Helicase HELQ: Molecular Characters Fit for DSB Repair Function
Abstract
1. Introduction
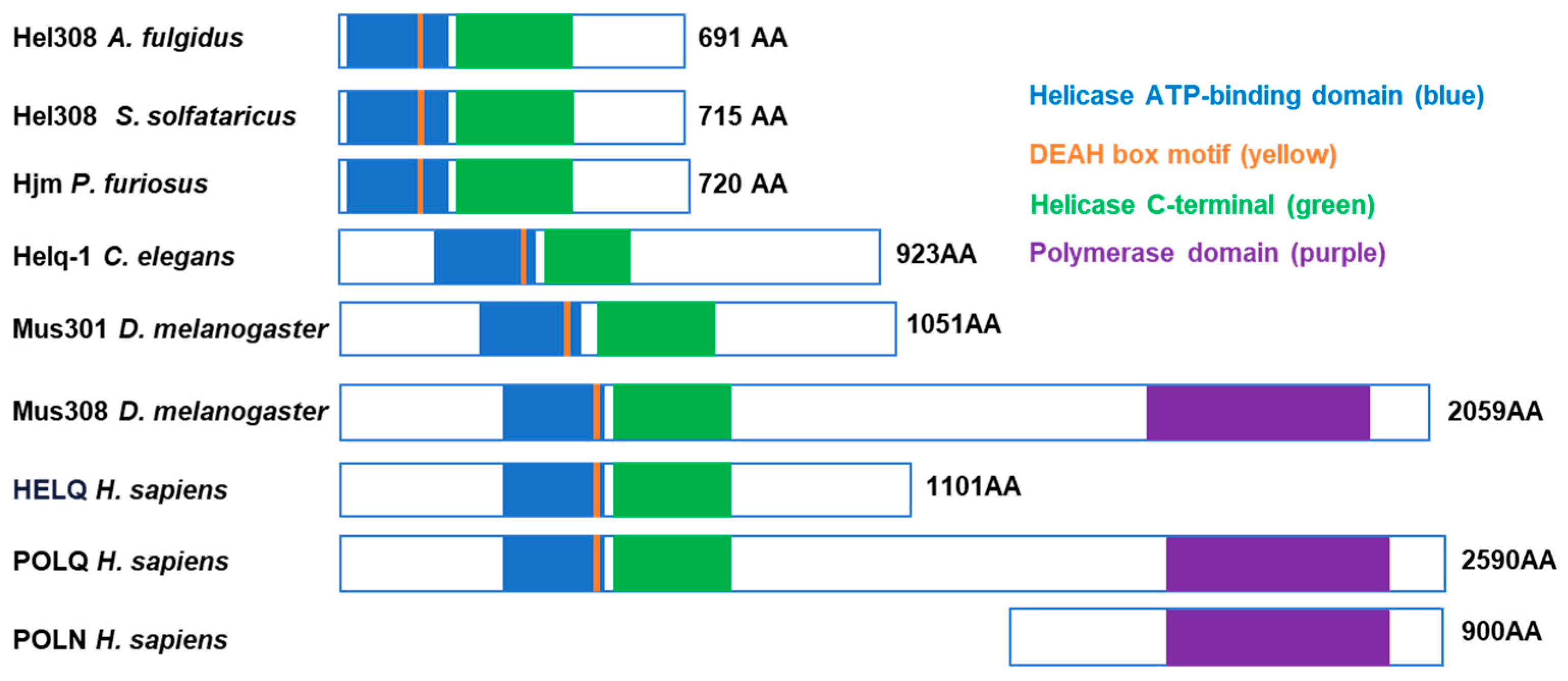
2. Molecular Architecture of HELQ Family Proteins
2.1. The HELQ Protein Sequence Is Conserved from Archaea to Humans
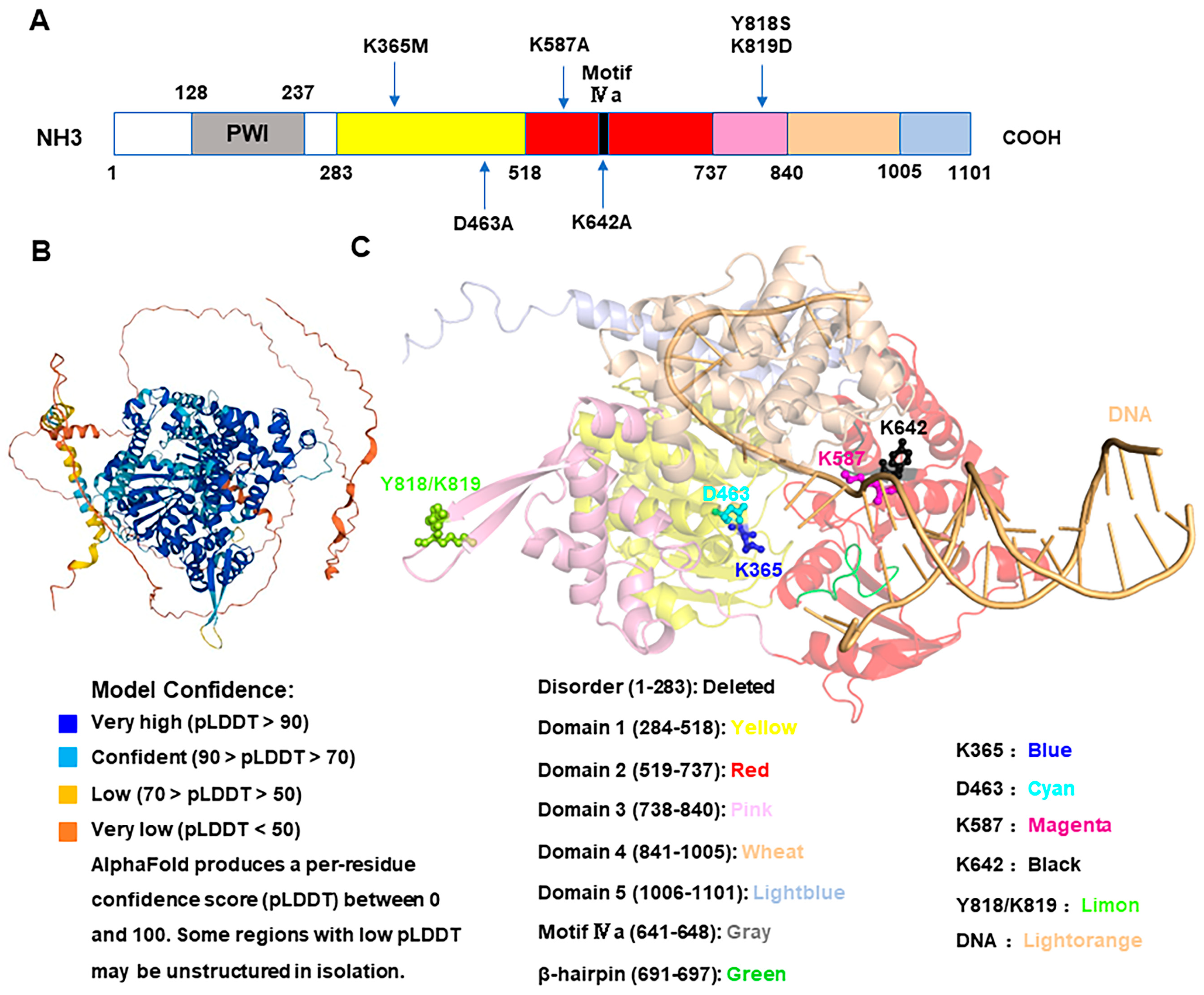
2.2. The HELQ 3–D Spatial Structure Is Conserved from Archaea to Humans
3. The Biochemical Function of HELQ
3.1. DNA Unwinding
3.2. DNA Binding
3.3. DNA Translocating
3.4. DNA Annealing
4. Biochemical Activity Fit for DSB Repair Function
4.1. DSB Repair
- (1)
- Homologous recombination (HR): HR utilizes sister chromatids as templates to repair DSBs and is considered the most accurate method of repair. HR requires a complex formed by MRE11–RAD50–NBS1 (MRN) and the CtBP–interacting protein (CtIP). The complex initiates short–range DNA end resection, forming 3′–terminated ssDNA, and then recruits exonuclease 1 (EXO1) or the DNA replication ATP–dependent helicase/nuclease 2 (DNA2) and Bloom syndrome protein (BLM) or Wemer syndrome protein (WRN) and other necessary factors to complete long–range end resection. This process generates longer single–stranded DNA (ssDNA) to facilitate recombination, which is conducive to accurate repair. Next, HR involves gene conversion (GC) and may result in synthesis–dependent strand annealing (SDSA) or a double Holliday junction (dHJ). During the SDSA process, one end of the DSB invades the homologous gene template and initiates replication from the donor. Subsequently, the DSB unwinds from its template to release the newly synthesized DNA strand. Then, the DSB anneals with the complementary DNA at the break sites and serves as a