenides, which present better electrical conductivity, have been synthesized on carbon fiber cloth via electrochemical deposition as CoNi2S4 [129]. Not only that, as mentioned in Section 3.1, electrodeposition can be combined with other synthesis routes to obtain hierarchical structures that involve more than one TMO, or a TMO and a derivative like Co9S8@NiCo2O4 [197], NiCo2O4@Ni(OH)2 [208], MoO2@MnO2 [209], or CuO@MnO2 [210]. As a summary, reported TMOs obtained on carbon fiber by ECD for supercapacitor electrode purposes are presented in Table 5.

Table 5.
TMOs obtained on carbon fiber by ECD for supercapacitor electrode applications.
Although TMOs obtained by electrodeposition are more often used for supercapacitors than batteries, TMO obtained in this way can also be employed as Li-ion battery anodes, i.e., Qiu and coworkers developed Fe3O4 nanotube arrays over a carbon fiber cloth via a ZnO sacrificial template obtained by electrodeposition. As Li-ion battery anodes, it exhibited a specific capacity of 930 mAh/g at a current density of 2 mA/g and a capacity retention of 73% after 200 GCD cycles [212].
4. Conclusions and Outlook
Currently, the development of all-solid-state energy-storage devices, such as Li-ion batteries and supercapacitors, has become a key objective for both researchers and industry. The interest in flexible devices stems not only from the latest trend in the electronic devices market but also from advancements in wearable biomedical devices. These new devices require energy-storage systems that are preferably flexible to ensure wearability.
On the other hand, the interest in structural devices interest is related to the development of multifunctional materials that can reduce the mass of vehicles. Electric vehicles, in particular, could greatly benefit from these multifunctional materials since their batteries are especially heavy. Partial or total substitution of traditional batteries with structural energy storage materials could mean an important improvement in autonomy, which currently is their main drawback to significantly enhancing vehicle range, addressing one of the main drawbacks of current electric vehicles.
Thereby, flexible and structural energy-storage materials are crucial for the sustainability of urban environments and transportation, as well as for the advancement of health monitoring technologies. However, the achievement of these devices is still far from having been accomplished, especially in the case of the structural ones. In this context, the use of composites has emerged as some of the most promising materials for these purposes due to the high potential of carbon fiber as a reinforcement and substrate that provides mechanical strength and electrical conductivity. Nevertheless, the carbon fiber electrochemical performance should be improved in order to become a functional energy-storage device component. This is where TMO coatings emerge as a possible solution due to their high theoretical capacitance as a supercapacitor electrode and capacity as a Li-ion battery anode. In this way, some techniques have been reported to obtain these coatings, with the most common being the following three:
- Solvothermal/hydrothermal synthesis: This technique offers the significant advantage of achieving a wide range of transition metal oxide compositions and nanostructure morphologies by varying only a few parameters. As Li-ion anodes, different transition metal oxides yield different theoretical capacities due to various conversion reactions. Additionally, different morphologies can affect cyclability. On the other hand, as supercapacitor electrodes, diverse transition metal oxides provide varying pseudocapacitive reactions and electrical conductivities, with different nanostructured morphologies offering different surface areas, a crucial factor for supercapacitor electrode capacitance. Consequently, the extensive possibilities offered by hydrothermal synthesis make it the most developed technique for these applications while also opening up a wide range of potential optimizations and new materials for future research.
- Metal–organic framework-derived synthesis: Using MOFs as precursors to obtain transition metal oxides results in more porous coatings. Increased porosity enhances the surface area of transition metal oxides, leading to higher supercapacitor electrode capacitance. On the other, for Li-ion battery anodes, increased porosity improves capacity and cyclability. However, a major drawback of this technique is that MOF-derived coatings are primarily limited to the zeolitic–imidazole frameworks series, resulting in a limited variety of available transition metal oxide structures. Therefore, the primary goal of this technique should be to develop new MOF coatings on the carbon fiber to expand the range of available materials.
- Electrochemical deposition: This is the least developed of the three techniques, particularly for Li-ion battery applications. Consequently, it presents significant potential for future research. One advantage of electrochemical deposition is the ability to deposit transition metal oxide alongside other compounds, such as conductive polymers, which can act synergistically with the oxides.
On the other hand, despite the synthesis method, the main problem of TMOs is their low electrical conductivity. Therefore, it is essential not only to control the composition and morphology of these coatings but also to develop strategies to improve their electrical conductivity. Some reported strategies are the combination of TMOs with conductive materials such as carbon-based nanostructures or conductive polymers, the creation of oxygen vacancies, the dopping with donor elements as P, the sulfurization or selenization, or the synthesis of the multivalence or multimetallic transition metal oxides. Hence, the development of these strategies on TMO coatings on carbon fiber is as crucial as the development of new coating compositions and morphologies. Moreover, the similar requirements for both supercapacitor electrodes and Li-ion battery anodes suggest that advances in materials for supercapacitor electrodes, which have been more extensively developed and studied, could be effectively applied to Li-ion battery applications.
Finally, some recent studies have used hierarchical structures as carbon fiber coatings for supercapacitor electrodes and Li-ion battery anode applications. This paves the way for a new generation of coatings that can take advantage of the synergy between more than one transition metal oxide or even between transition metal oxides and other materials, such as CNTs grown by CVD or vertical graphene.
Author Contributions
Conceptualization, A.G.-B., D.M.-D., M.S. and A.U.; writing—original draft preparation, A.G.-B.; writing—review and editing, A.G.-B., D.M.-D., M.S. and A.U.; visualization, A.G.-B. and D.M.-D.; supervision, M.S. and A.U.; funding acquisition, M.S. and A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agencia Estatal de Investigación of the Spanish Government (Project MULTISENS PID2022-136636OBI00) and (PRE2020-094255).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X.J.; Wang, J. Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Adv. Energy Mater. 2021, 11, 2100608. [Google Scholar] [CrossRef]
- Abdillah, O.B.; Bin Rus, Y.; Ulfa, M.; Dedi, F.; Iskandar, F. Recent progress on reduced graphene oxide and polypyrrole composites for high performance supercapacitors: A review. J. Energy Storage 2023, 74, 109300. [Google Scholar] [CrossRef]
- Battery Market Size, Share & Trends Analysis Report by Product (Lead Acid, Li-Ion, Nickle Metal Hydride, Ni-Cd), by Application, by End-Use, by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2020.
- Supercapacitors Market Size, Share, and Trends 2024 to 2034. Available online: https://www.precedenceresearch.com/supercapacitors-market (accessed on 23 July 2024).
