Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings
Abstract
1. Introduction
2. Requirements for Conferring Antibacterial Properties to Object Surfaces
- Landing: Bacteria initially land on the surface of the foreign material.
- Adhesion and aggregation: Bacteria adhere to the surface and begin to aggregate.
- Biofilm formation: Bacteria protect themselves by forming a biofilm, creating small colonies.
- Colony maturation: The bacterial colonies mature, enhancing their resilience.
- Dispersal: Bacteria disperse from the mature colonies to colonize new areas.
- Preventing bacterial landing: Implementing strategies to keep bacteria from initially landing on the surface.
- Inhibiting adhesion and aggregation: Developing methods to prevent bacteria from adhering to and aggregating on the surface.
- Suppressing biofilm formation: Employing techniques to inhibit biofilm formation, thereby preventing the formation and maturation of bacterial colonies.
- Free energy;
- Tribology (smoothness, lubricity, friction, and wear);
- Topography;
- Surface chemical characteristics;
- Surface electrical properties;
- Surface Elasticity.
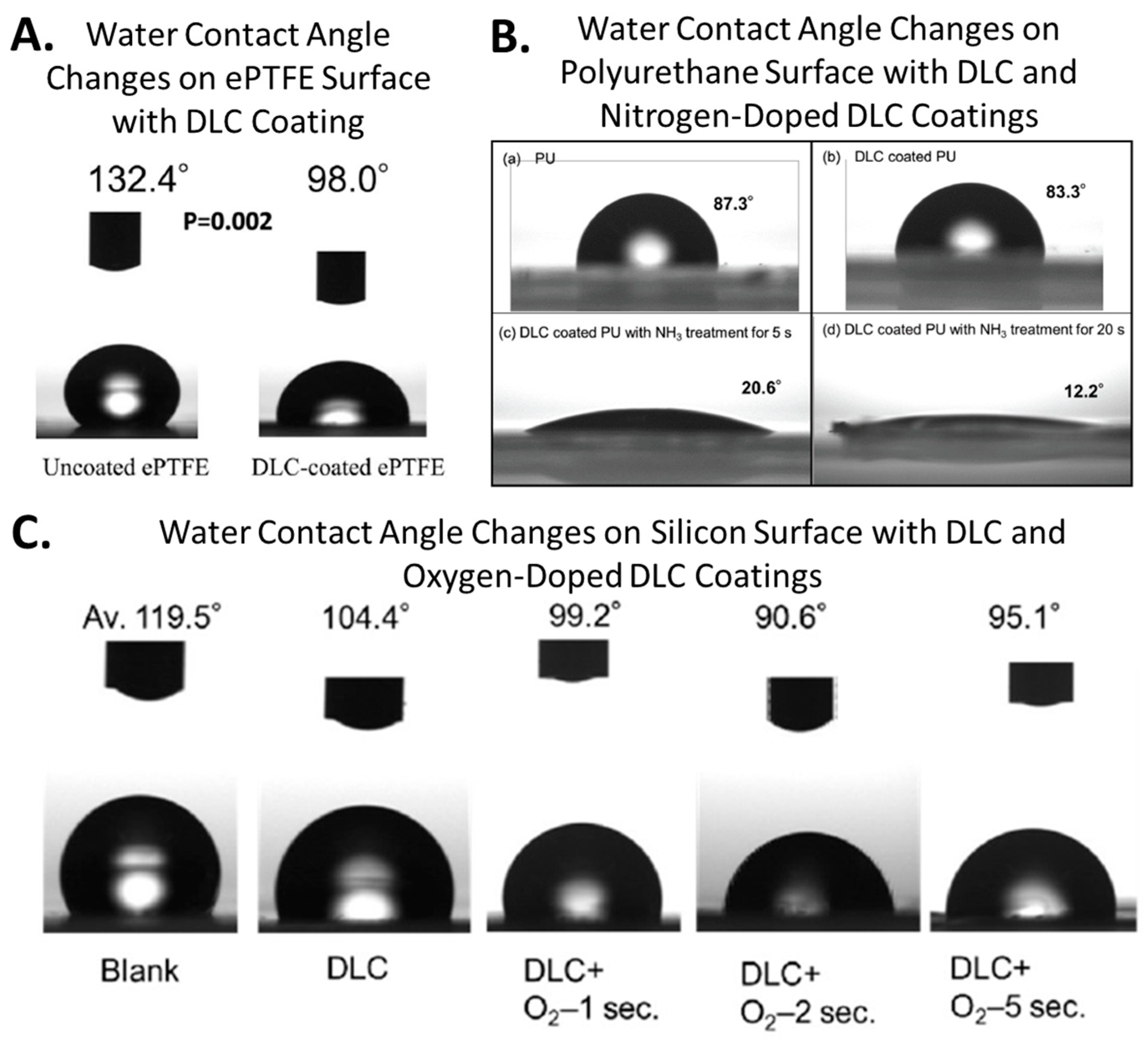
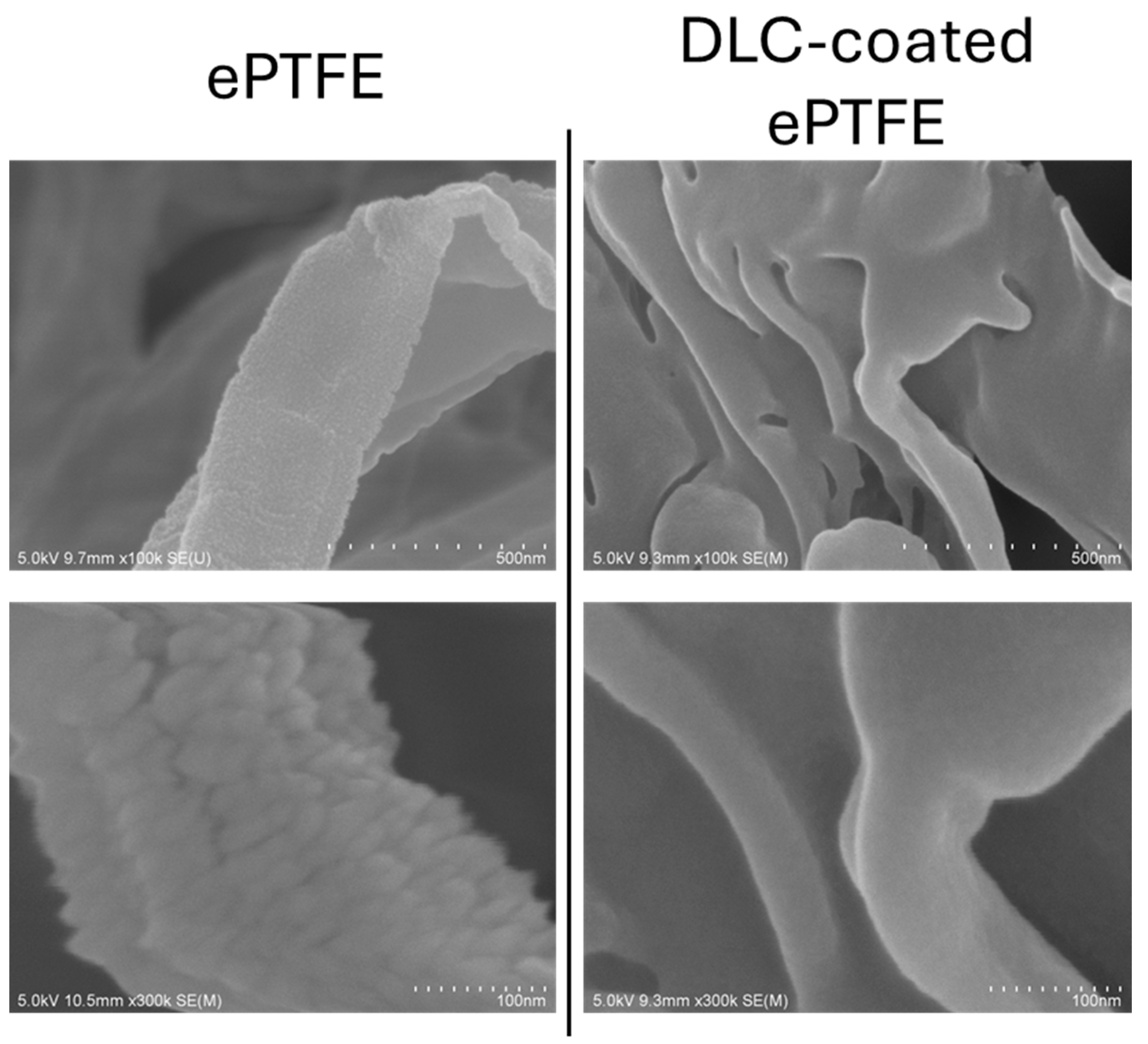
3. Surface Free Energy
4. Tribology
