The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China
Abstract
:1. Introduction
2. Results
2.1. Physical and Chemical Index Analysis
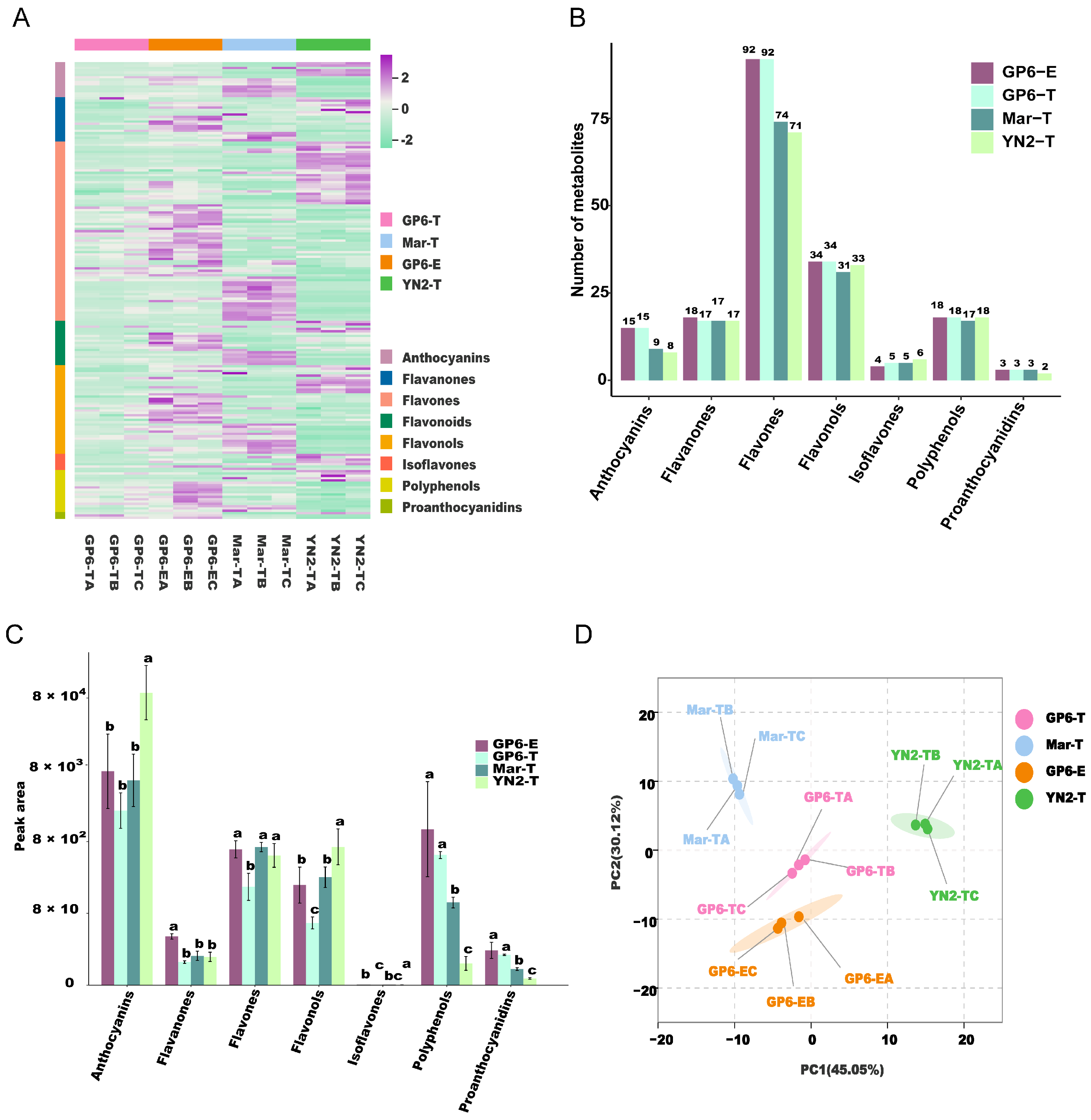
2.2. Overview of Metabolic Profiling
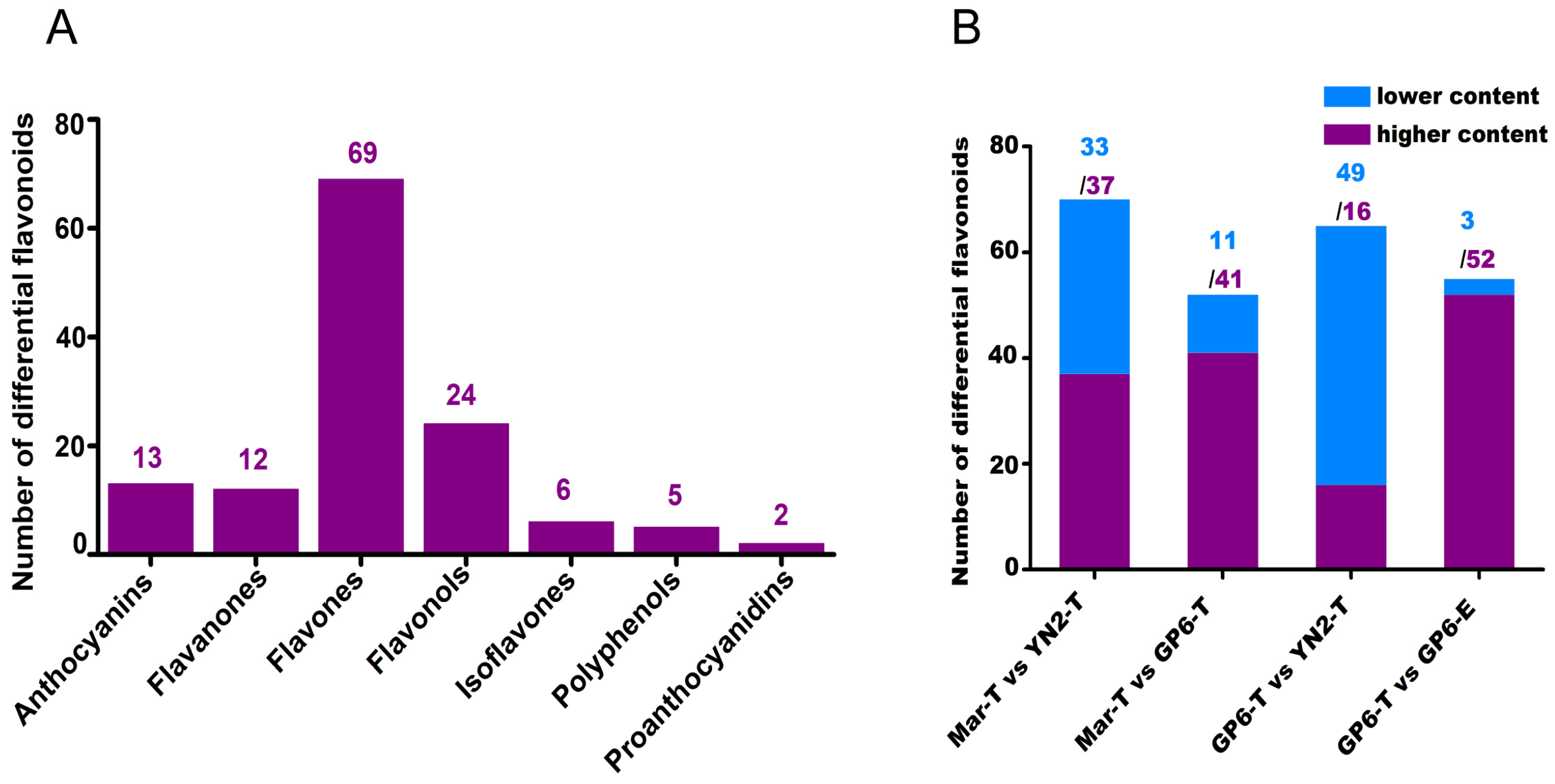
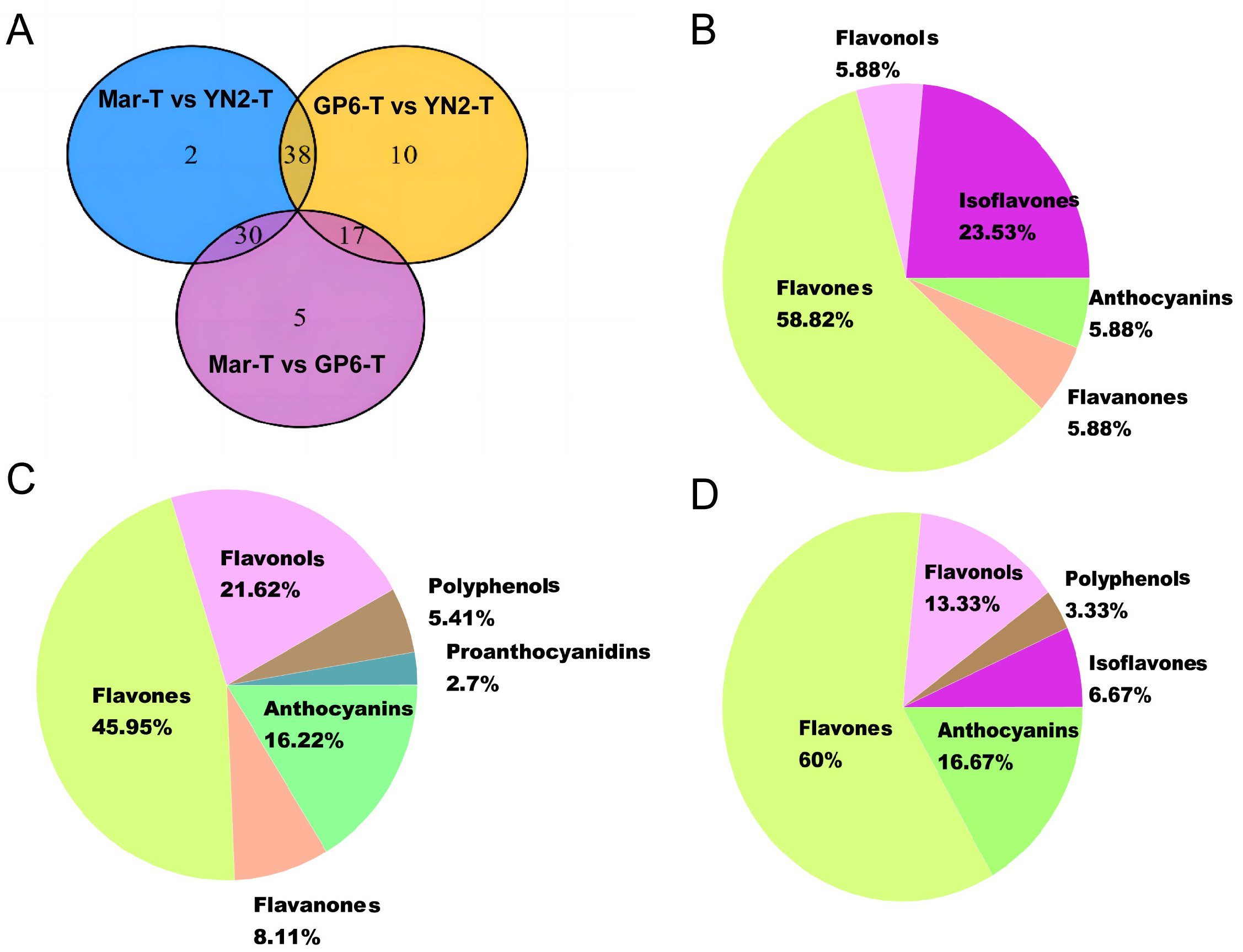
2.3. Identification of Different Flavonoid Metabolites
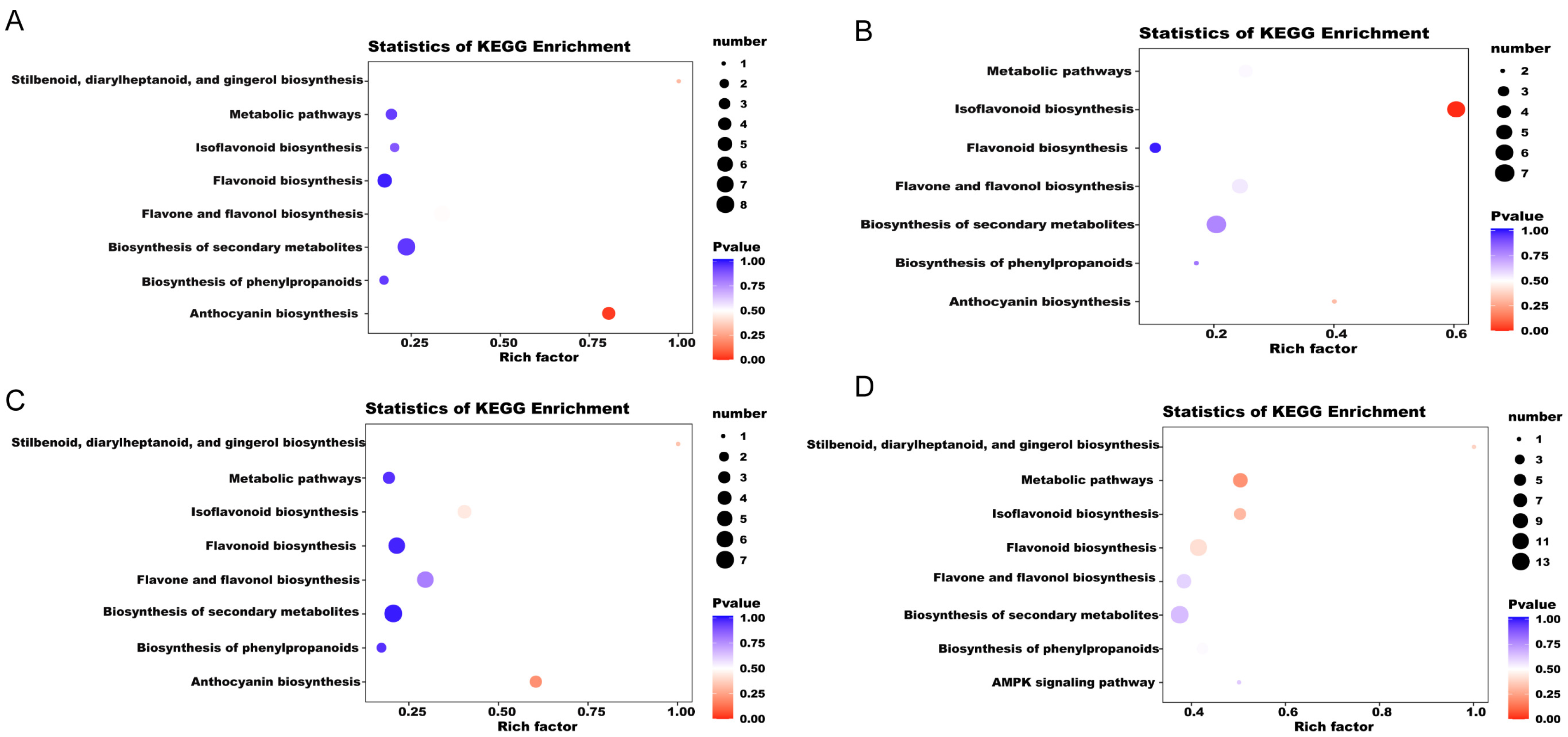
2.4. KEGG Annotation and Enrichment Analysis of Differential Metabolites
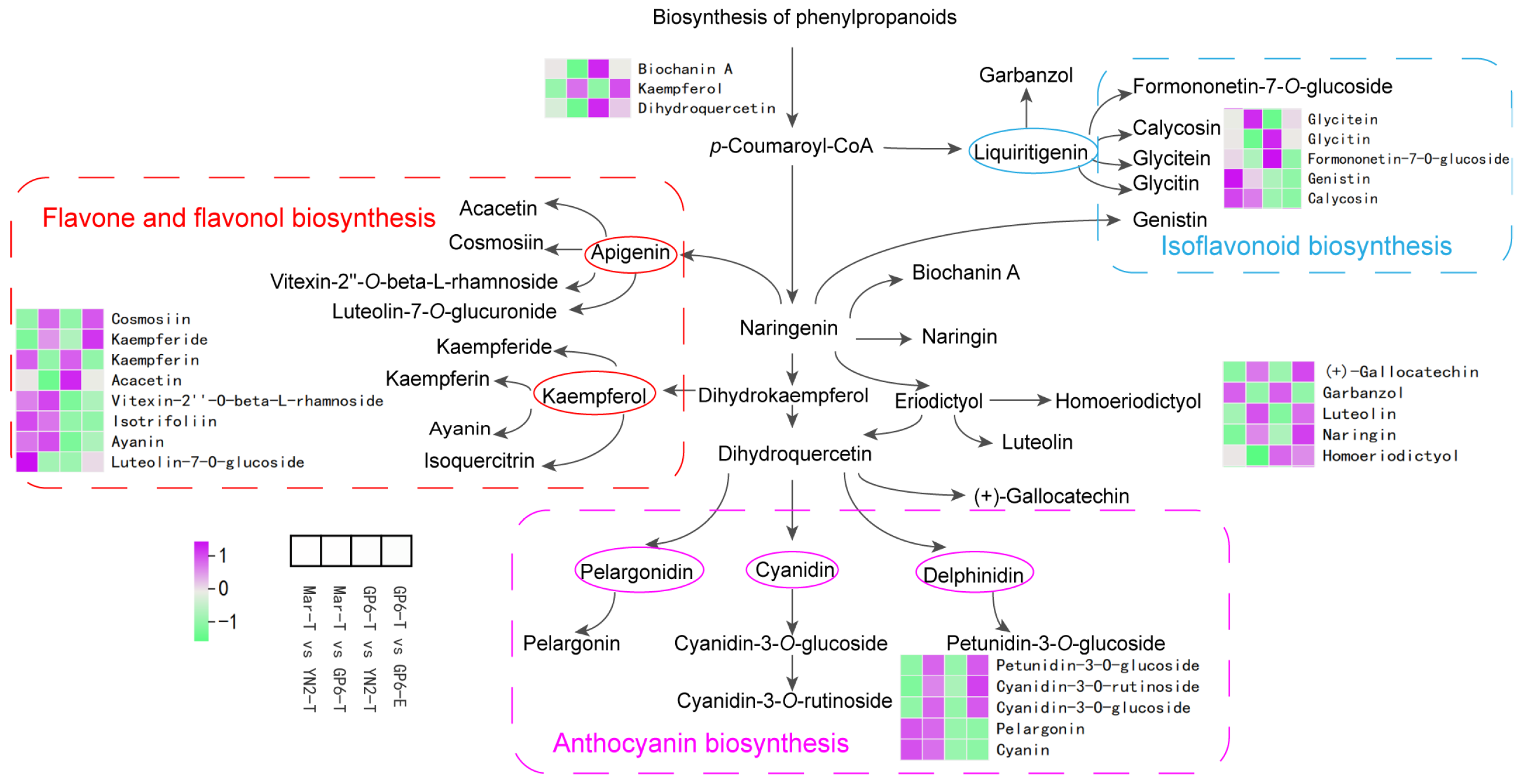
2.5. Differential Metabolites of Flavonoid Biosynthesis Pathways
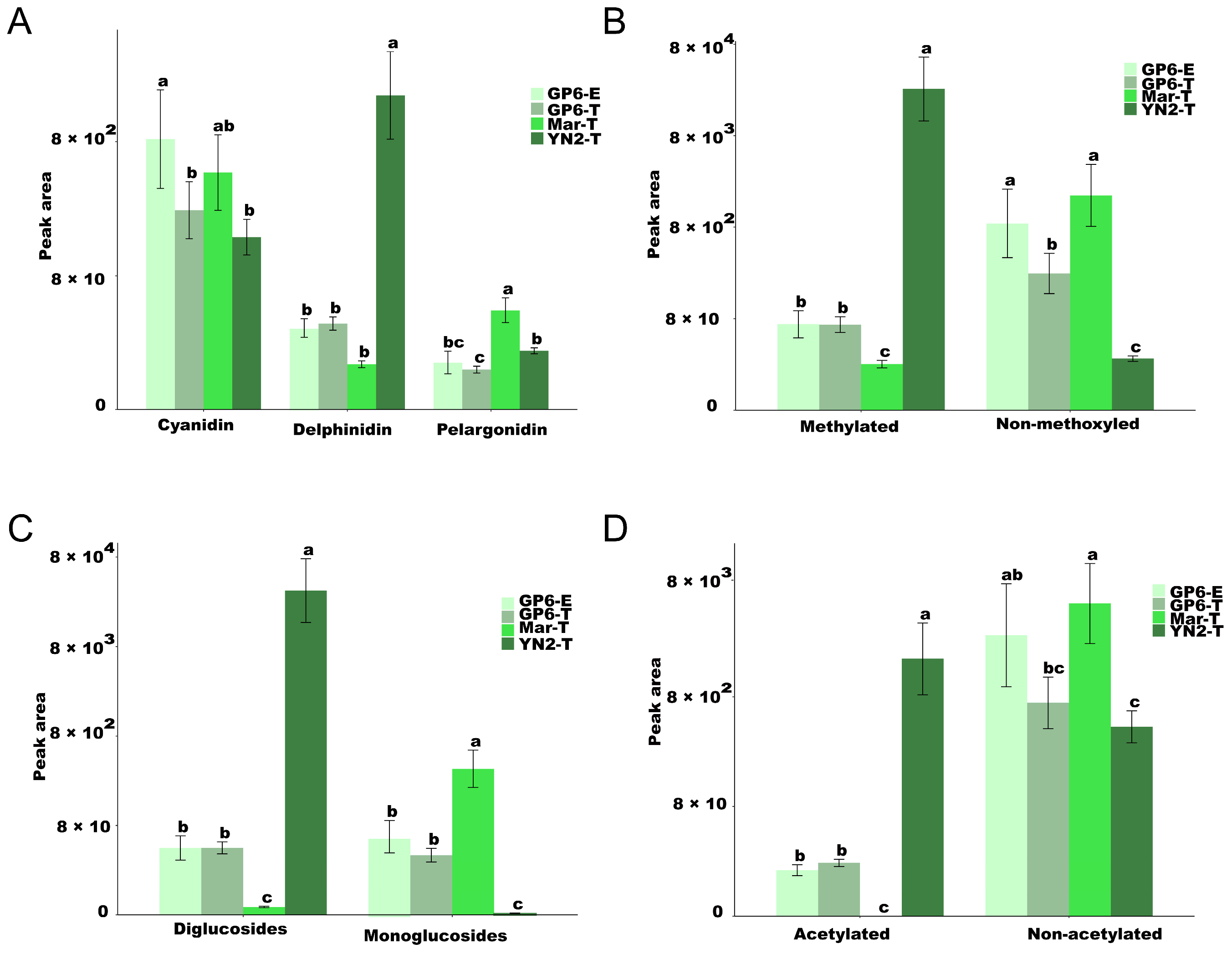
2.6. Differentially Modified Anthocyanins
3. Discussion
4. Materials and Methods
4.1. Experimental Vineyard Conditions
4.2. Berry Sampling and Physical Chemical Index Analysis
4.3. Sample Preparation and Metabolite Extraction
4.4. HPLC Conditions
4.5. ESI-Q TRAP-MS/MS
4.6. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
4.7. Differential Metabolite Analysis
4.8. Kyoto Encyclopedia of Genes and Genomes (KEGG) Annotation and Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atak, A.; Göksel, Z.; Yılmaz, Y. Changes in Major Phenolic Compounds of Seeds, Skins, and Pulps from Various Vitis spp. and the Effect of Powdery and Downy Mildew Diseases on Their Levels in Grape Leaves. Plants 2021, 10, 2554. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Gonçalves, M.I.V.Z.; Di Gaspero, G. Phenolics in Grape Berry and Key Antioxidants. In The Biochemistry of the Grape Berry; Geros, H., Chaves, M.M., Delrot, S., Eds.; Bentham Science Publishers: Sharja, United Arab Emirates, 2012; pp. 8–110. Available online: http://hdl.handle.net/10807/61282 (accessed on 6 February 2023).