- Aydin, A.; Zajonz, F.; Günther, T.; Dermenci, K.B.; Berecibar, M.; Urrutia, L. Lithium-Ion Battery Manufacturing: Industrial View on Processing Challenges, Possible Solutions and Recent Advances. Batteries 2023, 9, 555. [Google Scholar] [CrossRef]
- Hong, H.; Tu, H.; Jiang, L.; Du, Y.; Wong, C.-P. Advances in fabric-based supercapacitors and batteries: Harnessing textiles for next-generation energy storage. J. Energy Storage 2024, 75, 109561. [Google Scholar] [CrossRef]
- Hesar, M.E.; Seyedsadrkhani, N.S.; Khan, D.; Naghashian, A.; Piekarski, M.; Gall, H.; Schermuly, R.; Ghofrani, H.A.; Ingebrandt, S. AI-enabled epidermal electronic system to automatically monitor a prognostic parameter for hypertension with a smartphone. Biosens. Bioelectron. 2023, 241, 115693. [Google Scholar] [CrossRef] [PubMed]
- del Bosque, A.; Sánchez–Romate, X.F.; Patrizi, D.; Sáez, J.S.d.R.; Wang, D.-Y.; Sánchez, M.; Ureña, A. Ultrasensitive flexible strain sensors based on graphene nanoplatelets doped poly(ethylene glycol) diglycidyl ether: Mask breathing monitoring for the Internet of Things. Sensors Actuators A Phys. 2023, 358, 114448. [Google Scholar] [CrossRef]
- Pasta, M.; Armstrong, D.; Brown, Z.L.; Bu, J.; Castell, M.R.; Chen, P.; Cocks, A.; Corr, S.A.; Cussen, E.J.; Darnbrough, E.; et al. 2020 roadmap on solid-state batteries. J. Phys. Energy 2020, 2, 032008. [Google Scholar] [CrossRef]
- Electric Vehicle Market Size, Share & Trends Analysis Report by Product (BEV, PHEV, FCEV), by Application (Passenger Cars, Commercial Vehicles), by Region, and Segment Forecasts, 2023–2030, Grand View Research, n.d. Available online: https://www.grandviewresearch.com/industry-analysis/electric-vehicles-ev-market (accessed on 23 July 2024).
- Niri, A.J.; Poelzer, G.A.; Zhang, S.E.; Rosenkranz, J.; Pettersson, M.; Ghorbani, Y. Sustainability challenges throughout the electric vehicle battery value chain. Renew. Sustain. Energy Rev. 2024, 191, 114176. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.; Greshnyakov, V.; Yalovega, G.; Bashkin, I. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Shmatko, V.; Yalovega, G.; Krestinin, A.; Bashkin, I.; Bogoslavskaja, E. Electronic structure of hydrogenated carbon nanotubes studied by core level spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2014, 196, 99–103. [Google Scholar] [CrossRef]
- Ouramdane, O.; Elbouchikhi, E.; Amirat, Y.; Gooya, E.S. Optimal sizing and energy management of microgrids with Vehicle-to-Grid technology: A critical review and future trends. Energies 2021, 14, 4166. [Google Scholar] [CrossRef]
- González, C.; Vilatela, J.; Molina-Aldareguía, J.; Lopes, C.; Llorca, J. Structural composites for multifunctional applications: Current challenges and future trends. Prog. Mater. Sci. 2017, 89, 194–251. [Google Scholar] [CrossRef]
- Petrushenko, D.; Rahmati, Z.; Barazanchy, D.; De Backer, W.; Mustain, W.E.; White, R.E.; Ziehl, P.; Coman, P.T. Dip-Coating of Carbon Fibers for the Development of Lithium Iron Phosphate Electrodes for Structural Lithium-Ion Batteries. Energy Fuels 2023, 37, 711–723. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, S.; Li, D.; Liao, J.; Ji, F.; Liu, H.; Ci, L. Commercial carbon cloth: An emerging substrate for practical lithium metal batteries. Energy Storage Mater. 2022, 48, 172–190. [Google Scholar] [CrossRef]
- Seo, M.-K.; Pandey, P.; Sohn, J.I. Metal-free flexible triboelectric nanogenerator based on bifunctional carbon fiber for mechanical energy harvesting and human activity monitoring. Sens. Actuators A Phys. 2024, 370, 115247. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, W.; Xu, G.; Chou, T.-W. Structural supercapacitor composites: A review. Compos. Sci. Technol. 2021, 204, 108636. [Google Scholar] [CrossRef]
- Asp, L.E.; Bouton, K.; Carlstedt, D.; Duan, S.; Harnden, R.; Johannisson, W.; Johansen, M.; Johansson, M.K.G.; Lindbergh, G.; Liu, F.; et al. A Structural Battery and its Multifunctional Performance. Adv. Energy Sustain. Res. 2021, 2, 2000093. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Danzi, F.; Salgado, R.M.; Oliveira, J.E.; Arteiro, A.; Camanho, P.P.; Braga, M.H. Structural Batteries: A Review. Molecules 2021, 26, 2203. [Google Scholar] [CrossRef]
- Kalnaus, S.; Asp, L.E.; Li, J.; Veith, G.M.; Nanda, J.; Daniel, C.; Chen, X.C.; Westover, A.; Dudney, N.J. Multifunctional approaches for safe structural batteries. J. Energy Storage 2021, 40, 102747. [Google Scholar] [CrossRef]
- Deng, R.; He, T. Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies 2023, 16, 4549. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Xu, J.; Xia, Z.; Sun, J.; Asp, L.E.; Palermo, V. Electrophoretic coating of LiFePO4/Graphene oxide on carbon fibers as cathode electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2021, 208, 108768. [Google Scholar] [CrossRef]
- Moyer, K.; Carter, R.; Hanken, T.; Douglas, A.; Oakes, L.; Pint, C.L. Electrophoretic deposition of LiFePO4 onto 3-D current collectors for high areal loading battery cathodes. Mater. Sci. Eng. B 2019, 241, 42–47. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Lu, Y.-C.; Hou, Y.; Li, Q. Electrophoretic lithium iron phosphate/reduced graphene oxide composite for lithium ion battery cathode application. J. Power Sources 2015, 284, 236–244. [Google Scholar] [CrossRef]
- Hagberg, J.; Maples, H.A.; Alvim, K.S.; Xu, J.; Johannisson, W.; Bismarck, A.; Zenkert, D.; Lindbergh, G. Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2018, 162, 235–243. [Google Scholar] [CrossRef]
- Mishra, A.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Carbon Cloth-based Hybrid Materials as Flexible Electrochemical Supercapacitors. ChemElectroChem 2019, 6, 5771–5786. [Google Scholar] [CrossRef]
- Artigas-Arnaudas, J.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Effect of electrode surface treatment on carbon fiber based structural supercapacitors: Electrochemical analysis, mechanical performance and proof-of-concept. J. Energy Storage 2023, 59, 106599. [Google Scholar] [CrossRef]
- Jin, T.; Singer, G.; Liang, K.; Yang, Y. Structural batteries: Advances, challenges and perspectives. Mater. Today 2023, 62, 151–167. [Google Scholar] [CrossRef]
- O’Brien, D.J.; Baechle, D.M.; Wetzel, E.D. Design and performance of multifunctional structural composite capacitors. J. Compos. Mater. 2011, 45, 2797–2809. [Google Scholar] [CrossRef]
- Snyder, J.; Gienger, E.; Wetzel, E. Performance metrics for structural composites with electrochemical multifunctionality. J. Compos. Mater. 2015, 49, 1835–1848. [Google Scholar] [CrossRef]
- Meena, D.; Kumar, R.; Gupta, S.; Khan, O.; Gupta, D.; Singh, M. Energy storage in the 21st century: A comprehensive review on factors enhancing the next-generation supercapacitor mechanisms. J. Energy Storage 2023, 72, 109323. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Show, Y. Electric double-layer capacitor fabricated with addition of carbon nanotube to polarizable electrode. J. Nanomater. 2012, 2012, 929343. [Google Scholar] [CrossRef]