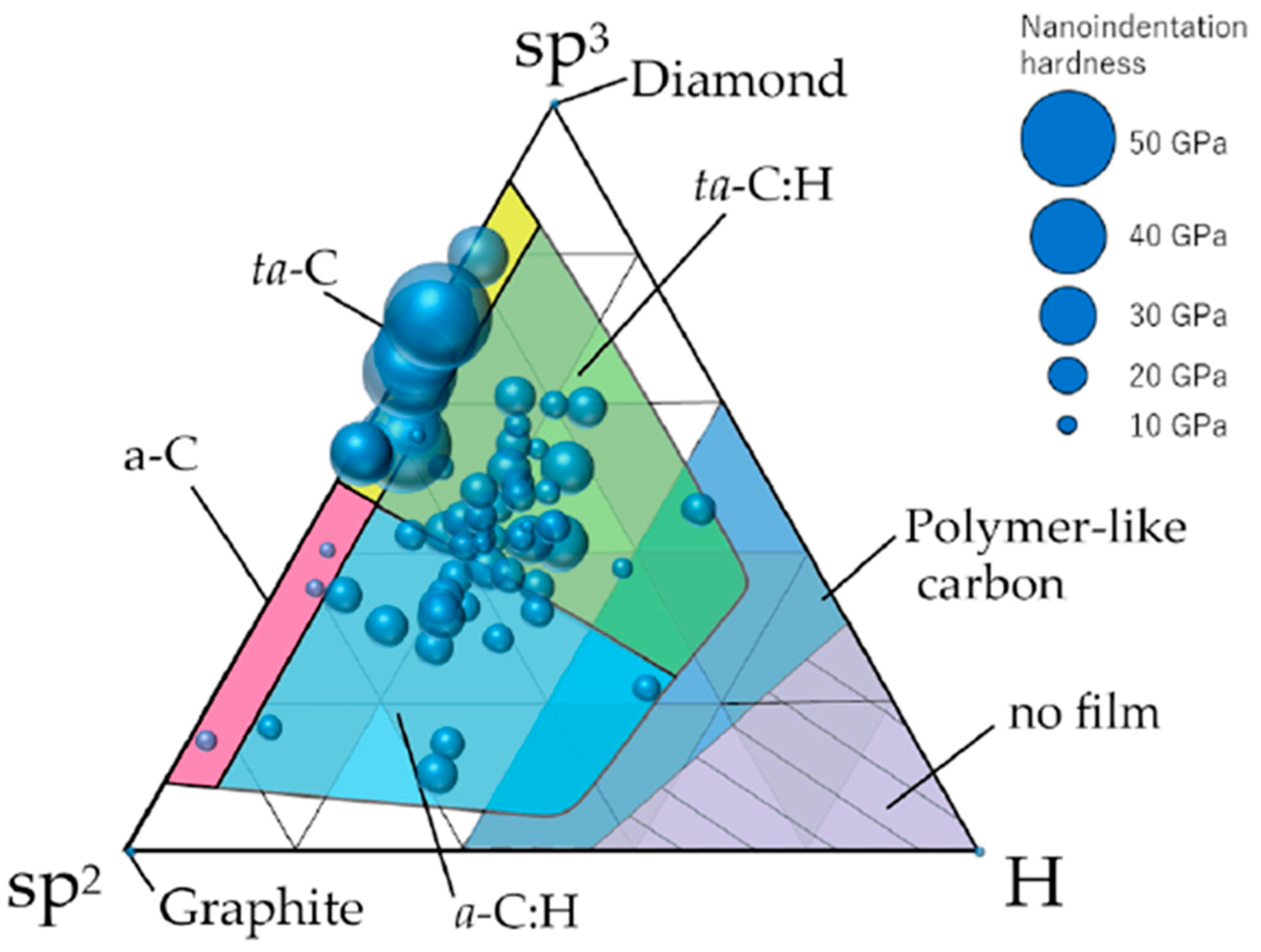
5. Surface Chemical Composition and Hardness
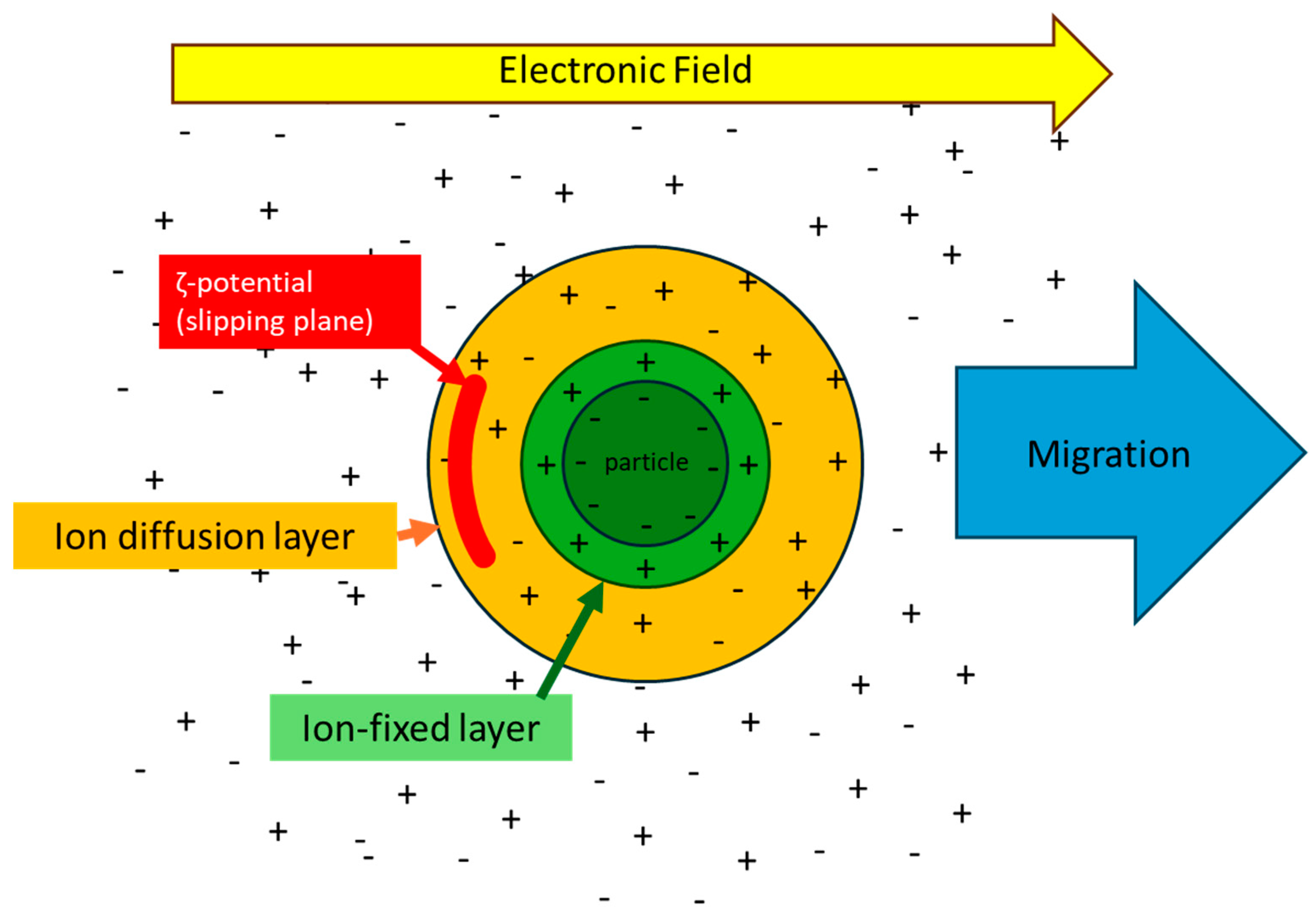
6. Surface Electrical Properties
7. Thickness and Density of the Biofunctional DLC Membrane
8. Surface Diffusion
9. Biocompatibility
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Precedence Reserch. Available online: https://www.precedenceresearch.com/medical-devices-market (accessed on 15 May 2024).
