Focal Adhesion Maturation Responsible for Behavioral Changes in Human Corneal Stromal Fibroblasts on Fibrillar Substrates
Abstract
:1. Introduction
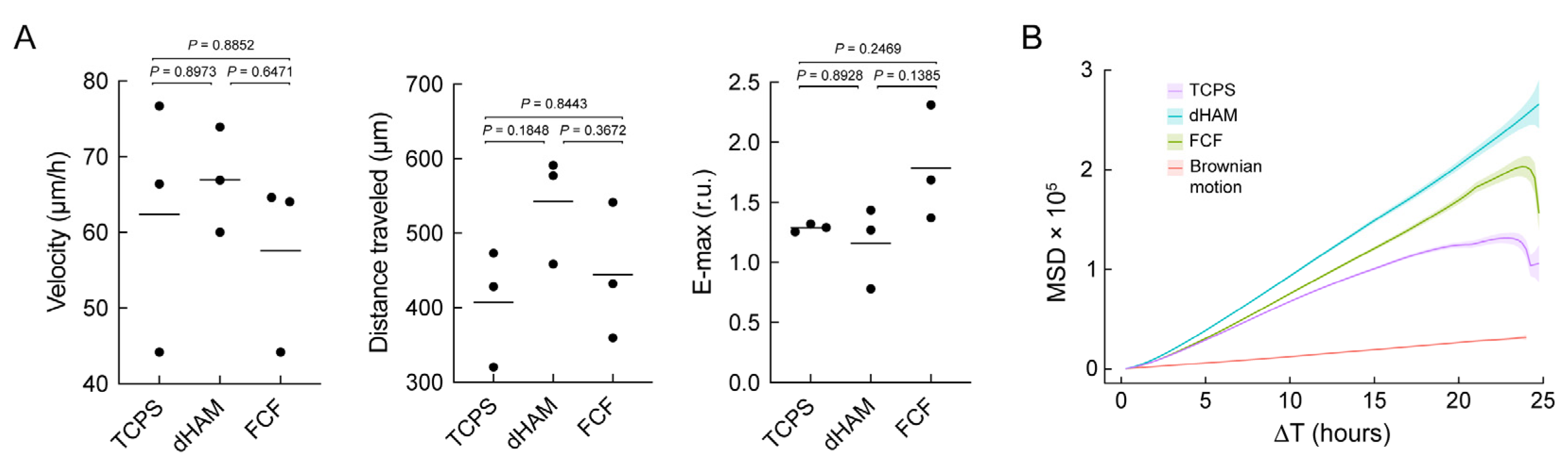
2. Results
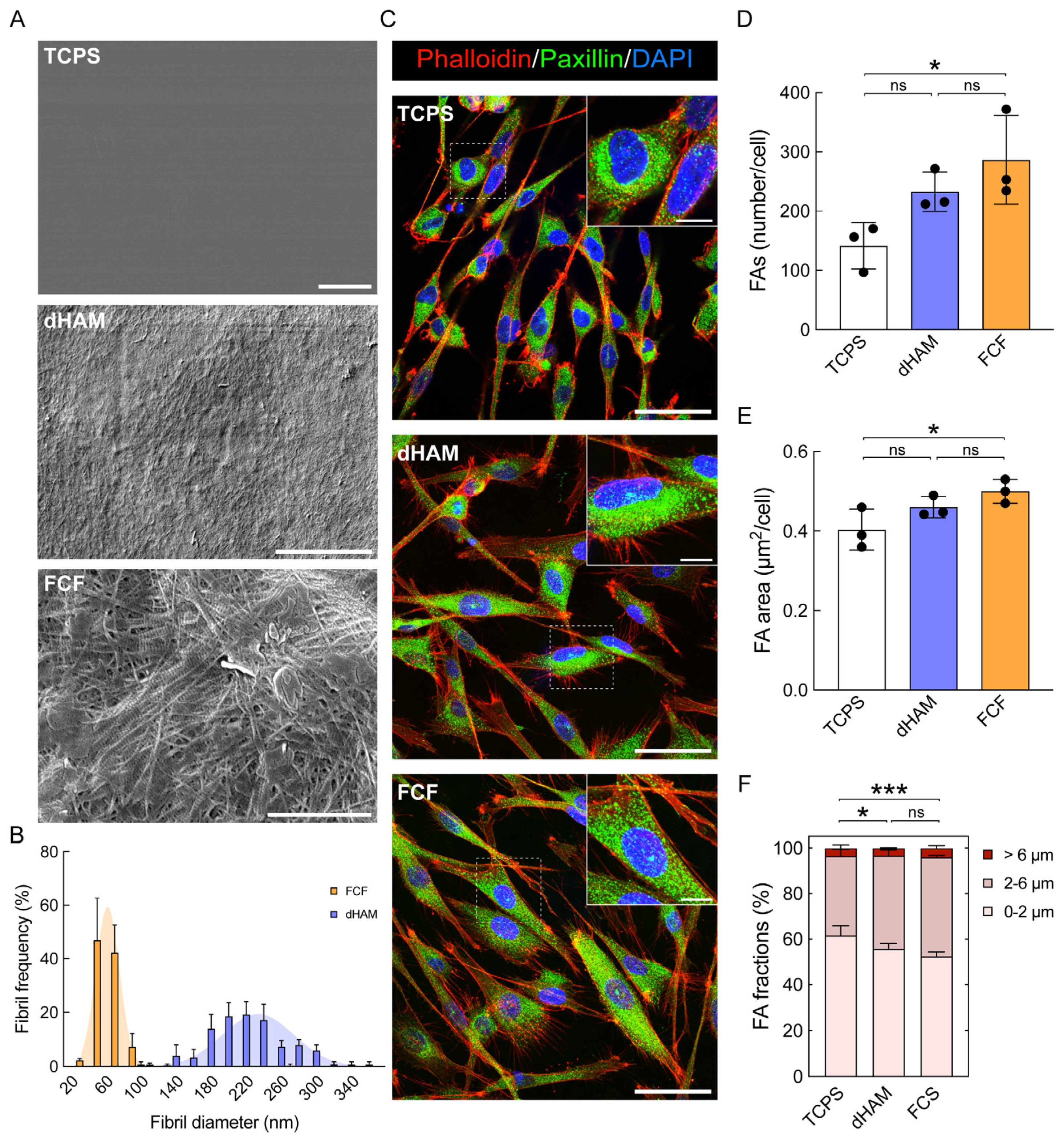
2.1. Focal Adhesion Maturation Is Enhanced in Cultured Human CSFs on FCF
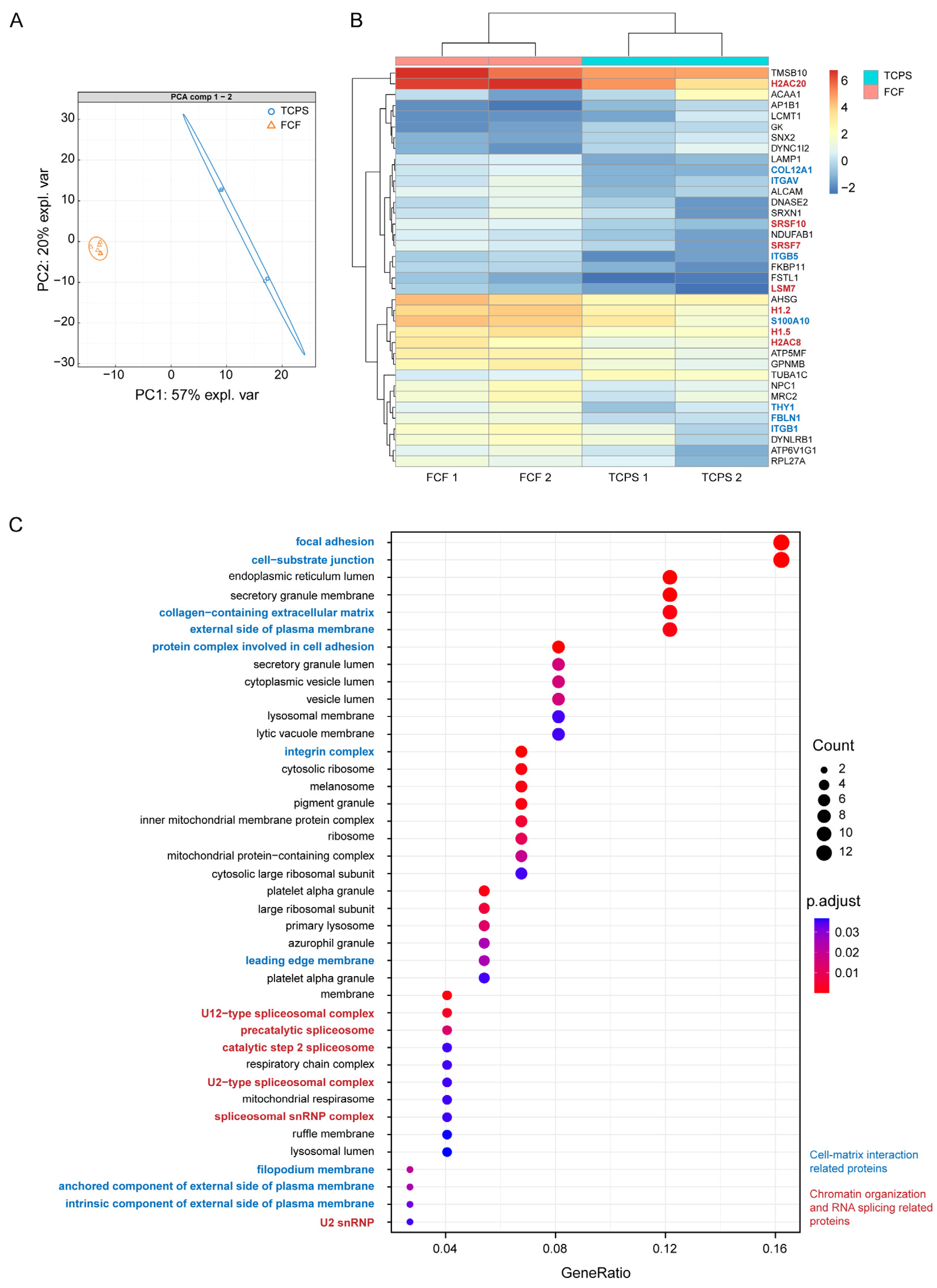
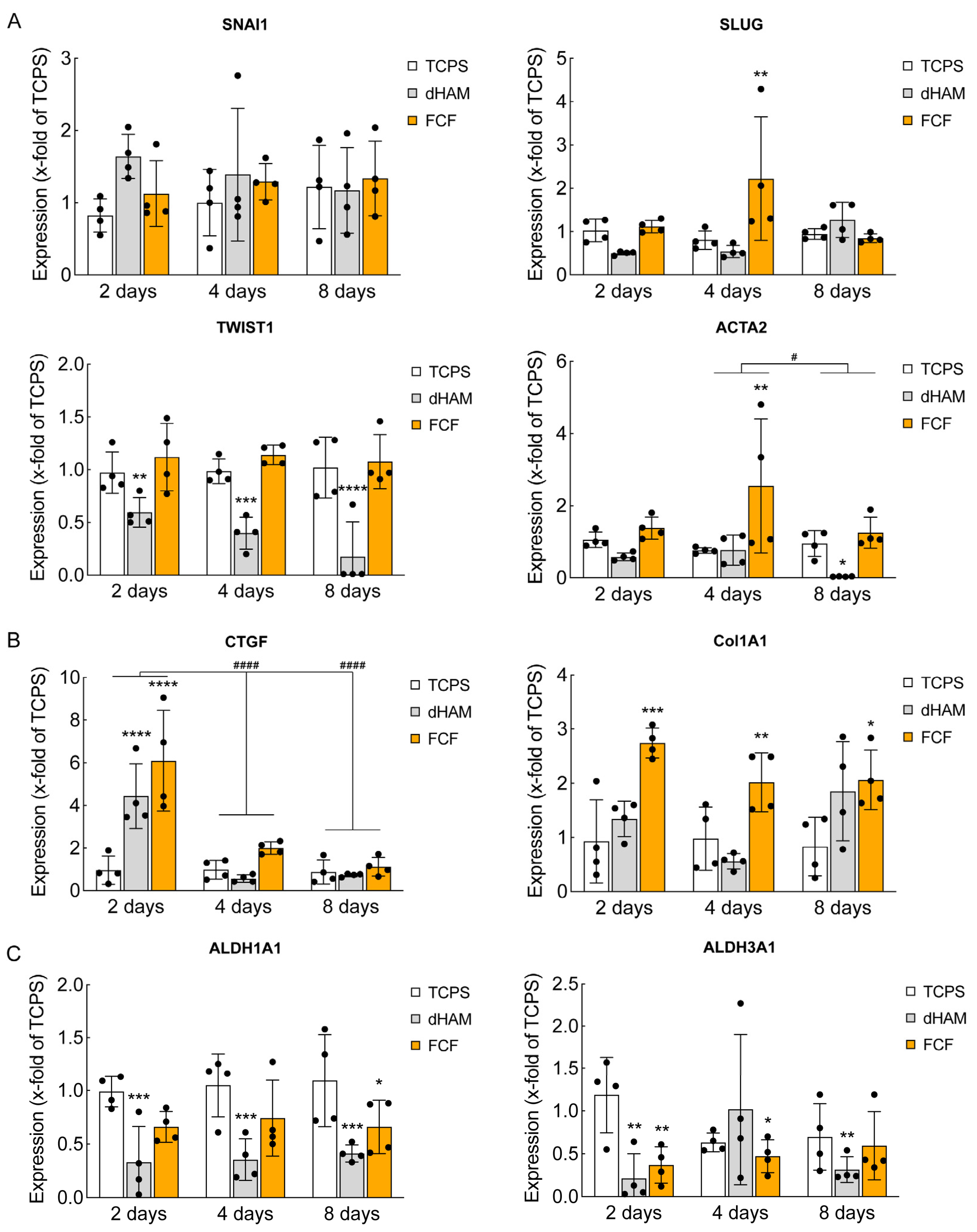
2.2. FCF Induces Changes in Cell Matrix Interaction-Related Protein Expression and Early Mechanotransduction in Cultured Human CSFs
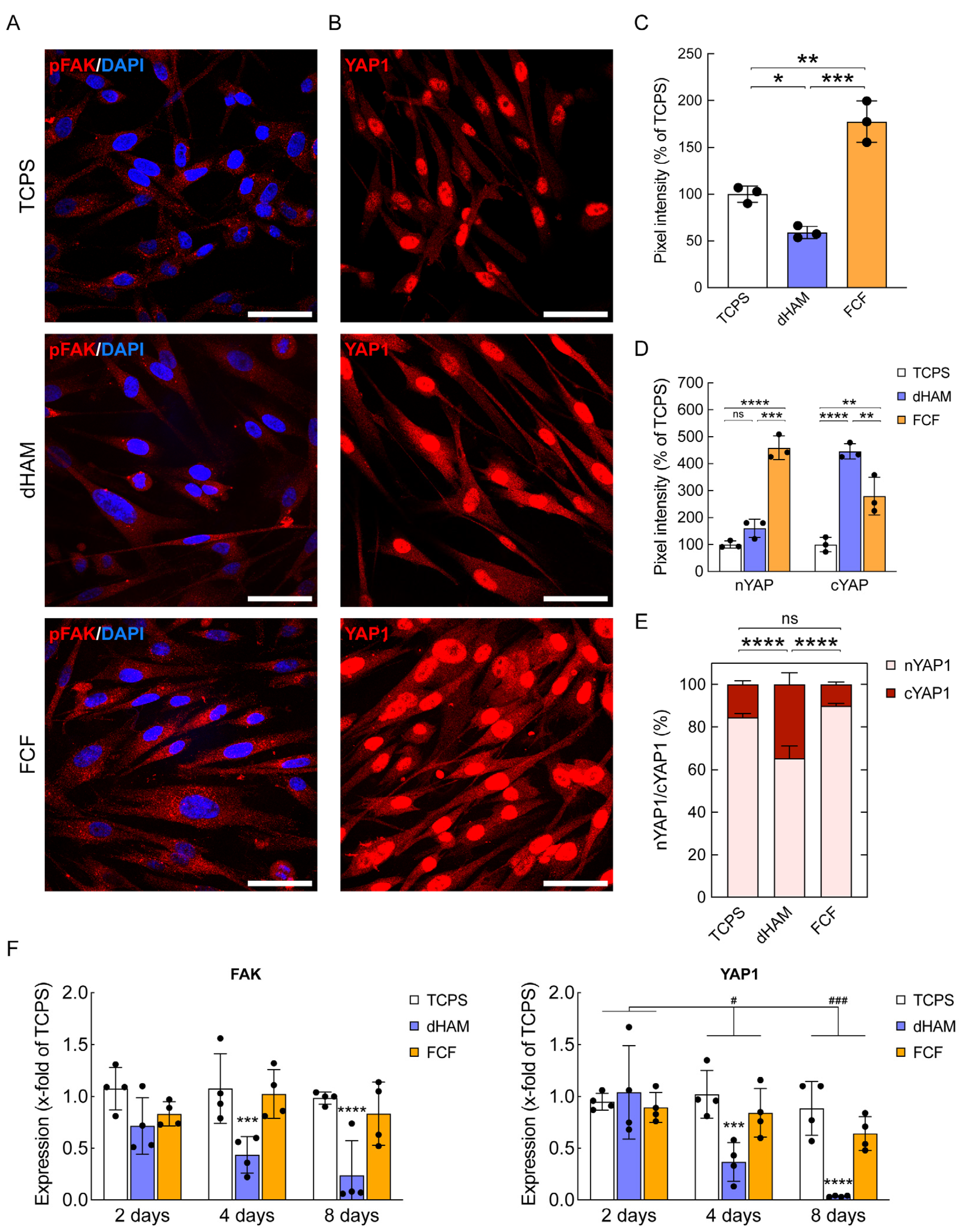
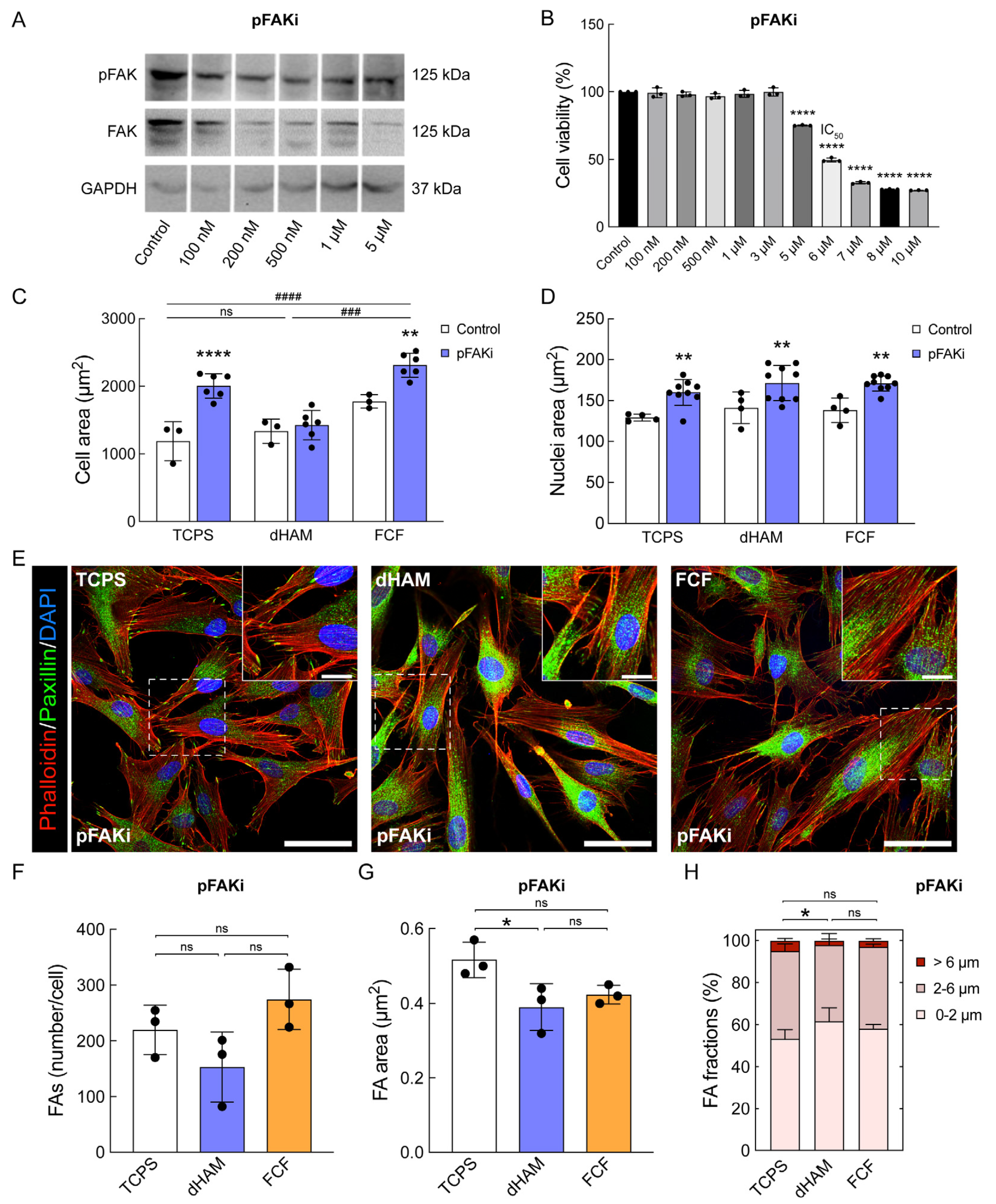
2.3. pFAK Inhibition Overrules Early Mechanotransduction in Human CSFs Cultured on FCF
3. Discussion
4. Materials and Methods
4.1. Human Tissue Sourcing
4.2. Isolation and Culture of Human Corneal Stromal Fibroblasts
4.3. Flow Cytometry
4.4. Human Amniotic Membrane Preparation
4.5. Fibrillar Collagen Film Fabrication
4.6. Scanning Electron Microscopy
4.7. Immunostaining
4.8. Quantitative Immunofluorescence Analysis
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Cell Motility Analysis
4.11. Proteomic Profiling