- Rajkumar, M.; Hsu, C.-T.; Wu, T.-H.; Chen, M.-G.; Hu, C.-C. Advanced materials for aqueous supercapacitors in the asymmetric design. Prog. Nat. Sci. Mater. Int. 2015, 25, 527–544. [Google Scholar] [CrossRef]
- Reveles-Miranda, M.; Ramirez-Rivera, V.; Pacheco-Catalán, D. Hybrid energy storage: Features, applications, and ancillary benefits. Renew. Sustain. Energy Rev. 2024, 192, 114196. [Google Scholar] [CrossRef]
- Biswas, S.; Chowdhury, A. Organic Supercapacitors as the Next Generation Energy Storage Device: Emergence, Opportunity, and Challenges. Chemphyschem 2023, 24, e202200567. [Google Scholar] [CrossRef]
- George, J.; Balachandran, M. Extrinsic pseudocapacitance: Tapering the borderline between pseudocapacitive and battery type electrode materials for energy storage applications. J. Energy Storage 2023, 74, 109292. [Google Scholar] [CrossRef]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Arumugam, B.; Mayakrishnan, G.; Manickavasagam, S.K.S.; Kim, S.C.; Vanaraj, R. An Overview of Active Electrode Materials for the Efficient High-Performance Supercapacitor Application. Crystals 2023, 13, 1118. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium-Ion Batteries with High Rate Capabilities. ACS Sustain. Chem. Eng. 2017, 5, 2799–2816. [Google Scholar] [CrossRef]
- Parveen, N. Resent Development of Binder-Free Electrodes of Transition Metal Oxides and Nanohybrids for High Performance Supercapacitors—A Review. Chem. Rec. 2023, 24, e202300065. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Shahzad, A.; Danish, M.; Fatima, M.; Adnan, M.; Atiq, S.; Asim, M.; Khan, M.A.; Ain, Q.U.; Perveen, R. Recent developments in transition metal oxide-based electrode composites for supercapacitor applications. J. Energy Storage 2024, 81, 110430. [Google Scholar] [CrossRef]
- Zhu, X. Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies. J. Energy Storage 2022, 49, 104148. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Brzhezinskaya, M.; Dmitriev, V.O.; Shmatko, V.A.; Ershov, I.V.; Ulyankina, A.A.; Chernysheva, D.V.; Smirnova, N.V. Interfacial Interaction in MeOx/MWNTs (Me–Cu, Ni) Nanostructures as Efficient Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Shmatko, V.; Leontyeva, D.; Nevzorova, N.; Smirnova, N.; Brzhezinskaya, M.; Yalovega, G. Interaction between NiOx and MWCNT in NiOx/MWCNTs composite: XANES and XPS study. J. Electron Spectrosc. Relat. Phenom. 2017, 220, 76–80. [Google Scholar] [CrossRef]
- Sankar, B.D.; Sekar, S.; Sathish, S.; Dhanasekaran, S.; Nirmala, R.; Kim, D.Y.; Lee, Y.; Lee, S.; Navamathavan, R. Recent advancements in MXene with two-dimensional transition metal chalcogenides/oxides nanocomposites for supercapacitor application—A topical review. J. Alloys Compd. 2024, 978, 173481. [Google Scholar] [CrossRef]
- Rahat, S.S.M.; Hasan, K.M.Z.; Mondol, M.H.; Mallik, A.K. A comprehensive review of carbon nanotube-based metal oxide nanocomposites for supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Xie, Q.; Ou, H.; Lin, X.; Zeb, A.; Hu, L.; Wu, Y.; Ma, G. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2022, 96, 262–284. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.; Olvera-Neria, O.; Hernández-Pérez, I.; Rubio-Ponce, A. Localized electronic states induced by oxygen vacancies on anatase TiO2 (101) surface. Surf. Sci. 2013, 616, 115–119. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Li, Y.; Liu, Y. Exploring transition metal oxide-based oxygen vacancy supercapacitors: A review. J. Energy Storage 2024, 80, 110350. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Sun, M.; Ni, W.; Wang, M.; Yu, M.; Yu, D.; Ling, M.; Liang, C. Superior lithium storage in Fe2O3 nanoporous arrays endowed by surface phosphorylation and bulk phosphorous doping. Appl. Surf. Sci. 2022, 604, 154668. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Feng, R.; Zhu, K.; Zhao, F.; Wang, C.; Wang, J.; Wang, L.; Chu, P.K. Fabrication of bimetallic oxides (MCo2O4: M=Cu, Mn) on ordered microchannel electro-conductive plate for high-performance hybrid supercapacitors. Sustainability 2021, 13, 9896. [Google Scholar] [CrossRef]
- Liu, J.; Dong, L.; Chen, D.; Han, Y.; Liang, Y.; Yang, M.; Han, J.; Yang, C.; He, W. Metal Oxides with Distinctive Valence States in an Electron-Rich Matrix Enable Stable High-Capacity Anodes for Li Ion Batteries. Small Methods 2020, 4, 1900753. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices System Power Ratings, Module Size, n.d. Available online: www.sciencemag.org (accessed on 23 July 2024).
- Palacín, M.R. Understanding ageing in Li-ion batteries: A chemical issue. Chem. Soc. Rev. 2018, 47, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Meng, J.; Wu, J.; Deng, Z.; Lin, M.; Mao, S.; Stroe, D.-I. A comprehensive overview and comparison of parameter benchmark methods for lithium-ion battery application. J. Energy Storage 2023, 71, 108197. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Gittins, J.W.; Chen, Y.; Arnold, S.; Augustyn, V.; Balducci, A.; Brousse, T.; Frackowiak, E.; Gómez-Romero, P.; Kanwade, A.; Köps, L.; et al. Interlaboratory study assessing the analysis of supercapacitor electrochemistry data. J. Power Sources 2023, 585, 233637. [Google Scholar] [CrossRef]
- Melot, B.C.; Tarascon, J.-M. Design and Preparation of Materials for Advanced Electrochemical Storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef]
- Zhu, J.; Ding, Y.; Ma, Z.; Tang, W.; Chen, X.; Lu, Y. Recent Progress on Nanostructured Transition Metal Oxides As Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 2022, 51, 3391–3417. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Li, S.; Nechache, R.; Davalos, I.A.V.; Goupil, G.; Nikolova, L.; Nicklaus, M.; Laverdiere, J.; Ruediger, A.; Rosei, F. Ultrafast Microwave Hydrothermal Synthesis of BiFeO3 Nanoplates. J. Am. Ceram. Soc. 2013, 96, 3155–3162. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Chen, J.; Ge, W.; Liu, M.; Lu, Y. Hydrothermal synthesis and formation mechanism of Aurivillius Bi5Fe0.9Co0.1Ti3O15nanosheets. CrystEngComm 2016, 18, 7449–7456. [Google Scholar] [CrossRef]
- Wu, L.; Sun, L.; Li, X.; Zhang, Q.; Si, H.; Zhang, Y.; Wang, K.; Zhang, Y. Mesoporous ZnCo2O4-CNT microflowers as bifunctional material for supercapacitive and lithium energy storage. Appl. Surf. Sci. 2020, 506, 144964. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, J.; Ma, X.; Li, J.; Xiong, S. Formation of quasi-mesocrystal ZnMn2O4 twin microspheres via an oriented attachment for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14236–14244. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Zhang, Z.; Hai, Z.; Xu, H.; Cui, D.; Zhuiykov, S.; Xue, C. NiCo2O4 particles with diamond-shaped hexahedron structure for high-performance supercapacitors. Appl. Surf. Sci. 2018, 436, 242–251. [Google Scholar] [CrossRef]