- Aisenberg, S.; Chabot, J. ion-beam deposition of thin films of diamondlike carbon. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Bull, S.J. Tribology of carbon coatings: DLC, diamond and beyond. Diam. Relat. Mater. 1995, 4, 827–836. [Google Scholar] [CrossRef]
- Thomson, L.A.; Law, S.C.; Rushton, N.; Franks, J. Biocompatibility of diamond-like carbon coating. Biomaterials 1991, 12, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Goyama, T.; Fujii, Y.; Muraoka, G.; Nakatani, T.; Ousaka, D.; Imai, Y.; Kuwada, N.; Tsuji, T.; Shuku, T.; Uchida, H.A.; et al. Comprehensive hemocompatibility analysis on the application of Diamond-like carbon to ePTFE artificial vascular prosthesis. Sci. Rep. 2023, 13, 8386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Su, Z.; Guo, J.; Li, K.; Chen, K.; Li, W.; Yi, A.; Liao, Z.; Luo, Y.; Hu, Y.; et al. Preparation and characterization of diamond-like carbon (DLC) film on 316L stainless steel by microwave plasma chemical vapor deposition (MPCVD). Diam. Relat. Mater. 2022, 122, 108802. [Google Scholar] [CrossRef]
- Kuwada, N.; Fujii, Y.; Nakatani, T.; Ousaka, D.; Tsuji, T.; Imai, Y.; Kobayashi, Y.; Oozawa, S.; Kasahara, S.; Tanemoto, K. Diamond-like carbon coating to inner surface of polyurethane tube reduces Staphylococcus aureus bacterial adhesion and biofilm formation. J. Artif. Organs 2023, 27, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Watari, S.; Wada, K.; Araki, M.; Sadahira, T.; Ousaka, D.; Oozawa, S.; Nakatani, T.; Imai, Y.; Kato, J.; Kariyama, R.; et al. Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters. Int. J. Urol. 2021, 28, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Zhang, M.; Ma, S.; Chu, P.K. Biological response of endothelial cells to diamond-like carbon-coated NiTi alloy. J. Biomed. Mater. Res. A 2012, 100, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Movahed, S.; Nguyen, A.K.; Goering, P.L.; Skoog, S.A.; Narayan, R.J. Argon and oxygen plasma treatment increases hydrophilicity and reduces adhesion of silicon-incorporated diamond-like coatings. Biointerphases 2020, 15, 041007. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Fukue, H.; Nakatani, T.; Kunitsugu, S.; Kanda, K.; Suzuki, T.; Watari, S.; Fujii, Y.; Ousaka, D.; Oozawa, S.; et al. Biomimetic diamond-like carbon coating on a lumen of small-diameter long-sized tube modified surface uniformly with carboxyl group using oxygen plasma. J. Photopolym. Sci. Technol. 2022, 4, 289–297. [Google Scholar] [CrossRef]
- Menegazzo, N.; Kahn, M.; Berghauser, R.; Waldhauser, W.; Mizaikoff, B. Nitrogen-doped diamond-like carbon as optically transparent electrode for infrared attenuated total reflection spectroelectrochemistry. Analyst 2011, 136, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Fukue, H.; Nakatani, T.; Kunitsugu, S.; Kuwada, N.; Fujii, Y.; Oozawa, S.; Uchi, T. Ultra-hydrophilic Diamond-like Carbon Coating on an Inner Surface of a Small-diameter Long Tube with an Amino Group by AC High-voltage Plasma Discharge. J. Photopolym. Sci. Technol. 2023, 5, 379–384. [Google Scholar] [CrossRef]
- Birkett, M.; Zia, A.W.; Devarajan, D.K.; Soni; Panayiotidis, M.I.; Joyce, T.J.; Tambuwala, M.M.; Serrano-Aroca, Á. Multi-functional bioactive silver- and copper-doped diamond-like carbon coatings for medical implants. Acta Biomater. 2023, 167, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, X.; Zhang, Z.; Shu, W.; Li, J. Attempting AG-Doped Diamond-Like Carbon Film to Improve Seal Performance of Hydraulic Servo-Actuator. Materials 2020, 13, 2618. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, T.; Yohena, S.; Kamijo, A.; Okazaki, Y.; Hotta, A.; Takahashi, K.; Suzuki, T. Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J. Biomed. Mater. Res. 2007, 83, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Mitra, M.K.; Chattopadhyay, K.K. Fluorinated diamond like carbon as an electron field emission material. J. Nanosci. Nanotechnol. 2009, 9, 5545–5549. [Google Scholar] [CrossRef] [PubMed]