4.12. Y397-FAK Autophosphorylation Inhibition
4.13. Western Blot Analysis
4.14. Cell Viability Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; You, J.; Liu, X.; Cooper, S.; Hodge, C.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomaterials for Corneal Bioengineering. Biomed. Mater. 2018, 13, 032002. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Chai, D.; Kriling, S.; Nien, C.J.; Brown, D.J.; Jester, B.; Juhasz, T.; Jester, J.V. Nonlinear Optical Macroscopic Assessment of 3-D Corneal Collagen Organization and Axial Biomechanics. Investig. Opthalmol. Vis. Sci. 2011, 52, 8818. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Espana, E.M.; Birk, D.E. Composition, Structure and Function of the Corneal Stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Budge, A.; Fisher, S.; Huang, J. Corneal Keratocytes: Phenotypic and Species Differences in Abundant Protein Expression and In Vitro Light-Scattering. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2369. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Liu, T.; Jia, C.; Qi, X.; Wang, Y. Physiological Expression of Lens α-, β-, and γ-Crystallins in Murine and Human Corneas. Mol. Vis. 2010, 16, 2745–2752. [Google Scholar] [PubMed]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast–Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing Nanotopography and Integrin–Matrix Interactions to Influence Stem Cell Fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial Contact Guidance on Well-Defined Micro- and Nanostructured Substrates. J. Cell Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Karamichos, D.; Funderburgh, M.L.; Hutcheon, A.E.K.; Zieske, J.D.; Du, Y.; Wu, J.; Funderburgh, J.L. A Role for Topographic Cues in the Organization of Collagenous Matrix by Corneal Fibroblasts and Stem Cells. PLoS ONE 2014, 9, e86260. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Martín, E.; Gallego-Muñoz, P.; Mar, S.; Fernández, I.; Cidad, P.; Martínez-García, M.C. Dynamic Changes of the Extracellular Matrix during Corneal Wound Healing. Exp. Eye Res. 2019, 186, 107704. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Gao, H.; Qin, L.; Jia, Y.; Gao, M.; Ren, L. Microgrooved Collagen-Based Corneal Scaffold for Promoting Collective Cell Migration and Antifibrosis. RSC Adv. 2019, 9, 29463–29473. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Lawrence, B.D.; Gao, X.R.; Guaiquil, V.H.; Liu, A.; Rosenblatt, M.I. The Effect of Micro- and Nanoscale Surface Topographies on Silk on Human Corneal Limbal Epithelial Cell Differentiation. Sci. Rep. 2019, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The Engineering of Organized Human Corneal Tissue through the Spatial Guidance of Corneal Stromal Stem Cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A Review on Tissue Engineering, Application, and Storage. J. Biomed. Mater. Res. 2021, 109, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Deng, S.X. The Application of Human Amniotic Membrane in the Surgical Management of Limbal Stem Cell Deficiency. Ocul. Surf. 2019, 17, 221–229. [Google Scholar] [CrossRef]
- Last, J.A.; Thomasy, S.M.; Croasdale, C.R.; Russell, P.; Murphy, C.J. Compliance Profile of the Human Cornea as Measured by Atomic Force Microscopy. Micron 2012, 43, 1293–1298. [Google Scholar] [CrossRef]
- Pei, Y.; Reins, R.Y.; McDermott, A.M. Aldehyde Dehydrogenase (ALDH) 3A1 Expression by the Human Keratocyte and Its Repair Phenotypes. Exp. Eye Res. 2006, 83, 1063–1073. [Google Scholar] [CrossRef]