- Liao, W.; Chen, H.; Zeng, Y.; Liu, L. Recent progress in the fabrication of nanostructured zinc-based ternary metal oxides for high-performance lithium-ion batteries. J. Appl. Electrochem. 2023, 53, 1077–1107. [Google Scholar] [CrossRef]
- Redekar, R.; Avatare, A.; Chouhan, J.; Patil, K.; Pawar, O.; Patil, S.; Bhoite, A.; Patil, V.; Patil, P.; Tarwal, N. Review on recent advancements in chemically synthesized manganese cobalt oxide (MnCo2O4) and its composites for energy storage application. Chem. Eng. J. 2022, 450, 137425. [Google Scholar] [CrossRef]
- Hu, L.; Qu, B.; Li, C.; Chen, Y.; Mei, L.; Lei, D.; Chen, L.; Li, Q.; Wang, T. Facile synthesis of uniform mesoporous ZnCo2O4 microspheres as a high-performance anode material for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5596–5602. [Google Scholar] [CrossRef]
- Dang, W.; Wang, F.; Ding, Y.; Feng, C.; Guo, Z. Synthesis and electrochemical properties of ZnMn2O4 microspheres for lithium-ion battery application. J. Alloys Compd. 2017, 690, 72–79. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, M.; Zong, B.; Guo, J.; Gong, J.; Wang, L.; Liang, G. Urea-assisted hydrothermal synthesis of a hollow hierarchical LiNi0.5Mn1.5O4 cathode material with tunable morphology characteristics. RSC Adv. 2018, 8, 30087–30097. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Elizondo, L.; Rendón-Ángeles, J.; Matamoros-Veloza, Z.; López-Cuevas, J.; Yanagisawa, K. Urea decomposition enhancing the hydrothermal synthesis of lithium iron phosphate powders: Effect of the lithium precursor. Adv. Powder Technol. 2017, 28, 1593–1602. [Google Scholar] [CrossRef]
- Nataraj, N.; Kubendhiran, S.; Gan, Z.-W.; Chen, S.-M.; Sakthivel, R. HMTA-assisted synthesis of praseodymium oxide nanostructures integrated multiwalled carbon nanotubes for efficient levofloxacin electrochemical sensing. Mater. Today Chem. 2022, 26, 101136. [Google Scholar] [CrossRef]
- Kong, K.; Deka, B.K.; Seo, J.W.; Park, Y.-B.; Park, H.W. Effect of CuO nanostructure morphology on the mechanical properties of CuO/woven carbon fiber/vinyl ester composites. Compos. Part A Appl. Sci. Manuf. 2015, 78, 48–59. [Google Scholar] [CrossRef]
- Kong, K.; Deka, B.K.; Kwak, S.K.; Oh, A.; Kim, H.; Park, Y.-B.; Park, H.W. Processing and mechanical characterization of ZnO/polyester woven carbon–fiber composites with different ZnO concentrations. Compos. Part A Appl. Sci. Manuf. 2013, 55, 152–160. [Google Scholar] [CrossRef]
- Zou, J.; Liu, B.; Liu, H.; Ding, Y.; Xin, T.; Wang, Y. Facile synthesis of interconnected mesoporous ZnMn2O4 nano-peanuts for Li-storage via distinct structure design. Mater. Res. Bull. 2018, 107, 468–476. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, B.; Wang, Q.; Tan, D.; Song, W.; Hou, X.; Chen, D.; Shen, G. Advanced rechargeable lithium-ion batteries based on bendable ZnCo2O4-urchins-on-carbon-fibers electrodes. Nano Res. 2013, 6, 525–534. [Google Scholar] [CrossRef]
- Chen, H.; Deng, L.; Luo, S.; Ren, X.; Li, Y.; Sun, L.; Zhang, P.; Chen, G.; Gao, Y. Flexible Three-Dimensional Heterostructured ZnO-Co3O4 on Carbon Cloth as Free-Standing Anode with Outstanding Li/Na Storage Performance. J. Electrochem. Soc. 2018, 165, A3932–A3942. [Google Scholar] [CrossRef]
- Mo, Y.; Ru, Q.; Chen, J.; Song, X.; Guo, L.; Hu, S.; Peng, S. Three-dimensional NiCo2O4 nanowire arrays: Preparation and storage behavior for flexible lithium-ion and sodium-ion batteries with improved electrochemical performance. J. Mater. Chem. A 2015, 3, 19765–19773. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, J.; Dong, Z.; Lin, H.; Han, S. Flexible carbon fiber/reduced-TiO2 composites for constructing remarkable performance supercapacitors. J. Power Sources 2022, 550, 232169. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Shah, H.U.; Asim, S.; Raza, R.; Mai, W. Achieving high rate and high energy density in an all-solid-state flexible asymmetric pseudocapacitor through the synergistic design of binder-free 3D ZnCo2O4 nano polyhedra and 2D layered Ti3C2Tx-MXenes. J. Mater. Chem. A 2019, 7, 24543–24556. [Google Scholar] [CrossRef]
- Ye, S.; Liu, F.; Xu, R.; Yao, Y.; Zhou, X.; Feng, Y.; Cheng, X.; Yu, Y. RuO2 Particles Anchored on Brush-Like 3D Carbon Cloth Guide Homogenous Li/Na Nucleation Framework for Stable Li/Na Anode. Small 2019, 15, e1903725. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, W.; Han, Z.; Wang, F.; Geng, D.; Li, X.; Li, Y.; Zhang, X. Preparation of PAN-based carbon fiber@MnO2 composite as an anode material for structural lithium-ion batteries. J. Mater. Sci. 2019, 54, 11972–11982. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Yu, H.; Wu, H.; Wang, J.; Liu, A. MnO2 nanowires-decorated carbon fiber cloth as electrodes for aqueous asymmetric supercapacitor. Funct. Mater. Lett. 2018, 11, 1850034. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.-L.; Wang, G.-G.; Zhang, H.-Y.; Zhang, B.; Li, G.-Z.; Wu, Z.-P.; Dang, L.-Y.; Han, J.-C. Few-layered Ti3C2Tx MXenes coupled with Fe2O3 nanorod arrays grown on carbon cloth as anodes for flexible asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 22631–22641. [Google Scholar] [CrossRef]
- Peng, S.; Yu, L.; Lan, B.; Sun, M.; Cheng, G.; Liao, S.; Cao, H.; Deng, Y. Low-cost superior solid-state symmetric supercapacitors based on hematite nanocrystals. Nanotechnology 2016, 27, 505404. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Shi, M.; Han, Z.; Zhang, W.; Li, Y.; Zhang, X.; Sheng, Y. Synthesis of one-dimensional PAN-based carbon fiber/NiO composite as an anode material for structural lithium-ion batteries. Ionics 2020, 26, 5935–5940. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, R.; Dong, Z.; Lin, H.; Han, S. An illumination-assisted supercapacitor of rice-like CuO nanosheet coated flexible carbon fiber. Electrochim. Acta 2022, 430, 140789. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Liu, G.; Xi, Y.; Hu, C.; Wang, X. CuO Nanoflowers growing on Carbon Fiber Fabric for Flexible High-Performance Supercapacitors. Electrochim. Acta 2016, 203, 1–8. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, M.; Hu, H.; Zhang, Y.; Qi, J.; Wei, F.; Meng, Q.; He, Y.; Ren, Y.; Sun, Z. ZnO@Ni-Co-S Core-Shell Nanorods-Decorated Carbon Fibers as Advanced Electrodes for High-Performance Supercapacitors. Nano 2018, 13, 1850148. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kwon, O.; Kim, D.; Park, Y.-B.; Park, H.W. Multifunctional enhancement of woven carbon fiber/ZnO nanotube-based structural supercapacitor and polyester resin-domain solid-polymer electrolytes. Chem. Eng. J. 2017, 325, 672–680. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Z.; Huang, F.; Gong, C.; Liu, H.; Zheng, G.; Wen, S. Carbon cloth supported anatase TiO2 aligned arrays as a high-performance anode material for Li-ion batteries. Mater. Lett. 2016, 171, 150–153. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S.; Razeeb, K.M. Ultra-fast rate capability of a symmetric supercapacitor with a hierarchical Co3O4 nanowire/nanoflower hybrid structure in non-aqueous electrolyte. RSC Adv. 2015, 5, 12700–12709. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Wang, J.; Zhang, B.; Yang, R. Hierarchical structure of CoFe2O4 core-shell microsphere coating on carbon fiber cloth for high-performance asymmetric flexible supercapacitor applications. Ionics 2019, 25, 4905–4914. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Wang, J.; Zhang, B.; Yang, R. Bifunctional Conducting Polymer Coated CoFe2O4 Core-Shell Nanolayer on Carbon Fiber Cloth for 2.0 V Wearable Aqueous Supercapacitors. ChemistrySelect 2019, 4, 1685–1695. [Google Scholar] [CrossRef]