- Kinnari, T.J.; Soininen, A.; Esteban, J.; Zamora, N.; Alakoski, E.; Kouri, V.P.; Lappalainen, R.; Konttinen, Y.T.; Gomez-Barrena, E.; Tiainen, V.M. Adhesion of staphylococcal and Caco-2 cells on diamond-like carbon polymer hybrid coating. J. Biomed. Mater. Res. A 2008, 86, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Sasai, Y.; Ousaka, D.; Fujii, Y.; Isono, A.; Yamauchi, Y.; Kondo, S.; Nakatiani, T. Surface functionalization of diamond-like carbon film with biocompatible polymer brushes. J. Photopolym. Sci. Technol. 2022, 4, 303–308. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Lin, X.; Zhu, Y.; Bai, X. A super-hydrophilic partially reduced graphene oxide membrane with improved stability and antibacterial properties. Water Sci. Technol. 2022, 86, 1426–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Zhang, H.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Anti-bacterial and super-hydrophilic bamboo charcoal with amidoxime modified for efficient and selective uranium extraction from seawater. J. Colloid Interface Sci. 2021, 598, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Q.; Zhang, Z.; Wang, X.; Niu, H. Recent advances in superhydrophobic and antibacterial cellulose-based fibers and fabrics: Bio-inspiration, strategies, and applications. Adv. Fiber Mater. 2023, 5, 1555–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Yang, Y.; Zhang, W.; Lv, H.; Yang, F.; Lin, C.; Tang, P. Inhibitory effect of super-hydrophobicity on silver release and antibacterial properties of super-hydrophobic Ag/TiO2 nanotubes. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- BinAhmed, S.; Hasane, A.; Wang, Z.; Mansurov, A.; Castrillón, S.R.V. Bacterial adhesion to ultrafiltration membranes: Role of hydrophilicity, natural organic matter, and cell-surface macromolecules. Environ. Sci. Technol. 2018, 52, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Stallard, C.P.; McDonnell, K.A.; Onayemi, O.D.; O’Gara, J.P.; Dowling, D.P. Evaluation of protein adsorption on atmospheric plasma deposited coatings exhibiting superhydrophilic to superhydrophobic properties. Biointerphases 2012, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.B.; Lee, J.W.; Khang, G. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohara, T.; Inaba, H.; Kano, M.; et al. Properties and classification of diamond-like carbon films. Materials 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, Y.; Hamada, T.; Izumi, H.; Yasuda, Y.; Kano, M. The Development of Hydrogen-free DLC-coated Valve-lifter. J. Mater. Manufact. 2007, 116, 788–794. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Papazoglou, E.L.; Karmiris-Obratański, P.; Karkalos, N.E.; Markopoulos, A.P. Surface antibacterial properties enhanced through engineered textures and surface roughness: A review. Colloids Surf. B Biointerfaces 2023, 231, 113584. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.H.; Su, Y.L.; Lai, Y.C. Antibacterial and tribological performance of carbonitride coatings doped with W, Ti, Zr, or Cr deposited on AISI 316L stainless steel. Materials 2017, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Senesi, G.S.; D’Aloia, E.; Gristina, R.; Favia, P.; d’Agostino, R. Surface characterization of plasma deposited nano-structured fluorocarbon coatings for promoting in vitro cell growth. Surf. Sci. 2007, 601, 1019–1025. [Google Scholar] [CrossRef]
- Sousa, C.; Rodrigues, D.; Oliveira, R.; Song, W.; Mano, J.; Azeredo, J. Superhydrophobic poly(L-lactic acid) surface as potential bacterial colonization substrate. AMB Express 2011, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Poelstra, K.A.; Barekzi, N.A.; Rediske, A.M.; Felts, A.G.; Slunt, J.B.; Grainger, D.W. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J. Biomed. Mater. Res. 2002, 60, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Choi, S.; Kim, J. Analysis of contact area between water and irregular fibrous surface for prediction of wettability. RSC Adv. 2016, 6, 73313–73322. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Bioinspired multiscale surfaces with special wettability. MRS Bull. 2013, 38, 375–382. [Google Scholar] [CrossRef]
- Wong, T.S.; Sun, T.; Feng, L.; Aizenberg, J. Interfacial materials with special wettability. MRS Bull. 2013, 38, 366–371. [Google Scholar] [CrossRef]
- Kota, A.K.; Choi, W.; Tuteja, A. Superomniphobic surfaces: Design and durability. MRS Bull. 2013, 38, 383–390. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bioinspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Chang, Y.R.; Hoseinabad, H.K.; Pruden, A.; Ducker, W.A. Colloidal crystals delay formation of early stage bacterial biofilms. ACS Biomater. Sci. Eng. 2016, 2, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’Reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [PubMed]