- Marigliano, L.E.; Yu, R.; Torres, N.; Tanner, J.D.; Battany, M.; Kurtural, S.K. Photoselective Shade Films Mitigate Heat Wave Damage by Reducing Anthocyanin and Flavonol Degradation in Grapevine (Vitis vinifera L.) Berries. Front. Agron. 2022, 4, 898870. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; He, F.; Zhu, B.Q.; Liu, B.; Sun, R.Z.; Duan, C.Q.; Reeves, M.J.; Wang, J. Comparison of distinct transcriptional expression patterns of flavonoid biosynthesis in Cabernet Sauvignon grapes from east and west China. Plant Physiol. Biochem. 2014, 84, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Martínez-lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Sadeghnezhad, E.; Hakeem, A.; Zhong, R.; Wang, P.; Shangguan, L.; Fang, J. Integrated transcriptomic and metabolic analyses unveil anthocyanins biosynthesis metabolism in three different color cultivars of grape (Vitis vinifera L.). Sci. Hortic. 2022, 305, 111418. [Google Scholar] [CrossRef]
- Kőrösi, L.; Molnár, S.; Teszlák, P.; Dörnyei, Á.; Maul, E.; Töpfer, R.; Marosvölgyi, T.; Szabó, É.; Röckel, F. Comparative Study on Grape Berry Anthocyanins of Various Teinturier Varieties. Foods 2022, 11, 3668. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, S.; Bai, X.; Li, X.; Wang, Q.; Wang, L.; Wang, W.; Li, Z. Transcriptomic and Non-Targeted Metabolomic Analyses Reveal the Flavonoid Biosynthesis Pathway in Auricularia cornea. Molecules 2022, 27, 2334. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Z.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2019, 307, 125518. [Google Scholar] [CrossRef] [PubMed]
- Blancquart, E.H.; Oberholster, A.; Ricardo-da-silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Brillante, L.; Martínez-Lüscher, J.; Kurtural, S.K. Spatial variability of soil and plant water status and their cascading effects on grapevine physiology are linked to berry and wine chemistry. Front. Plant Sci. 2020, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous Melatonin and Abscisic Acid Expedite the Flavonoids Biosynthesis in Grape Berry of Vitis vinifera cv. Kyoho. Molecules 2020, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating Heat Wave and Exposure Damage to ‘Cabernet Sauvignon’ Wine Grape with Partial Shading under Two Irrigation Amounts. Front. Plant Sci. 2020, 11, 579192. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; Li, D.; Zhang, J.; Zhang, Y.; Zhan, Z.; Wang, Z. Evolution of Volatile Aroma Compounds and Amino Acids in Cabernet Gernischt Grape Berries(Vitis vinifera L.): Comparison of Different Training Systems for Mechanical Soil Burial. Foods 2022, 11, 1568. [Google Scholar] [CrossRef] [PubMed]
- Andabaka, Ž.; Stupić, D.; Tomaz, I.; Marković, Z.; Karoglan, M.; Zdunić, G.; Kontić, J.K.; Maletić, E.; Šikuten, I.; Preiner, D. Characterization of Berry Skin Phenolic Profiles in Dalmatian Grapevine Varieties. Appl. Sci. 2022, 12, 7822. [Google Scholar] [CrossRef]
