Physical Activity and Epigenetic Aging in Breast Cancer Treatment
Abstract
:1. Introduction
2. Results
2.1. Participant Baseline Characteristics
2.2. Physical Activity Improves Functional Parameters in Breast Cancer Patients
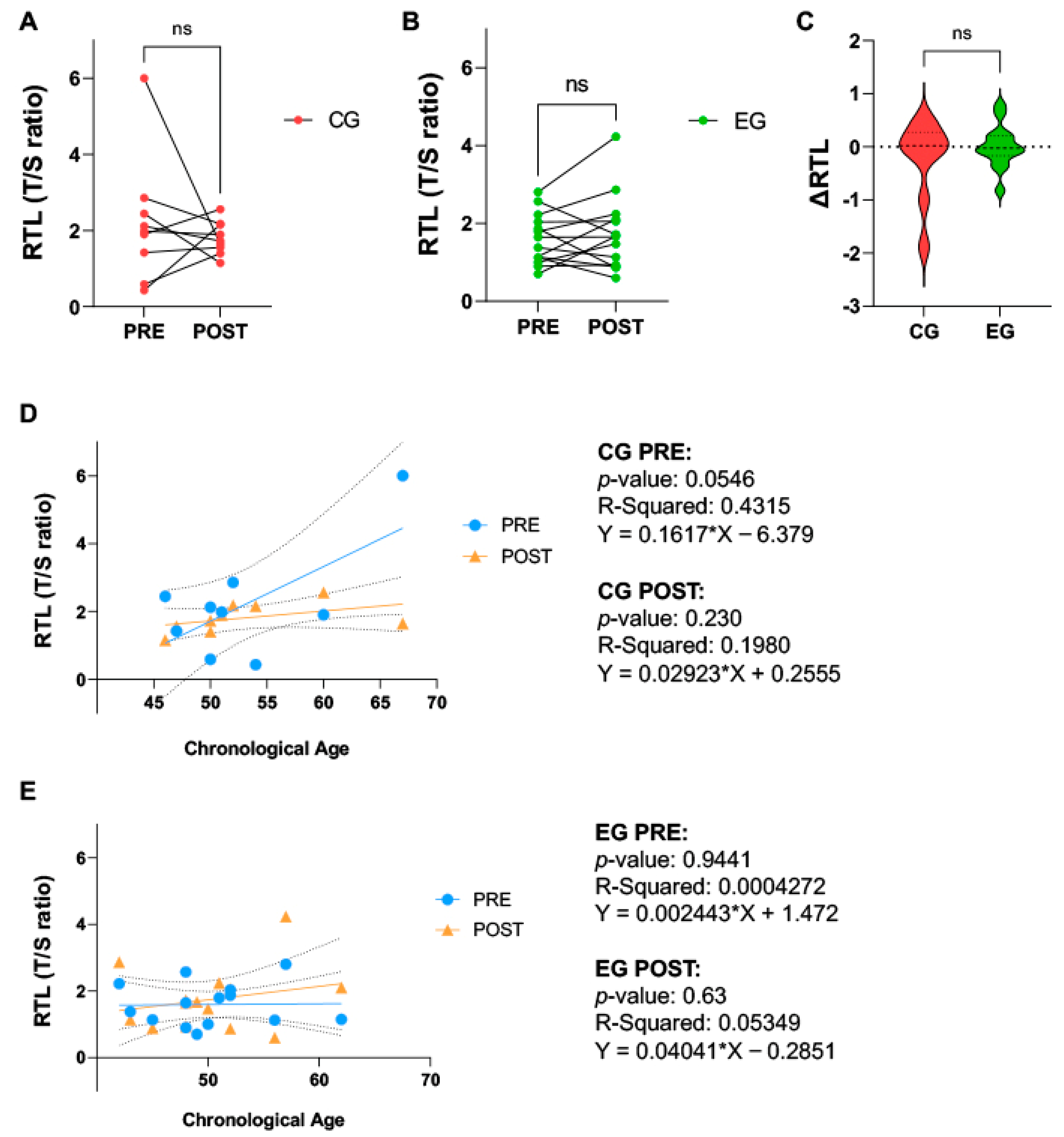
2.3. Physical Activity Does Not Affect Relative Telomere Length in Breast Cancer Patients
2.4. Health-Related Quality of Life
2.5. Physical Activity Improves ELOVL2-Based Epigenetic Clock in Breast Cancer Patients
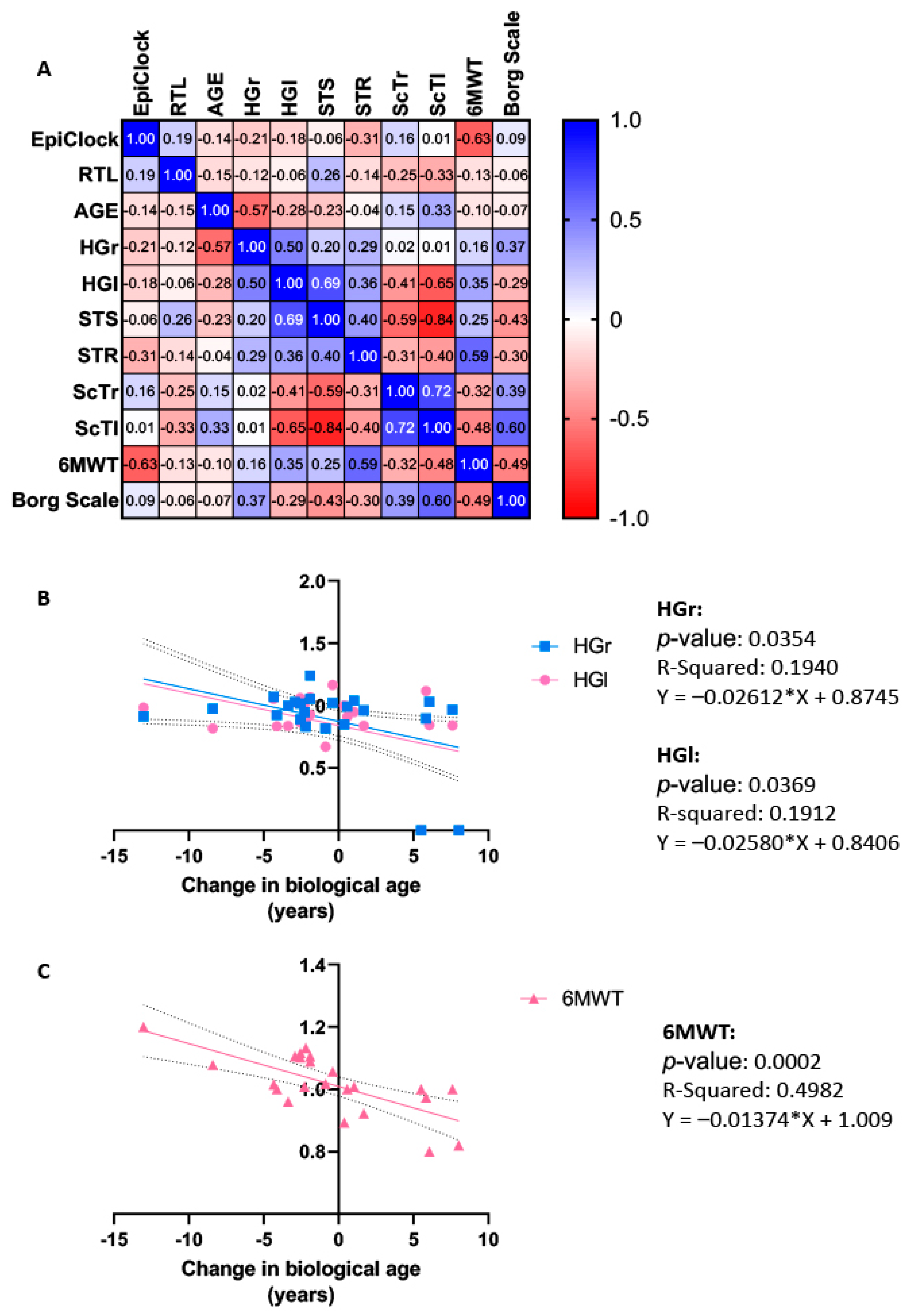
2.6. Correlation and Regression Analysis
3. Discussion
4. Future Perspectives, Limitations, and Clinical Considerations
5. Materials and Methods
5.1. Ethical Approval
5.2. Study Design
5.3. Exercise Training Program
5.4. Blood Sampling and DNA Extraction
5.5. Measurement of Relative Telomere Length
5.6. Measurement of ELOVL2-Based Epigenetic Clock
5.7. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, B.J. Aging and Cancer. J. Clin. Oncol. 1988, 6, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Mandelblatt, J.S.; Ahles, T.A.; Lippman, M.E.; Isaacs, C.; Adams-Campbell, L.; Saykin, A.J.; Cohen, H.J.; Carroll, J. Applying a Life Course Biological Age Framework to Improving the Care of Individuals With Adult Cancers: Review and Research Recommendations. JAMA Oncol. 2021, 7, 1692. [Google Scholar] [CrossRef] [PubMed]
- Rentscher, K.E.; Bethea, T.N.; Zhai, W.; Small, B.J.; Zhou, X.; Ahles, T.A.; Ahn, J.; Breen, E.C.; Cohen, H.J.; Extermann, M.; et al. Epigenetic Aging in Older Breast Cancer Survivors and Noncancer Controls: Preliminary Findings from the Thinking and Living with Cancer Study. Cancer 2023, 129, 2741–2753. [Google Scholar] [CrossRef]
- Hurria, A.; Jones, L.; Muss, H.B. Cancer Treatment as an Accelerated Aging Process: Assessment, Biomarkers, and Interventions. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e516–e522. [Google Scholar] [CrossRef] [PubMed]
- Cupit-Link, M.C.; Kirkland, J.L.; Ness, K.K.; Armstrong, G.T.; Tchkonia, T.; LeBrasseur, N.K.; Armenian, S.H.; Ruddy, K.J.; Hashmi, S.K. Biology of Premature Ageing in Survivors of Cancer. ESMO Open 2017, 2, e000250. [Google Scholar] [CrossRef]
- Hodes, R.J.; Sierra, F.; Austad, S.N.; Epel, E.; Neigh, G.N.; Erlandson, K.M.; Schafer, M.J.; LeBrasseur, N.K.; Wiley, C.; Campisi, J.; et al. Disease Drivers of Aging. Ann. N. Y. Acad. Sci. 2016, 1386, 45–68. [Google Scholar] [CrossRef]