- Luo, G.; Diao, G.; He, Q.; Han, S.; Dang, D.; Su, X. Morphology-controlled synthesis of MnO2 grown on carbon fiber paper for high-rate supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2022, 33, 18284–18293. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Tang, J.; Jiang, X.; Bao, Y.; Chen, Y. Preparation of ternary composite CF@γ-MnO2/PANI material in electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 25300–25317. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Zhang, M.; Li, M.; Li, T.; Yuan, G.; Liu, Y.; Zhang, X.; Cheng, X. Li-ion charge storage performance of wood-derived carbon fibers@MnO as a battery anode. Chin. Chem. Lett. 2021, 33, 1091–1094. [Google Scholar] [CrossRef]
- Sha, Z.; Zhou, Y.; Huang, F.; Yang, W.; Yu, Y.; Zhang, J.; Wu, S.; Brown, S.A.; Peng, S.; Han, Z.; et al. Carbon fibre electrodes for ultra long cycle life pseudocapacitors by engineering the nano-structure of vertical graphene and manganese dioxides. Carbon 2021, 177, 260–270. [Google Scholar] [CrossRef]
- Subhani, K.; Hameed, N.; Al-Qatatsheh, A.; Ince, J.; Mahon, P.J.; Lau, A.; Salim, N.V. Multifunctional structural composite supercapacitors based on MnO2-nanowhiskers decorated carbon fibers. J. Energy Storage 2022, 56, 105936. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, G.; Long, Z.; Lu, X.; He, Z.; Li, J.; Wang, Y.; Zhang, D. Core–shell structure γ-MnO2-PANI carbon fiber paper-based flexible electrode material for high-performance supercapacitors. J. Ind. Eng. Chem. 2021, 99, 317–325. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, G.; Zhang, X.; Shi, Z. Mace-like carbon fibers@Fe3O4@carbon composites as anode materials for lithium-ion batteries. Ionics 2020, 26, 5923–5934. [Google Scholar] [CrossRef]
- Zhang, K.; Cen, Z.; Yang, F.; Xu, K. Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors. Prog. Nat. Sci. Mater. Int. 2021, 31, 19–24. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Z.; Zhang, H.; Shen, A.; Zhao, Y.; Zhou, Y.; Weng, Y. Binder-Free Charantia-Like Metal-Oxide Core/Shell Nanotube Arrays for High-Performance Lithium-Ion Anodes. Front. Chem. 2020, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, S.; Yu, Y.; Feng, Q.; Yang, J.; Zhang, B. Carboxyl functionalized carbon fibers with preserved tensile strength and electrochemical performance used as anodes of structural lithium-ion batteries. Appl. Surf. Sci. 2017, 392, 27–35. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Zhu, Y.; Fu, W.; Zhang, X.; Zhao, P.; Wu, S. Graphene enhanced anchoring of nanosized Co3O4 particles on carbon fiber cloth as free-standing anode for lithium-ion batteries with superior cycling stability. Electrochim. Acta 2017, 247, 125–131. [Google Scholar] [CrossRef]
- Deka, B.K.; Kong, K.; Seo, J.; Kim, D.; Park, Y.-B.; Park, H.W. Controlled growth of CuO nanowires on woven carbon fibers and effects on the mechanical properties of woven carbon fiber/polyester composites. Compos. Part A Appl. Sci. Manuf. 2015, 69, 56–63. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on Carbon Fibers Modified with Carbon Nanotubes for Ultrafast Flexible Supercapacitors in Ionic Liquid Electrolytes with Wide Voltage Windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, K.; Ye, P.; Huang, Q.; Wang, L.; Li, S. Optimized NiCo2O4/rGO hybrid nanostructures on carbon fiber as an electrode for asymmetric supercapacitors. RSC Adv. 2018, 8, 37550–37556. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.M.; Kitiphatpiboon, N.; An, X.; Hao, X.; Li, S.; Hao, X.; Abudula, A.; Guan, G. Fabrication of High Energy Flexible All-Solid-State Supercapacitor Using Pseudocapacitive 2D-Ti3C2Tx-MXene and Battery-Type Reduced Graphene Oxi-de/Nickel-Cobalt Bimetal Oxide Electrode Materials. ACS Appl. Mater. Interfaces 2020, 12, 52749–52762. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, H.; Xiong, T.; Adekoya, D.; Qiu, W.; Wang, Z.; Zhang, S.; Balogun, M.-S. Adsorption energy engineering of nickel oxide hybrid nanosheets for high areal capacity flexible lithium-ion batteries. Energy Storage Mater. 2020, 25, 41–51. [Google Scholar] [CrossRef]
- Razali, S.; Majid, S. Electrochemical performance of binder-free NiO-PANI on etched carbon cloth as active electrode material for supercapacitor. Mater. Des. 2018, 153, 24–35. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Wei, X.; Chen, S.; Hu, Q.; Liu, Y.; Zhi, C.; Lu, W.; Zapien, J.A.; Huang, H. Towards high areal capacitance, rate capability, and tailorable supercapacitors: Co3O4@polypyrrole core–shell nanorod bundle array electrodes. J. Mater. Chem. A 2018, 6, 19058–19065. [Google Scholar] [CrossRef]
- Chen, T.; Fan, Y.; Wang, G.; Zhang, J.; Chuo, H.; Yang, R. Rationally Designed Carbon Fiber@NiCo2O4@Polypyrrole Core–Shell Nanowire Array for High-Performance Supercapacitor Electrodes. Nano 2016, 11, 1650015. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Chi, H.Z.; Zhu, H.; Gao, L. Boron-doped MnO2/carbon fiber composite electrode for supercapacitor. J. Alloys Compd. 2015, 645, 199–205. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J. MoS2/MoOx-Nanostructure-Decorated Activated Carbon Cloth for Enhanced Supercapacitor Performance. ChemSusChem 2018, 11, 897–906. [Google Scholar] [CrossRef]
- Govindasamy, M.; Shanthi, S.; Elaiyappillai, E.; Wang, S.-F.; Johnson, P.M.; Ikeda, H.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C. Fabrication of hierarchical NiCo2S4@CoS2 nanostructures on highly conductive flexible carbon cloth substrate as a hybrid electrode material for supercapacitors with enhanced electrochemical performance. Electrochim. Acta 2019, 293, 328–337. [Google Scholar] [CrossRef]
- Tian, Z.; Yin, J.; Wang, X.; Wang, Y. Construction of Ni3S2 wrapped by rGO on carbon cloth for flexible supercapacitor application. J. Alloys Compd. 2019, 777, 806–811. [Google Scholar] [CrossRef]