- Sikuten, I.; Stambuk, P.; Tomaz, I.; Marchal, C.; Kontic, J.K.; Lacombe, T.; Maletic, E.; Preiner, D. Discrimination of genetic and geographical groups of grape varieties (Vitis vinifera L.) based on their polyphenolic profiles. J. Food Compos. Anal. 2021, 102, 104062. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Narduzzi, L.; Stanstrup, J.; Mattivi, F. Comparing wild American grapes with Vitis vinifera: A metabolomics study of grape composition. J. Agric. Food Chem. 2015, 63, 6823–6834. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.B.; Yue, T.X.; Ai, L.L.; Cheng, Y.F.; Meng, J.F.; Li, M.H.; Zhang, Z.W. Phenolic Compound Profiles in Grape Skins of Cabernet Sauvignon, Merlot, Syrah and Marselan Cultivated in the Shacheng Area (China). S. Afr. J. Enol. Vitic. 2016, 37, 132–138. [Google Scholar] [CrossRef]
- Revilla, E.; Carrasco, D.; Benito, A.; Arroyo-García, R.A. Anthocyanin fingerprint of different genotypes of wild grapes (Vitis vinifera spp. sylvestris (Gmelin) Hegi). J. Berry Res. 2019, 9, 63–82. [Google Scholar] [CrossRef]
- Guo, D.L.; Wang, Z.G.; Li, Q.; Gu, S.C.; Zhang, G.H.; Yu, Y.H. Hydrogen peroxide treatment promotes early ripening of Kyoho grape. Aust. J. Grape Wine Res. 2019, 25, 357–362. [Google Scholar] [CrossRef]