- Ness, K.K.; Wogksch, M.D. Frailty and Aging in Cancer Survivors. Transl. Res. 2020, 221, 65–82. [Google Scholar] [CrossRef]
- Wang, S.; Prizment, A.; Thyagarajan, B.; Blaes, A. Cancer Treatment-Induced Accelerated Aging in Cancer Survivors: Biology and Assessment. Cancers 2021, 13, 427. [Google Scholar] [CrossRef]
- Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Gori, D.; Giuliani, C.; Mari, D.; Di Blasio, A.M.; Gentilini, D.; Vitale, G.; Collino, S.; et al. Methylation of ELOVL2 Gene as a New Epigenetic Marker of Age. Aging Cell 2012, 11, 1132–1134. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L. Relationship between Telomere Length and the Prognosis of Breast Cancer Based on Estrogen Receptor Status: A Mendelian Randomization Study. Front. Oncol. 2022, 12, 1024772. [Google Scholar] [CrossRef] [PubMed]
- Paparazzo, E.; Lagani, V.; Geracitano, S.; Citrigno, L.; Aceto, M.A.; Malvaso, A.; Bruno, F.; Passarino, G.; Montesanto, A. An ELOVL2-Based Epigenetic Clock for Forensic Age Prediction: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2254. [Google Scholar] [CrossRef] [PubMed]
- Kipling, D. The Telomere; Oxford University Press: Oxford, UK, 1995; ISBN 0-19-963600-1. [Google Scholar]
- Nomikos, N.N.; Nikolaidis, P.T.; Sousa, C.V.; Papalois, A.E.; Rosemann, T.; Knechtle, B. Exercise, Telomeres, and Cancer: “The Exercise-Telomere Hypothesis”. Front. Physiol. 2018, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Shammas, M.A. Telomeres, Lifestyle, Cancer, and Aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cleal, K.; Norris, K.; Baird, D. Telomere Length Dynamics and the Evolution of Cancer Genome Architecture. Int. J. Mol. Sci. 2018, 19, 482. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; De Lange, T. Telomeres in Cancer: Tumour Suppression and Genome Instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, E.; Kim, H. Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2022, 58, 242. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Gouveira, É.R.; Peralta, M.; Martins, J.; Venturini, J.; Henriques-Neto, D.; Sarmento, H. Cardiorespiratory Fitness and Telomere Length: A Systematic Review. J. Sports Sci. 2020, 38, 1690–1697. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic Clock: A Promising Biomarker and Practical Tool in Aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef]