- Huang, F.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. Ni3S4 supported on carbon cloth for high-performance flexible all-solid-state asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 2525–2536. [Google Scholar] [CrossRef]
- Xu, X.; Tian, X.; Li, X.; Yang, T.; He, Y.; Wang, K.; Song, Y.; Liu, Z. Structural and chemical synergistic effect of NiCo2S4 nanoparticles and carbon cloth for high performance binder-free asymmetric supercapacitors. Appl. Surf. Sci. 2019, 465, 635–642. [Google Scholar] [CrossRef]
- Su, C.; Xu, S.; Zhang, L.; Chen, X.; Guan, G.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z.; et al. Hierarchical CoNi2S4 nanosheet/nanotube array structure on carbon fiber cloth for high-performance hybrid supercapacitors. Electrochim. Acta 2019, 305, 81–89. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, T.; Zhang, Y.; Chen, J.; Xie, M.; Du, C. 1D-on-1D core–shell cobalt iron selenide @ cobalt nickel carbonate hydroxide hybrid nanowire arrays as advanced battery-type supercapacitor electrode. J. Colloid Interface Sci. 2022, 621, 149–159. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, K.; Yu, X.; Yang, M.; Cao, X.; Lei, B.; Pan, D.; Jiang, H.; Wu, M. α-Fe2O3 Nanoparticles Decorated C@MoS2 Nanosheet Arrays with Expanded Spacing of (002) Plane for Ultrafast and High Li/Na-Ion Storage. Small 2019, 15, e1901083. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Maiti, S.; Paul, T.; Besra, N.; Sarkar, S.; Chattopadhyay, K.K. Geometrically intricate sheet-on-pillar/flake hierarchy embracing cobaltosic and manganese oxides over flexible carbon scaffold for binder-free high-energy-density supercapacitor. CrystEngComm 2018, 20, 6183–6196. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Hu, Z.; Miao, Y.; Sui, Y.; Qi, J.; Wei, F.; Ren, Y.; Zhan, Z.; Liu, J.; et al. In-situ growth of core-shell NiCo2O4@Ni-Co layered double hydroxides for all-solid-state flexible hybrid supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125417. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, R.; Wang, Q.; Xu, J.; Chen, D.; Shen, G. Efficient synthesis of hierarchical NiO nanosheets for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2014, 2, 10917–10922. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, M.; Ren, G.; Wu, S.; Shen, J. Facilely prepared oxidized carbon Fiber@Co3O4@RGO as negative electrode for a novel asymmetric supercapacitor with high areal energy and power density. Appl. Surf. Sci. 2018, 450, 66–76. [Google Scholar] [CrossRef]
- Abbas, Q.; Javed, M.S.; Ahmad, A.; Siyal, S.H.; Asim, I.; Luque, R.; Albaqami, M.D.; Tighezza, A.M. Zno nano-flowers assembled on carbon fiber textile for high-performance supercapacitor’s electrode. Coatings 2021, 11, 1337. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Chen, B.; Dai, G.; Situ, Y.; Huang, H. High-performance adjustable manganese oxides hybrid nanostructure for supercapacitors. Electrochim. Acta 2021, 381, 138213. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Xu, J.; Pan, Y.; Huang, Y.; Han, S.; Li, Y. Preparation of high-performance asymmetric supercapacitors based on NiCo2S4 nanospheres and CuO-MOF nanosheets on carbon fibers. Fuel 2024, 356, 129542. [Google Scholar] [CrossRef]
- Song, Z.; Shu, K.; Hu, H.; Wu, X.; Tang, X.; Zhou, X.; Li, Y.; Zhang, Y. Facile synthesis and modification of Fe2O3 nanorod arrays on carbon paper as efficient negative electrodes for supercapacitors. J. Alloys Compd. 2024, 979, 173578. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Hydrothermal Synthesis of a Cellular NiO Film on Carbon Paper as a Promising Way to Obtain a Hierarchically Organized Electrode for a Flexible Supercapacitor. Materials 2023, 16, 5208. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Yang, W.-D. Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes. Nanomaterials 2023, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, B.K.; González-Banciella, A.; Ureña, D.; Sánchez, M.; Ureña, A. Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4. Polymers 2023, 15, 4036. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.-H.; Liu, X. SnO2 nanospheres and V2O5/SnO2 nanoparticles with mesoporous structures for flexible asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 1–19. [Google Scholar] [CrossRef]
- Radhi, A.A.; Al-Rubaiey, S.I.J.; Al-Rubaye, S. NH4F-assisted and morphology-controlled fabrication of iron cobaltite FeCo2O4 nanoparticles on carbon fiber cloth for supercapacitor applications. Chem. Pap. 2023, 78, 733–746. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Liu, J.; Lu, Y.; Yang, Q.; Yang, L.-Y.; Yang, H.-R.; Liu, S.; Lei, M.; Han, M. Carbon fiber cloth@VO2(B): Excellent binder-free flexible electrodes with ultrahigh mass-loading. J. Mater. Chem. A 2016, 4, 6426–6432. [Google Scholar] [CrossRef]
- Pan, G.; Xia, X.; Cao, F.; Chen, J.; Zhang, Y. Carbon cloth supported vanadium pentaoxide nanoflake arrays as high-performance cathodes for lithium ion batteries. Electrochim. Acta 2014, 149, 349–354. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, X.; Zhang, W.; Li, Y.; Zhang, Z. Preparation of multifunctional structural P-CF@ZnCo2O4 composites used as structural anode materials. J. Alloys Compd. 2020, 842, 155743. [Google Scholar] [CrossRef]
- Han, Q.; Sheng, Y.; Zhang, X. Preparation of a multifunctional P-CF@Mn3O4 composite as a structural anode material. New J. Chem. 2021, 45, 15808–15817. [Google Scholar] [CrossRef]
- Purvika, A.; Yadav, S.; Jijoe, S.P.; Tenzin, T.; Divya, V.; Shahmoradi, B.; Wantala, K.; Jenkins, D.; McKay, G.; Shivaraju, H.P. Improved metal-organic frameworks (MOFs) and their application in catalytic CO2 reduction: A review. Mater. Today Sustain. 2024, 26, 100745. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Huang, X.; Qiu, S.; Liu, X.; Liu, L.; Zhao, M.; Qu, J.; Zou, J.; Zhang, J. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. J. Environ. Chem. Eng. 2023, 11, 111217. [Google Scholar] [CrossRef]
- Meskher, H.; Belhaouari, S.B.; Sharifianjazi, F. Mini review about metal organic framework (MOF)-based wearable sensors: Challenges and prospects. Heliyon 2023, 9, e21621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Wang, K.B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. Energy Chem. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Young, C.; Tang, J.; Takei, T.; Ide, Y.; Kobayashi, N.; Yamauchi, Y. A high-performance supercapacitor cell based on ZIF-8-derived nanoporous carbon using an organic electrolyte. Chem. Commun. 2016, 52, 4764–4767. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, S.; Li, J.; Ma, L.; He, F.; Liu, E.; He, C.; Shi, C.; Li, Q.; Zhao, N. Ball-in-cage nanocomposites of metal–organic frameworks and three-dimensional carbon networks: Synthesis and capacitive performance. Nanoscale 2017, 9, 6478–6485. [Google Scholar] [CrossRef]