- Li, S.C.; Sun, L.; Fan, X.C.; Zhang, Y.; Jiang, J.F.; Liu, C.H. Polymorphism of anthocyanin concentration and composition in Chinese wild grapes. Aust. J. Grape Wine Res. 2021, 27, 34–41. [Google Scholar] [CrossRef]
- Favre, G.; Gómez-Alonso, S.; Pérez-Navarro, J.; García Romero, E.; Mena Morales, A.; Piccardo, D.; González Neves, G. Seed and skin-derived flavanols in red wine: A study of Syrah, Marselan, and Tannat cultivars. Eur. Food Res. Technol. 2024, 250, 845–857. [Google Scholar] [CrossRef]
- Cheng, G.; Wu, D.D.; Guo, R.R.; Li, H.Y.; Wei, R.F.; Zhang, J.; Wei, Z.Y.; Meng, X.; Yu, H.; Xie, T.L.; et al. Chromosome-scale genomics, metabolomics, and transcriptomics provide insight into the synthesis and regulation of phenols in Vitis adenoclada grapes. Front. Plant Sci. 2023, 14, 1124046. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, H.Y.; Wei, R.F.; Cheng, G.; Zhou, Y.M.; Liu, L.J.; Xie, T.L.; Guo, R.R.; Zhou, S.H. Widely Targeted Metabolomics Profiling Reveals the Effect of Powdery Mildew on Wine Grape Varieties with Different Levels of Tolerance to the Disease. Foods 2022, 11, 2461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhou, S.H.; Huang, Y.; Zhang, Y.; Guan, J.X.; Yang, Y.; Wen, R.D.; Zhang, J. Analysis of anthocyanin composition characteristics and gene expression patterns of ‘Guipu No. 6’ grape. Plant Physiol. J. 2017, 53, 103–114. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, J.; Wei, L.; Zheng, T.; Wu, J. Metabolomic Profiling and Assessment of Phenolic Compounds Derived from Vitis davidii Foex Cane and Stem Extracts. Int. J. Mol. Sci. 2022, 23, 14873. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Cao, X.Y.; Fang, Y.L. Targeted metabolomic and transcript level analysis reveals quality characteristic of Chinese wild grapes (Vitis davidii Foex). Foods 2020, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yang, L.; Yue, X.F.; He, R.; Deng, S.L.; Yang, X.; Liu, X.; Fang, Y.L. The condensed tannin chemistry and astringency properties of fifteen Vitis davidii Foex grapes and wines. Food Chem. X 2021, 11, 100125. [Google Scholar] [CrossRef] [PubMed]
- Revilla, E.; Arroyo-Garcia, R.; Bellido, A.; Carrasco, D.; Puig, A.; Ruiz-Garcia, L. 2018. Fingerprints of Anthocyanins and Flavonols in Wild Grapes (Vitis vinifera L. ssp. sylvestris (Gmelin) Hegi). In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; António Manuel, J., Fernanda, C., Eds.; InTech: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Sun, X.; Fang, Y. Oenological potential and health benefits of Chinese non-Vitis vinifera species: An opportunity to the revalorization and to breed new varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, X.; Hu, X.X.; Li, X.Y.; Lv, S.S.; Zhan, C.; Chen, Y.H.; Wang, C.Y.; Xu, J.Y. Phenolic features and anthocyanin profiles in winemaking pomace and fresh berries of grapes with different pedigrees. Food Sci. Biotechnol. 2023, 32, 145–156. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Rustioni, L.; Pozzi, C.; Failla, O. Disfunctions in the anthocyanin accumulation of Vitis vinifera L. varieties studied by a targeted resequencing approach. J. Berry Res. 2020, 10, 345–363. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar] [CrossRef]