- Li Piani, L.; Vigano’, P.; Somigliana, E. Epigenetic Clocks and Female Fertility Timeline: A New Approach to an Old Issue? Front. Cell Dev. Biol. 2023, 11, 1121231. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E. A Comparison of Epigenetic Mitotic-like Clocks for Cancer Risk Prediction. Genome Med. 2020, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Voisin, S. Exercise and/or Stress Effects on the Epigenetic Clock. In Stress: Genetics, Epigenetics and Genomics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 275–278. ISBN 978-0-12-813156-5. [Google Scholar]
- Fransquet, P.D.; Wrigglesworth, J.; Woods, R.L.; Ernst, M.E.; Ryan, J. The Epigenetic Clock as a Predictor of Disease and Mortality Risk: A Systematic Review and Meta-Analysis. Clin. Epigenet. 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Robertson, N.A.; Wang, T.; Havas, A.; Ideker, T.; Adams, P.D. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol. Cell 2018, 71, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kabacik, S.; Lowe, D.; Fransen, L.; Leonard, M.; Ang, S.-L.; Whiteman, C.; Corsi, S.; Cohen, H.; Felton, S.; Bali, R.; et al. The Relationship between Epigenetic Age and the Hallmarks of Aging in Human Cells. Nat. Aging 2022, 2, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Zbieć-Piekarska, R.; Spólnicka, M.; Kupiec, T.; Makowska, Ż.; Spas, A.; Parys-Proszek, A.; Kucharczyk, K.; Płoski, R.; Branicki, W. Examination of DNA Methylation Status of the ELOVL2 Marker May Be Useful for Human Age Prediction in Forensic Science. Forensic Sci. Int. Genet. 2015, 14, 161–167. [Google Scholar] [CrossRef]
- Zbieć-Piekarska, R.; Spólnicka, M.; Kupiec, T.; Parys-Proszek, A.; Makowska, Ż.; Pałeczka, A.; Kucharczyk, K.; Płoski, R.; Branicki, W. Development of a Forensically Useful Age Prediction Method Based on DNA Methylation Analysis. Forensic Sci. Int. Genet. 2015, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Wang, L.; Gao, Y.; Feng, G.; Li, G.; Zou, J.; Yu, M.; Li, Y.F.; Liu, C.; et al. Lipid Metabolism Dysfunction Induced by Age-Dependent DNA Methylation Accelerates Aging. Sig. Transduct. Target. Ther. 2022, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, C.; Chorin, F.; Beltran, E.E.M.; Neuzillet, C.; Cardot-Ruffino, V. Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives. Cancers 2022, 14, 5402. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cañamero, S.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M.; Pozuelo-Carrascosa, D.P.; Santacruz-Salas, E.; Rabanales-Sotos, J.A.; Cuesta-Mateos, T.; Laredo-Aguilera, J.A. Impact of Physical Exercise in Advanced-stage Cancer Patients: Systematic Review and Meta-analysis. Cancer Med. 2022, 11, 3714–3727. [Google Scholar] [CrossRef]
- Schmidt, A.; Cross, G.; Pitoia, F. Metástasis a distancia en cáncer diferenciado de tiroides: Diagnóstico y tratamiento. Rev. Argent. Endocrinol. Metab. 2017, 54, 92–100. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Giobbie-Hurder, A.; Shockro, L.; Campbell, N.; Partridge, A.H.; Tolaney, S.M.; Lin, N.U.; Winer, E.P. Randomized Trial of a Physical Activity Intervention in Women with Metastatic Breast Cancer. Cancer 2016, 122, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Álvaro Sanz, E.; Abilés, J.; Garrido Siles, M.; Pérez Ruíz, E.; Alcaide García, J.; Rueda Domínguez, A. Impact of Weight Loss on Cancer Patients’ Quality of Life at the Beginning of the Chemotherapy. Support. Care Cancer 2021, 29, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Vangelov, B.; Venchiarutti, R.L.; Smee, R.I. Critical Weight Loss in Patients With Oropharynx Cancer During Radiotherapy (± Chemotherapy). Nutr. Cancer 2017, 69, 1211–1218. [Google Scholar] [CrossRef]
- Albrecht, T.A.; Taylor, A.G. Physical Activity in Patients with Advanced-Stage Cancer: A Systematic Review of the Literature. Clin. J. Oncol. Nurs. 2012, 16, 293–300. [Google Scholar] [CrossRef]
- Sellami, M.; Bragazzi, N.; Prince, M.S.; Denham, J.; Elrayess, M. Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes Over a Lifetime. Front. Genet. 2021, 12, 652497. [Google Scholar] [CrossRef] [PubMed]
- Światowy, W.J.; Drzewiecka, H.; Kliber, M.; Sąsiadek, M.; Karpiński, P.; Pławski, A.; Jagodziński, P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021, 22, 12989. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Cánovas-Cervera, I.; Pallardó, F.V. Oxidative Stress and Metabolism Meet Epigenetic Modulation in Physical Exercise. Free. Radic. Biol. Med. 2024, 213, 123–137. [Google Scholar] [CrossRef]