- Huang, J.; Hao, F.; Zhang, X.; Chen, J. N-doped porous carbon sheets derived from ZIF-8: Preparation and their electrochemical capacitive properties. J. Electroanal. Chem. 2018, 810, 86–94. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal–organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Gao, Q.; Guo, H. Porous carbons prepared by using metal–organic framework as the precursor for supercapacitors. Carbon 2010, 48, 3599–3606. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Lin, X.; Zeb, A.; Xu, X.; Luo, Y.; Liu, J. Application of MOF-derived transition metal oxides and composites as anodes for lithium-ion batteries. Inorg. Chem. Front. 2020, 7, 4939–4955. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, B.; Tian, S.; Yang, X.; Ye, H.; Xia, Z.; Zheng, G. Application of MOFs-derived mixed metal oxides in energy storage. J. Electroanal. Chem. 2020, 878, 114576. [Google Scholar] [CrossRef]
- Bajwa, R.A.; Farooq, U.; Ullah, S.; Salman, M.; Haider, S.; Hussain, R. Metal-organic framework (MOF) attached and their derived metal oxides (Co, Cu, Zn and Fe) as anode for lithium ion battery: A review. J. Energy Storage 2023, 72, 108708. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. Highly flexible and large areal/volumetric capacitances for asymmetric supercapacitor based on ZnCo2O4 nanorods arrays and polypyrrole on carbon cloth as binder-free electrodes. Mater. Lett. 2019, 234, 1–4. [Google Scholar] [CrossRef]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni–Co–O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Feng, M.; Yang, J.; Zhang, B. MOF-derived ZnCo2O4/C wrapped on carbon fiber as anode materials for structural lithium-ion batteries. Chin. Chem. Lett. 2019, 30, 529–532. [Google Scholar] [CrossRef]
- Wang, F.; Han, Q.; Yi, Z.; Geng, D.; Li, X.; Wang, Z.; Wang, L. Synthesis and performances of carbon fiber@Co3O4 based on metal organic frameworks as anode materials for structural lithium-ion battery. J. Electroanal. Chem. 2017, 807, 196–202. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Dai, S.; Han, F.; Tang, J.; Tang, W. MOF-derived Co3O4 nanosheets rich in oxygen vacancies for efficient all-solid-state symmetric supercapacitors. Electrochim. Acta 2019, 328, 135103. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Yao, J. Synthesis of 2D nanoporous zeolitic imidazolate framework nanosheets for diverse applications. Co-ord. Chem. Rev. 2021, 431, 213677. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, H.; Hu, Z.; Yin, S.; Zhou, L. Temperature-induced microstructure optimization of Co3O4 for the achievement of a high-areal-capacity carbon cloth-based lithium ion battery anode. Compos. Commun. 2020, 22, 100446. [Google Scholar] [CrossRef]
- Wu, F.; Bai, J.; Feng, J.; Xiong, S. Porous mixed metal oxides: Design, formation mechanism, and application in lithium-ion batteries. Nanoscale 2015, 7, 17211–17230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Bin Wu, H.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Kavinkumar, T.; Vinodgopal, K.; Neppolian, B. Development of nanohybrids based on porous spinel MCo2O4 (M = Zn, Cu, Ni and Mn)/reduced graphene oxide/carbon nanotube as promising electrodes for high performance energy storage devices. Appl. Surf. Sci. 2020, 513, 145781. [Google Scholar] [CrossRef]
- Qi, J.; Wang, P.; Yan, Y.; Zheng, X.; Cao, J.; Feng, J. MCo2O4 (M = Co, Mn, Ni, Zn) nanosheet arrays constructed by two-dimension metal-organic frameworks as binder-free electrodes for lithium-ion batteries. Vacuum 2019, 169, 108959. [Google Scholar] [CrossRef]
- Liu, X.; Zang, W.; Guan, C.; Zhang, L.; Qian, Y.; Elshahawy, A.M.; Zhao, D.; Pennycook, S.J.; Wang, J. Ni-Doped Cobalt–Cobalt Nitride Heterostructure Arrays for High-Power Supercapacitors. ACS Energy Lett. 2018, 3, 2462–2469. [Google Scholar] [CrossRef]
- Lim, G.J.; Liu, X.; Guan, C.; Wang, J. Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim. Acta 2018, 291, 177–187. [Google Scholar] [CrossRef]
- Huang, T.; Lou, Z.; Lu, Y.; Li, R.; Jiang, Y.; Shen, G.; Chen, D. Metal-Organic-Framework-Derived MCo2O4 (M = Mn and Zn) Nanosheet Arrays on Carbon Cloth as Integrated Anodes for Energy Storage Applications. ChemElectroChem 2019, 6, 5836–5843. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational Design of Metal-Organic Framework Derived Hollow NiCo2O4 Arrays for Flexible Supercapacitor and Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Lai, Z.; Li, X.; Sun, S.; Zhang, H.; Cheetham, A.K.; Wang, J. Cobalt oxide and N-doped carbon nanosheets derived from a single two-dimensional metal–organic framework precursor and their application in flexible asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhou, Y.; Duan, H.; Guo, Y.; Li, H.; Chen, K.; Xue, D.; Liu, H. MOF-Derived Hollow Co3S4 Quasi-polyhedron/MWCNT Nanocomposites as Electrodes for Advanced Lithium Ion Batteries and Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 402–410. [Google Scholar] [CrossRef]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, C.; Xiao, D.; Kopold, P.; Gu, L.; van Aken, P.A.; Maier, J.; Yu, Y. MOF-Derived Hollow Co9S8 Nanoparticles Embedded in Graphitic Carbon Nanocages with Superior Li-Ion Storage. Small 2016, 12, 2354–2364. [Google Scholar] [CrossRef]
- Li, H.; Wu, P.; Xiao, Y.; Shao, M.; Shen, Y.; Fan, Y.; Chen, H.; Xie, R.; Zhang, W.; Li, S.; et al. Metal–Organic Frameworks as Metal Ion Precursors for the Synthesis of Nanocomposites for Lithium-Ion Batteries. Angew. Chem. 2020, 132, 4793–4799. [Google Scholar] [CrossRef]
- Aslam, M.K.; Shah, S.S.A.; Li, S.; Chen, C. Kinetically controlled synthesis of MOF nanostructures: Single-holed hollow core–shell ZnCoS@Co9S8/NC for ultra-high performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14083–14090. [Google Scholar] [CrossRef]
- Li, H.; Su, Y.; Sun, W.; Wang, Y. Carbon Nanotubes Rooted in Porous Ternary Metal Sulfide@N/S-Doped Carbon Dodecahedron: Bimetal-Organic-Frameworks Derivation and Electrochemical Application for High-Capacity and Long-Life Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 8345–8353. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Zheng, X.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Controlled synthesis of MOF-derived quadruple-shelled CoS2 hollow dodecahedrons as enhanced electrodes for supercapacitors. Electrochim. Acta 2019, 312, 54–61. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Guan, B.; Lou, X.W. Unusual Formation of CoSe@carbon Nanoboxes, which have an Inhomogeneous Shell, for Efficient Lithium Storage. Angew. Chem. 2016, 128, 9666–9670. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.; Men, S.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. CoSe2 Nanoparticles Encapsulated by N-Doped Carbon Framework Intertwined with Carbon Nanotubes: High-Performance Dual-Role Anode Materials for Both Li- and Na-Ion Batteries. Adv. Sci. 2018, 5, 1800763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, P.; Liu, H.; Yan, L.; Song, H.; Liu, D.; Zhao, X. Metal-organic framework derived porous flakes of cobalt chalcogenides (CoX, X = O, S, Se and Te) rooted in carbon fibers as flexible electrode materials for pseudocapacitive energy storage. Electrochim. Acta 2021, 369, 137681. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Yan, M.; Lei, Y.; Shi, W. MOF-derived hierarchical nanosheet arrays constructed by interconnected NiCo-alloy@NiCo-sulfide core-shell nanoparticles for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 666–676. [Google Scholar] [CrossRef]