- Tian, M.B.; Liu, Y.; Lu, H.C.; Hu, L.; Wang, Y.; Cheng, C.F.; Chen, W.; Li, S.D.; He, F.; Duan, C.Q.; et al. Cluster spatial positions varied the phenolics profiles of ‘Cabernet Sauvignon’ grapes and wines under a fan training system with multiple trunks. Food Chem. 2022, 387, 132930. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, N.N.; Karadoğan, B.; Kadioğlu, Z.; Esmek, İ.; Albayrak, S.; Kaya, O. Response of Karaerik Grape Cultivar (Vitis vinifera L.) to Two Training Systems and Three Trunk Heights. Erwerbs-Obstbau 2022, 64, 119–127. [Google Scholar] [CrossRef]
- Tian, M.B.; Liu, Y.; Lu, H.C.; Hu, L.; Wang, Y.; Cheng, C.F.; Chen, W.; Li, S.D.; He, F.; Duan, C.Q.; et al. Volatomics of ‘Cabernet Sauvignon’ grapes and wines under the fan training system revealed the nexus of microclimate and volatile compounds. Food Chem. 2023, 403, 134421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Guo, S.H.; Xiao, Q.H.; Du, Y.P.; Zhai, H. Effect of canopy micro-environment on anthocyanins of ‘Moldova’ grape. Food Sci. 2018, 39, 98–106. [Google Scholar] [CrossRef]
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Sun, X.; Fang, Y. Transcriptomics Integrated with Metabolomics Reveals the Effect of Regulated Deficit Irrigation on Anthocyanin Biosynthesis in Cabernet Sauvignon Grape Berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef] [PubMed]