- Manabe, K.; Nishizawa, S.; Shiratori, S. Porous surface structure fabricated by breath figures that suppresses Pseudomonas aeruginosa biofilm formation. ACS Appl. Mater. Interfaces 2013, 5, 11900–11905. [Google Scholar] [CrossRef] [PubMed]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface roughness mediated adhesion forces between borosilicate glass and gram-positive bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [PubMed]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.H.; Weibel, D.B. Bacteria–surface interactions. Soft Mater. 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun polystyrene fiber diameter influencing bacterial attachment, proliferation, and growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2015, 21, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Angus, J.C.; Hayman, C.C. Low-Pressure, Metastable Growth of Diamond and “Diamondlike” Phases. Science 1988, 241, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Kumagai, T. Hydrogen permeability of diamondlike amorphous carbons. J. Vac. Sci. Technol. A 2017, 35, 04D101. [Google Scholar] [CrossRef]
- Levon, J.; Myllymaa, K.; Kouri, V.P.; Rautemaa, R.; Kinnari, T.; Myllymaa, S.; Konttinen, Y.T.; Lappalainen, R. Patterned macroarray plates in comparison of bacterial adhesion inhibition of tantalum, titanium, and chromium compared with diamond-like carbon. J. Biomed. Mater. Res. A 2010, 92, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Del Prado, G.; Terriza, A.; Ortiz-Pérez, A.; Molina-Manso, D.; Mahillo, I.; Yubero, F.; Puértolas, J.A.; Manrubia-Cobo, M.; Gómez Barrena, E.; Esteban, J. DLC coatings for UHMWPE: Relationship between bacterial adherence and surface properties. J. Biomed. Mater. Res. A 2012, 100, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Harrasser, N.; Jüssen, S.; Banke, I.J.; Kmeth, R.; von Eisenhart-Rothe, R.; Stritzker, B.; Gollwitzer, H.; Burgkart, R. Antibacterial efficacy of titanium-containing alloy with silver-nanoparticles enriched diamond-like carbon coatings. AMB Express 2015, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Cazalini, E.M.; Miyakawa, W.; Teodoro, G.R.; Sobrinho, A.S.S.; Matieli, J.E.; Massi, M.; Koga-Ito, C.Y. Antimicrobial and anti-biofilm properties of polypropylene meshes coated with metal-containing DLC thin films. J. Mater. Sci. Mater. Med. 2017, 28, 97. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef] [PubMed]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials 2016, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Harrasser, N.; Jüssen, S.; Obermeir, A.; Kmeth, R.; Stritzker, B.; Gollwitzer, H.; Burgkart, R. Antibacterial potency of different deposition methods of silver and copper containing diamond-like carbon coated polyethylene. Biomater. Res. 2016, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.P.; Wang, D.Y.; Chen, L.K.; Kung, C.M.; Wu, Y.C.; Ou, K.L.; Yu, C.H. Antibacterial nanostructured composite films for biomedical applications: Microstructural characteristics, biocompatibility, and antibacterial mechanisms. Biofouling 2013, 29, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.S.; Oliveira, J.R.; Milani, J.; Oliveira, L.D.; Machado, J.P.B.; Trava-Airoldi, V.J.; Lobo, A.O.; Marciano, F.R. Biomineralized diamond-like carbon films with incorporated titanium dioxide nanoparticles improved bioactivity properties and reduced biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Kawaguchi, M.; Kaneuji, A.; Ichiseki, T.; Iinuma, Y.; Kawamura, K.; Shintani, K.; Oda, S.; Taki, M.; Kawahara, N. Evaluation of Antibacterial and Cytotoxic Properties of a Fluorinated Diamond-Like Carbon Coating for the Development of Antibacterial Medical Implants. Antibiotics 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Kocourek, T.; Zemek, J.; Mikšovský, J.; Kubinová, Š.; Remsa, J.; Kopeček, J.; Jurek, K. Chromium-doped DLC for implants prepared by laser-magnetron deposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Buchegger, S.; Kamenac, A.; Fuchs, S.; Herrmann, R.; Houdek, P.; Gorzelanny, C.; Obermeier, A.; Heller, S.; Burgkart, R.; Stritzker, B.; et al. Smart antimicrobial efficacy employing pH-sensitive ZnO-doped diamond-like carbon coatings. Sci. Rep. 2019, 9, 17246. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.E.; Kurt, M.Ş.; Aslan, N.; Kadi, A.; Öner, S.; Çobanoğlu, Ş.; Yazici, A. Structural, biocompatibility, and antibacterial properties of Ge-DLC nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface design for antibacterial materials: From fundamentals to advanced strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ziats, N.P.; Tierney, B.P.; Nakabayashi, N.; Anderson, J.M. Protein adsorption from human plasma is reduced on phospholipid polymers. J. Biomed. Mater. Res. 1991, 25, 1397–1407. [Google Scholar] [CrossRef]