- Yan, F.; Qian, M.; He, Q.; Zhu, H.; Yang, B. The Posttranslational Modifications of Hippo-YAP Pathway in Cancer. Biochim Biophys. Acta-Gen. Subj. 2020, 1864, 129397. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Ingber, D.E. Mechanical Control of Tissue and Organ Development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef]
- Blackburn, B.J.; Jenkins, M.W.; Rollins, A.M.; Dupps, W.J. A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking. Front. Bioeng. Biotechnol. 2019, 7, 66. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of Corneal Substrate Biomechanics and Its Effect on Epithelial Stem Cell Maintenance and Differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Xiong, S.; Gao, H.; Qin, L.; Jia, Y.-G.; Ren, L. Engineering Topography: Effects on Corneal Cell Behavior and Integration into Corneal Tissue Engineering. Bioact. Mater. 2019, 4, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K.F. Nanotopography Modulates Mechanotransduction of Stem Cells and Induces Differentiation through Focal Adhesion Kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Robinson, B.; Rice, A.; Rombouts, K.; Del Río Hernández, A.E. FAK Controls the Mechanical Activation of YAP, a Transcriptional Regulator Required for Durotaxis. FASEB J. 2018, 32, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Zarka, M.; Etienne, F.; Bourmaud, M.; Szondi, D.; Schwartz, J.-M.; Kampmann, K.; Helary, C.; Rannou, F.; Haÿ, E.; Cohen-Solal, M. Mechanical Loading Activates the YAP/TAZ Pathway and Chemokine Expression in the MLO-Y4 Osteocyte-like Cell Line. Lab. Investig. 2021, 101, 1597–1604. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Backman, L.J.; Malm, A.D.; Danielson, P. Surface Topography and Mechanical Strain Promote Keratocyte Phenotype and Extracellular Matrix Formation in a Biomimetic 3D Corneal Model. Adv. Healthc. Mater. 2017, 6, 1601238. [Google Scholar] [CrossRef]
- Pot, S.A.; Liliensiek, S.J.; Myrna, K.E.; Bentley, E.; Jester, J.V.; Nealey, P.F.; Murphy, C.J. Nanoscale Topography–Induced Modulation of Fundamental Cell Behaviors of Rabbit Corneal Keratocytes, Fibroblasts, and Myofibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple Roles of α-Smooth Muscle Actin in Mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Fu, X.; Ji, Y.; Li, M.; Shi, X.; Wang, Y. FAK- and YAP/TAZ Dependent Mechanotransduction Pathways Are Required for Enhanced Immunomodulatory Properties of Adipose-Derived Mesenchymal Stem Cells Induced by Aligned Fibrous Scaffolds. Biomaterials 2018, 171, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Nemec, S.; Kilian, K.A. Materials Control of the Epigenetics Underlying Cell Plasticity. Nat. Rev. Mater. 2020, 6, 69–83. [Google Scholar] [CrossRef]
- Murugan, N.J.; Cariba, S.; Abeygunawardena, S.; Rouleau, N.; Payne, S.L. Biophysical Control of Plasticity and Patterning in Regeneration and Cancer. Cell. Mol. Life Sci. 2024, 81, 9. [Google Scholar] [CrossRef] [PubMed]
- Dubovikov, A.; Gavrilyik, I.; Kulikov, A.; Churashov, S.; Chernysh, V.; Bezushko, A.; Aleksandrova, O.; Blinova, M. Method of Immobilization of Native Amniotic Membrane for Transportation, Preservation and Its Application as a Carrier of Cultured Cells. 2019, pp. 1–6. Available online: https://new.fips.ru/ofpstorage/Doc/IZPM/RUNWC1/000/000/002/680/471/ИЗ-02680471-00001/DOCUMENT.PDF (accessed on 29 June 2024).