- Voisin, S.; Eynon, N.; Yan, X.; Bishop, D.J. Exercise Training and DNA Methylation in Humans. Acta Physiol. 2015, 213, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.; Murri, A.; Benotti, G.; Fantini, C.; Duranti, G.; Ceci, R.; Grazioli, E.; Cerulli, C.; Sgrò, P.; Rossi, C.; et al. The Impact of Physical Activity on Promoter-Specific Methylation of Genes Involved in the Redox-Status and Disease Progression: A Longitudinal Study on Post-Surgery Female Breast Cancer Patients Undergoing Medical Treatment. Redox Biol. 2024, 70, 103033. [Google Scholar] [CrossRef] [PubMed]
- Fox, F.A.U.; Liu, D.; Breteler, M.M.B.; Aziz, N.A. Physical Activity Is Associated with Slower Epigenetic Ageing—Findings from the Rhineland Study. Aging Cell 2023, 22, e13828. [Google Scholar] [CrossRef] [PubMed]
- Jokai, M.; Torma, F.; McGreevy, K.M.; Koltai, E.; Bori, Z.; Babszki, G.; Bakonyi, P.; Gombos, Z.; Gyorgy, B.; Aczel, D.; et al. DNA Methylation Clock DNAmFitAge Shows Regular Exercise Is Associated with Slower Aging and Systemic Adaptation. GeroScience 2023, 45, 2805–2817. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Radak, Z.; Tabata, H.; Akiyama, H.; Nakamura, N.; Kawakami, R.; Ito, T.; Usui, C.; Jokai, M.; Torma, F.; et al. Associations between Cardiorespiratory Fitness and Lifestyle-related Factors with DNA Methylation-based Ageing Clocks in Older Men: WASEDA’S Health Study. Aging Cell 2024, 23, e13960. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Buelga, C.; Sanchez-Barroso, L.; Gallardo, M.; Apellániz-Ruiz, M.; Inglada-Pérez, L.; Yanowski, K.; Carrillo, J.; Garcia-Estevez, L.; Calvo, I.; Perona, R.; et al. Impact of Chemotherapy on Telomere Length in Sporadic and Familial Breast Cancer Patients. Breast Cancer Res. Treat. 2015, 149, 385–394. [Google Scholar] [CrossRef]
- Pearce, E.E.; Alsaggaf, R.; Katta, S.; Dagnall, C.; Aubert, G.; Hicks, B.D.; Spellman, S.R.; Savage, S.A.; Horvath, S.; Gadalla, S.M. Telomere Length and Epigenetic Clocks as Markers of Cellular Aging: A Comparative Study. GeroScience 2022, 44, 1861–1869. [Google Scholar] [CrossRef]