- Dai, S.; Yuan, Y.; Yu, J.; Tang, J.; Zhou, J.; Tang, W. Metal–organic framework-templated synthesis of sulfur-doped core–sheath nanoarrays and nanoporous carbon for flexible all-solid-state asymmetric supercapacitors. Nanoscale 2018, 10, 15454–15461. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Pu, W.; Zheng, Y.; Wu, H.; Li, L.; Wei, X. Excellent rate capability supercapacitor electrodes with highly hydroxyl ion adsorption capacity enabled by P-doped MnCo2O4 nanotube arrays. Appl. Surf. Sci. 2022, 599, 153908. [Google Scholar] [CrossRef]
- Gong, H.; Bie, S.; Zhang, J.; Ke, X.; Wang, X.; Liang, J.; Wu, N.; Zhang, Q.; Luo, C.; Jia, Y. In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor. Nanomaterials 2022, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mateen, A.; Javed, M.S.; Khan, S.; Saleem, A.; Majeed, M.K.; Khan, A.J.; Tahir, M.F.; Ahmad, M.A.; Assiri, M.A.; Peng, K.-Q. Metal-organic framework-derived walnut-like hierarchical Co-O-nanosheets as an advanced binder-free electrode material for flexible supercapacitor. J. Energy Storage 2022, 49, 104150. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Yi, M.; Chen, Q.; Xu, C.; Cai, D.; Zhan, H. Metal-organic framework derived porous ternary ZnCo2O4 nanoplate arrays grown on carbon cloth as binder-free electrodes for lithium-ion batteries. Chem. Eng. J. 2018, 354, 454–462. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Liang, C.; Pan, A.; Zhang, C.; Tang, Y.; Tan, X.; Liu, J.; Liang, S. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Wang, F.; Han, Z.; Geng, D.; Zhang, W.; Li, Y.; Deng, Y.; Zhang, J.; Niu, S.; et al. Carbon fiber@ pore-ZnO composite as anode materials for structural lithium-ion batteries. J. Electroanal. Chem. 2018, 833, 39–46. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, W.; Liu, Y.; Shi, W. Self-supported hierarchical core–shell Co9S8@NiCo2O4 hollow nanoneedle arrays for asymmetric supercapacitors. Inorg. Chem. Front. 2019, 6, 982–987. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, J.-H.; Gan, H.; Chen, B.-Z.; Shi, X.-C. Electrochemical growth of α-MnO2 on carbon fibers for high-performance binder-free electrodes of supercapacitors. J. Appl. Electrochem. 2018, 48, 105–113. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Liu, Y.-C.; Lai, J.-Y.; Chung, C.-C.; Lin, K.-J. Vertically Standing MnO2 Nanowalls Grown on AgCNT-Modified Carbon Fibers for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 669–678. [Google Scholar] [CrossRef]
- Sha, Z.; Huang, F.; Zhou, Y.; Zhang, J.; Wu, S.; Chen, J.; Brown, S.A.; Peng, S.; Han, Z.; Wang, C.-H. Synergies of vertical graphene and manganese dioxide in enhancing the energy density of carbon fibre-based structural supercapacitors. Compos. Sci. Technol. 2021, 201, 108568. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Zhang, M.; Yu, J.; Liu, H.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Continuous preparation of high performance flexible asymmetric supercapacitor with a very fast, low-cost, simple and scalable electrochemical co-deposition method. J. Power Sources 2019, 437, 226827. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, S.; Huang, Y.; Shi, T.; Tang, Z. High performance wire-shaped supercapacitive electrodes based onactivated carbon fibers core/manganese dioxide shell structures. Ceram. Int. 2017, 43, 7916–7921. [Google Scholar] [CrossRef]
- Velayutham, R.; Manikandan, R.; Raj, C.J.; Kale, A.M.; Kaya, C.; Palanisamy, K.; Kim, B.C. Electrodeposition of vanadium pentoxide on carbon fiber cloth as a binder-free electrode for high-performance asymmetric supercapacitor. J. Alloys Compd. 2021, 863, 158332. [Google Scholar] [CrossRef]
- Kazemi, S.; Asghari, A.; Kiani, M. High Performance Supercapacitors Based on the Electrodeposited Co3O4 Nanoflakes on Electro-etched Carbon Fibers. Electrochim. Acta 2014, 138, 9–14. [Google Scholar] [CrossRef]
- Ko, T.H.; Kwak, C.-S.; Seong, J.-G.; Cho, Y.-H.; Lei, D.; Choi, W.-K.; Kuk, Y.-S.; Seo, M.-K.; Kim, B.-S. Influence of electrochemically deposited polypyrrole layers on NiCo2O4-decorated carbon fiber paper electrodes for high-performance hybrid supercapacitor applications. Funct. Compos. Struct. 2019, 1, 045003. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, X.-B.; Wang, X.-C.; Ma, J.; Liu, S.; Wang, X.-J. Nickel oxide grown on carbon nanotubes/carbon fiber paper by electrodeposition as flexible electrode for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2015, 26, 7901–7908. [Google Scholar] [CrossRef]
- Li, H.; Feng, Z.; Che, H.; Liu, Y.; Guo, Z.; Zhang, X.; Zhang, Z.; Wang, Y.; Mu, J. Direct growth of NiCo2O4nanosheet arrays on 3D-Ni-modified CFs for enhanced electrochemical storage in flexible supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 17879–17891. [Google Scholar] [CrossRef]
- Li, W.; Xin, L.; Xu, X.; Liu, Q.; Zhang, M.; Ding, S.; Zhao, M.; Lou, X. Facile synthesis of three-dimensional structured carbon fiber-NiCo2O4-Ni(OH)2 high-performance electrode for pseudocapacitors. Sci. Rep. 2015, 5, srep09277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hu, Y.; Zhou, Y.; Li, N.; Ding, Y.; Guo, J.; Zhao, C.; Yang, Y. Aerobic Recovered Carbon Fiber Support-Based MoO2//MnO2 Asymmetric Supercapacitor with a Widened Voltage Window. Energy Fuels 2021, 35, 6909–6920. [Google Scholar] [CrossRef]
- Xie, H.; Liu, X.; Wu, R.; Liu, J.; Wu, J.; Li, L. High-Performance Supercapacitor with Faster Energy Storage and Long Cyclic Life Based on CuO@MnO2 Nano-Core–Shell Array on Carbon Fiber Surface. ACS Appl. Energy Mater. 2020, 3, 7325–7334. [Google Scholar] [CrossRef]
- Huang, F.; Zhou, Y.; Sha, Z.; Peng, S.; Chang, W.; Cheng, X.; Zhang, J.; Brown, S.A.; Han, Z.; Wang, C.-H. Surface Functionalization of Electrodes and Synthesis of Dual-Phase Solid Electrolytes for Structural Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 30857–30871. [Google Scholar] [CrossRef]
- Qiu, W.; Balogun, M.-S.; Luo, Y.; Chen, K.; Zhu, Y.; Xiao, X.; Lu, X.; Liu, P.; Tong, Y. Three-dimensional Fe3O4 Nanotube Array on Carbon Cloth Prepared from A Facile Route for Lithium ion Batteries. Electrochim. Acta 2016, 193, 32–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).