- Zhurenkov, K.E.; Alexander-Sinkler, E.I.; Gavrilyik, I.O.; Yartseva, N.M.; Aleksandrova, S.A.; Mashel, T.V.; Khorolskaya, J.I.; Blinova, M.I.; Kulikov, A.N.; Churashov, S.V.; et al. Labial Mucosa Stem Cells: Isolation, Characterization, and Their Potential for Corneal Epithelial Reconstruction. Investig. Ophthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef] [PubMed]
- Chandrakasan, G.; Torchia, D.A.; Piez, K.A. Preparation of Intact Monomeric Collagen from Rat Tail Tendon and Skin and the Structure of the Nonhelical Ends in Solution. J. Biol. Chem. 1976, 251, 6062–6067. [Google Scholar] [CrossRef]
- Darvish, D.M. Collagen Fibril Formation in Vitro: From Origin to Opportunities. Mater. Today Bio. 2022, 15, 100322. [Google Scholar] [CrossRef]
- Buskermolen, A.B.C.; Kurniawan, N.A.; Bouten, C.V.C. An Automated Quantitative Analysis of Cell, Nucleus and Focal Adhesion Morphology. PLoS ONE 2018, 13, e0195201. [Google Scholar] [CrossRef]
- Bobkov, D.; Polyanskaya, A.; Musorina, A.; Lomert, E.; Shabelnikov, S.; Poljanskaya, G. Replicative Senescence in MSCWJ-1 Human Umbilical Cord Mesenchymal Stem Cells Is Marked by Characteristic Changes in Motility, Cytoskeletal Organization, and RhoA Localization. Mol. Biol. Rep. 2020, 47, 3867–3883. [Google Scholar] [CrossRef] [PubMed]
- Bovet, P.; Benhamou, S. Spatial Analysis of Animals’ Movements Using a Correlated Random Walk Model. J. Theor. Biol. 1988, 131, 419–433. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Wang, S.; Li, W.; Hu, L.; Cheng, J.; Yang, H.; Liu, Y. NAguideR: Performing and Prioritizing Missing Value Imputations for Consistent Bottom-up Proteomic Analyses. Nucleic Acids Res. 2020, 48, e83. [Google Scholar] [CrossRef] [PubMed]
- Branden, K.V.; Verboven, S. Robust Data Imputation. Comput. Biol. Chem. 2009, 33, 7–13. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, Use R! 2nd ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Blighe, K.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. 2019. Available online: https://www.bioconductor.org/packages/release/bioc/vignettes/EnhancedVolcano/inst/doc/EnhancedVolcano.html (accessed on 29 June 2024).
- Kolde, R. Pheatmap: Pretty Heatmaps. 2012. Available online: https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (accessed on 29 June 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhurenkov, K.E.; Lobov, A.A.; Bildyug, N.B.; Alexander-Sinclair, E.I.; Darvish, D.M.; Lomert, E.V.; Kriger, D.V.; Zainullina, B.R.; Chabina, A.S.; Khorolskaya, J.I.; et al. Focal Adhesion Maturation Responsible for Behavioral Changes in Human Corneal Stromal Fibroblasts on Fibrillar Substrates. Int. J. Mol. Sci. 2024, 25, 8601. https://doi.org/10.3390/ijms25168601
Zhurenkov KE, Lobov AA, Bildyug NB, Alexander-Sinclair EI, Darvish DM, Lomert EV, Kriger DV, Zainullina BR, Chabina AS, Khorolskaya JI, et al. Focal Adhesion Maturation Responsible for Behavioral Changes in Human Corneal Stromal Fibroblasts on Fibrillar Substrates. International Journal of Molecular Sciences. 2024; 25(16):8601. https://doi.org/10.3390/ijms25168601
Chicago/Turabian StyleZhurenkov, Kirill E., Arseniy A. Lobov, Natalya B. Bildyug, Elga I. Alexander-Sinclair, Diana M. Darvish, Ekaterina V. Lomert, Daria V. Kriger, Bozhana R. Zainullina, Alina S. Chabina, Julia I. Khorolskaya, and et al. 2024. "Focal Adhesion Maturation Responsible for Behavioral Changes in Human Corneal Stromal Fibroblasts on Fibrillar Substrates" International Journal of Molecular Sciences 25, no. 16: 8601. https://doi.org/10.3390/ijms25168601