- Gallicchio, L.; Gadalla, S.M.; Murphy, J.D.; Simonds, N.I. The Effect of Cancer Treatments on Telomere Length: A Systematic Review of the Literature. J. Natl. Cancer Inst. 2018, 110, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, I.M.; Mirabello, L.; Pfeiffer, R.M.; Savage, S.A. The Association of Telomere Length and Cancer: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A Systematic Review of Leukocyte Telomere Length and Age in Adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Chen, B.H.; Hernandez, D.G.; Singleton, A.B.; Ferrucci, L.; Bandinelli, S.; Salfati, E.; Manson, J.E.; Quach, A.; et al. Menopause Accelerates Biological Aging. Proc. Natl. Acad. Sci. USA 2016, 113, 9327–9332. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.; Grazioli, E.; Antinozzi, C.; Fantini, C.; Cerulli, C.; Murri, A.; Duranti, G.; Ceci, R.; Vulpiani, M.C.; Pellegrini, P.; et al. Online Home-Based Physical Activity Counteracts Changes of Redox-Status Biomarkers and Fitness Profiles during Treatment Programs in Postsurgery Female Breast Cancer Patients. Antioxidants 2023, 12, 1138. [Google Scholar] [CrossRef]
- Joglekar, M.V.; Satoor, S.N.; Wong, W.K.M.; Cheng, F.; Ma, R.C.W.; Hardikar, A.A. An Optimised Step-by-Step Protocol for Measuring Relative Telomere Length. Methods Protoc. 2020, 3, 27. [Google Scholar] [CrossRef]

| CG (n = 9) | EG (n = 14) | p-Value | |
|---|---|---|---|
| Chronological age (years) | 53.00 ± 6.65 | 50.21 ± 5.47 | 0.2852 |
| Weight (kg) | 61.13 ± 11.77 | 62.82 ± 6.39 | 0.6592 |
| Height (cm) | 163.2 ± 7.17 | 162.4 ± 7.45 | 0.8028 |
| BMI (kg/m2) | 22.76 ± 2.30 | 27.16 ± 6.11 | 0.0747 |
| Type of surgery | |||
| Quadrantectomy | 5 | 8 | N.A. |
| Mastectomy | 4 | 5 | N.A. |
| Medical treatments | |||
| Chemo + hormonal + radio | 2 | 3 | N.A. |
| Hormonal + radio | 4 | 7 | N.A. |
| Hormonal | 3 | 4 | N.A. |
| Physical activity level | |||
| IPAQ (MET-min/week) | 834.6 ± 596.0 | 854.3 ± 587.5 | 0.9389 |
| PRE | POST | p-Value | Overall Change | ||
|---|---|---|---|---|---|
| HGr | CG: EG: | 29.30 ± 4.043 28.67 ± 3.584 | 28.91 ± 5.400 28.07 ± 3.984 | 0.1394 0.4983 | 0.8904 ± 0.2954 ▲ |
| HGl | CG: EG: | 24.12 ± 2.204 26.43 ± 3.995 | 20.80 ± 0.4967 25.43 ± 3.939 | 0.0019 0.3230 | 0.8563 ± 0.2939 ▲ |
| STS | CG: EG: | 17.44 ± 6.521 18.00 ± 3.843 | 18.43 ± 5.127 21.29 ± 4.906 | 0.3559 0.0413 | 1.032 ± 0.4021 |