| Physical and Chemical Index | YN2-T | Mar-T | GP6-T | GP6-E |
|---|---|---|---|---|
| Berry weight/(g) | 0.97 ± 0.13 b | 0.73 ± 0.06 c | 1.83 ± 0.06 a | 1.73 ± 0.19 a |
| Soluble solids concentration/(°Brix) | 16.53 ± 0.85 a | 16.2 ± 0.35 a | 17.27 ± 0.15 a | 16.9 ± 1.71 a |
| Titratable acidity/(g·L−1) | 14.34 ± 3.55 a | 19.29 ± 0.99 a | 18.27 ± 1.48 a | 18.55 ± 3.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, H.-Y.; Zhou, S.-H.; Cheng, G.; Wei, R.-F.; Zhou, Y.-M.; Zhang, Y.; Xie, T.-L.; Zhang, L. The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China. Int. J. Mol. Sci. 2024, 25, 8624. https://doi.org/10.3390/ijms25168624
Yu H, Li H-Y, Zhou S-H, Cheng G, Wei R-F, Zhou Y-M, Zhang Y, Xie T-L, Zhang L. The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China. International Journal of Molecular Sciences. 2024; 25(16):8624. https://doi.org/10.3390/ijms25168624
Chicago/Turabian StyleYu, Huan, Hong-Yan Li, Si-Hong Zhou, Guo Cheng, Rong-Fu Wei, Yong-Mei Zhou, Ying Zhang, Tai-Li Xie, and Lan Zhang. 2024. "The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China" International Journal of Molecular Sciences 25, no. 16: 8624. https://doi.org/10.3390/ijms25168624
APA StyleYu, H., Li, H.-Y., Zhou, S.-H., Cheng, G., Wei, R.-F., Zhou, Y.-M., Zhang, Y., Xie, T.-L., & Zhang, L. (2024). The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China. International Journal of Molecular Sciences, 25(16), 8624. https://doi.org/10.3390/ijms25168624