- Lv, J.; Jin, J.; Chen, J.; Cai, B.; Jiang, W. Antifouling and antibacterial properties constructed by quaternary ammonium and benzyl ester derived from lysine methacrylamide. ACS Appl. Mater. Interfaces 2019, 11, 25556–25568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Wang, H.; Newby, B.-M.Z.; Cheng, F.; Liu, L. Amino acid-based zwitterionic polymer surfaces highly resist long-term bacterial adhesion. Langmuir 2016, 32, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Lee, B.S.; Shin, H.S.; Park, K.; Han, D.K. Surface grafting of blood compatible zwitterionic poly(ethylene glycol) on diamond-like carbon-coated stent. J. Mater. Sci. Mater. Med. 2011, 22, 507–514. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus. 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Oura, K.; Katayama, M.; Zotov, A.V.; Lifshits, V.G.; Saranin, A.A. Elementary Processes at Surfaces II. Surface Diffusion. In Book Surface Science; Oura, K., Katayama, M., Zotov, V., Lifshits, V.G., Saranin, A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 325–356. [Google Scholar]
- Kreuzer, H.J. Kinetics of Adsorption, Desorption and Reactions at Surfaces. In Springer Handbook of Surface Science; Rocca, M., Rahman, T.S., Vattuone, L., Eds.; Springer Handbooks; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Castellino, M.; Stolojan, V.; Virga, A.; Rovere, M.; Cabiale, K.; Galloni, M.R.; Tagliaferro, A. Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents. Anal. Biochem. Chem. 2013, 405, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Kousar, Y.; Okpalugo, T.I.; Singh, V.; Pease, M.; Ogwu, A.A.; Gracio, J.; Titus, E.; Meletis, E.I.; Jackson, M.J. Human micro-vascular endothelial cell seeding on Cr-DLC thin films for mechanical heart valve applications. Thin Solid Films 2006, 515, 59–65. [Google Scholar] [CrossRef]
- Maguire, P.D.; McLaughlin, J.A.; Okpalugo, T.I.T.; Lemoine, P.; Papakonstantinou, P.; McAdams, E.T.; Needham, M.; Ogwu, A.A.; Ball, M.; Abbas, G.A. Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam. Relat. Mater. 2005, 14, 1277–1288. [Google Scholar] [CrossRef]
- Ando, K.; Ishii, K.; Tada, E.; Kataoka, K.; Hirohata, A.; Goto, K.; Kobayashi, K.; Tsutsui, H.; Nakahama, M.; Nakashima, H.; et al. Prospective multi-center registry to evaluate efficacy and safety of the newly developed diamond-like carbon-coated cobalt-chromium coronary stent system. Cardiovasc. Interv. Ther. 2017, 32, 225–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zia, A.W.; Anestopoulos, I.; Panayiotidis, M.I.; Birkett, M. Soft diamond-like carbon coatings with superior biocompatibility for medical applications. Ceram. Int. 2023, 49, 17203–17211. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.X.; Cui, F.Z.; Lee, I.S.; Lee, G.H. Characterization and degradation comparison of DLC film on different magnesium alloys. Surf. Coat. Technol. 2010, 205, 15–20. [Google Scholar] [CrossRef]
- Sokołowska, A.; Rudnicki, J.; Niedzielski, P.; Boczkowska, A.; Bogusławski, G.; Wierzchoń, T.; Mitura, S. TiN–NCD composite coating produced on the Ti6Al4V alloy for medical applications. Surf. Coat. Technol. 2005, 200, 87–89. [Google Scholar] [CrossRef]
- Mitura, S.; Niedzielski, P.; Jachowicz, D.; Langer, M.; Marciniak, J.; Stanishevsky, A.; Tochitsky, E.; Louda, P.; Couvrat, P.; Denis, M.; et al. Influence of carbon coatings origin on the properties important for biomedical application. Diam. Relat. Mater. 1996, 5, 1185–1188. [Google Scholar] [CrossRef]
- Malisz, K.; Świeczko-Żurek, B.; Sionkowska, A. Preparation and Characterization of Diamond-like Carbon Coatings for Biomedical Applications-A Review. Materials 2023, 16, 3420. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Nakatani, T.; Ousaka, D.; Oozawa, S.; Sasai, Y.; Kasahara, S. Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings. Int. J. Mol. Sci. 2024, 25, 8593. https://doi.org/10.3390/ijms25168593
Fujii Y, Nakatani T, Ousaka D, Oozawa S, Sasai Y, Kasahara S. Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings. International Journal of Molecular Sciences. 2024; 25(16):8593. https://doi.org/10.3390/ijms25168593
Chicago/Turabian StyleFujii, Yasuhiro, Tatsuyuki Nakatani, Daiki Ousaka, Susumu Oozawa, Yasushi Sasai, and Shingo Kasahara. 2024. "Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings" International Journal of Molecular Sciences 25, no. 16: 8593. https://doi.org/10.3390/ijms25168593
APA StyleFujii, Y., Nakatani, T., Ousaka, D., Oozawa, S., Sasai, Y., & Kasahara, S. (2024). Development of Antimicrobial Surfaces Using Diamond-like Carbon or Diamond-like Carbon-Based Coatings. International Journal of Molecular Sciences, 25(16), 8593. https://doi.org/10.3390/ijms25168593