| STR | CG: EG: | 2.278 ± 12.32 −0.5714 ± 9.277 | 2.571 ± 14.02 4.929 ± 8.265 | 0.3078 0.0035 | 3.952 ± 5.298 ■ |
| ScTr | CG: EG: | 25.22 ± 8.657 27.23 ± 9.418 | 22.00 ± 7.483 20.92 ± 7.609 | 0.33559 0.0020 | −5 ± 5.944 ■ |
| ScTl | CG: EG: | 29.44 ± 11.95 27.46 ± 5.897 | 28.86 ± 12.85 22.77 ± 5.988 | 0.3559 0.0002 | −2.947 ± 4.54 ■ |
| 6MWT | CG: EG: | 533.2 ± 40.07 597.1 ± 67.05 | 486.4 ± 59.00 628.6 ± 63.20 | 0.1250 0.0025 | 1.017 ± 0.09696 ▲ |
| Borg scale | CG: EG: | 1.556 ± 0.7265 2.67 ± 0.4875 | 1.857 ± 0.8997 1.571 ± 1.072 | 0.5000 0.0127 | −0.5 ± 1.36 ■ |
| PRE (Mean ± SD) | POST (Mean ± SD) | % Change | p-Value | |
|---|---|---|---|---|
| EORTC QLQ C-30 | ||||
| Physical function CG EG | 90.01 ± 2.35 88.81 ± 7.04 | 91.21 ± 3.99 93.99 ± 4.01 | +1.33 +5.83 | 0.323 0.040 |
| Emotional function CG EG | 88.23 ± 7.62 73.15 ± 11.52 | 87.61 ± 9.92 84.01 ± 21.11 | −0.69 +14.84 | 0.498 0.342 |
| Cognitive function CG EG | 89.57 ± 12.01 85.08 ± 17.29 | 87.10 ± 10.30 87.41 ± 15.53 | −2.76 +2.73 | 0.397 0.881 |
| Social function CG EG | 87.12 ± 13.95 82.78 ± 20.34 | 84.09 ± 13.99 92.09 ± 15.13 | −2.55 +11.24 | 0.488 0.269 |
| Global health CG EG | 73.88 ± 10.90 64.66 ± 10.42 | 66.34 ± 21.92 71.44 ± 14.09 | −10.21 +10.48 | 0.385 0.350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulton, C.; Grazioli, E.; Ibáñez-Cabellos, J.S.; Murri, A.; Cerulli, C.; Silvestri, M.; Caporossi, D.; Pallardó, F.V.; García-Giménez, J.L.; Magno, S.; et al. Physical Activity and Epigenetic Aging in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 8596. https://doi.org/10.3390/ijms25168596
Moulton C, Grazioli E, Ibáñez-Cabellos JS, Murri A, Cerulli C, Silvestri M, Caporossi D, Pallardó FV, García-Giménez JL, Magno S, et al. Physical Activity and Epigenetic Aging in Breast Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(16):8596. https://doi.org/10.3390/ijms25168596
Chicago/Turabian StyleMoulton, Chantalle, Elisa Grazioli, José Santiago Ibáñez-Cabellos, Arianna Murri, Claudia Cerulli, Monica Silvestri, Daniela Caporossi, Federico V. Pallardó, José Luis García-Giménez, Stefano Magno, and et al. 2024. "Physical Activity and Epigenetic Aging in Breast Cancer Treatment" International Journal of Molecular Sciences 25, no. 16: 8596. https://doi.org/10.3390/ijms25168596