template for synthesizing the second strand, thereby completing the DNA replication process. The initiation of dHJ is similar to that of SDSA, but the D–loop “captures” the second broken DNA strand via annealing, thus forming the Holliday junction. The Holliday junction determines the outcomes of crossovers and non–crossovers [25].
- (2)
- Non-homologous end joining (NHEJ): NHEJ directly joins the two ends of the break, which can easily lead to base deletions or introduce some DNA fragment insertions. As a result, its error–proness rate greatly increases. Because NHEJ repair is fast and efficient, it occurs at various stages of the cell cycle and becomes the primary method of cell repair [26].
- (3)
- Microhomology–mediated end joining (MMEJ): Firstly, MMEJ requires limited DNA end resection to expose short ssDNAs. Secondly, the microhomology DNA strands are annealed, or single–stranded DNA overhangs (Flaps) need to be removed. Lastly, gaps are populated, and DNAs are ligated [27].
- (4)
- Single–strand annealing (SSA): Compared with MMEJ, SSA requires end resection to generate a longer 3′ ssDNA tail, which is then annealed between homologous regions. The repair is completed by removing the 3′ flaps, filling the gaps, and ligating the DNA [24].
- (1)
- Break–induced replication (BIR): BIR needs to undergo end resection to form a 3′ ssDNA that invades the homologous donor and then initiates bubble migration DNA synthesis, which can travel to the end of the chromosome [24].
- (2)
- Mitotic DNA synthesis (MiDAS): A BIR–like process, MiDAS is observed at common fragile sites, where the DNA frequently remains unreplicated after the S phase [28].
- (3)
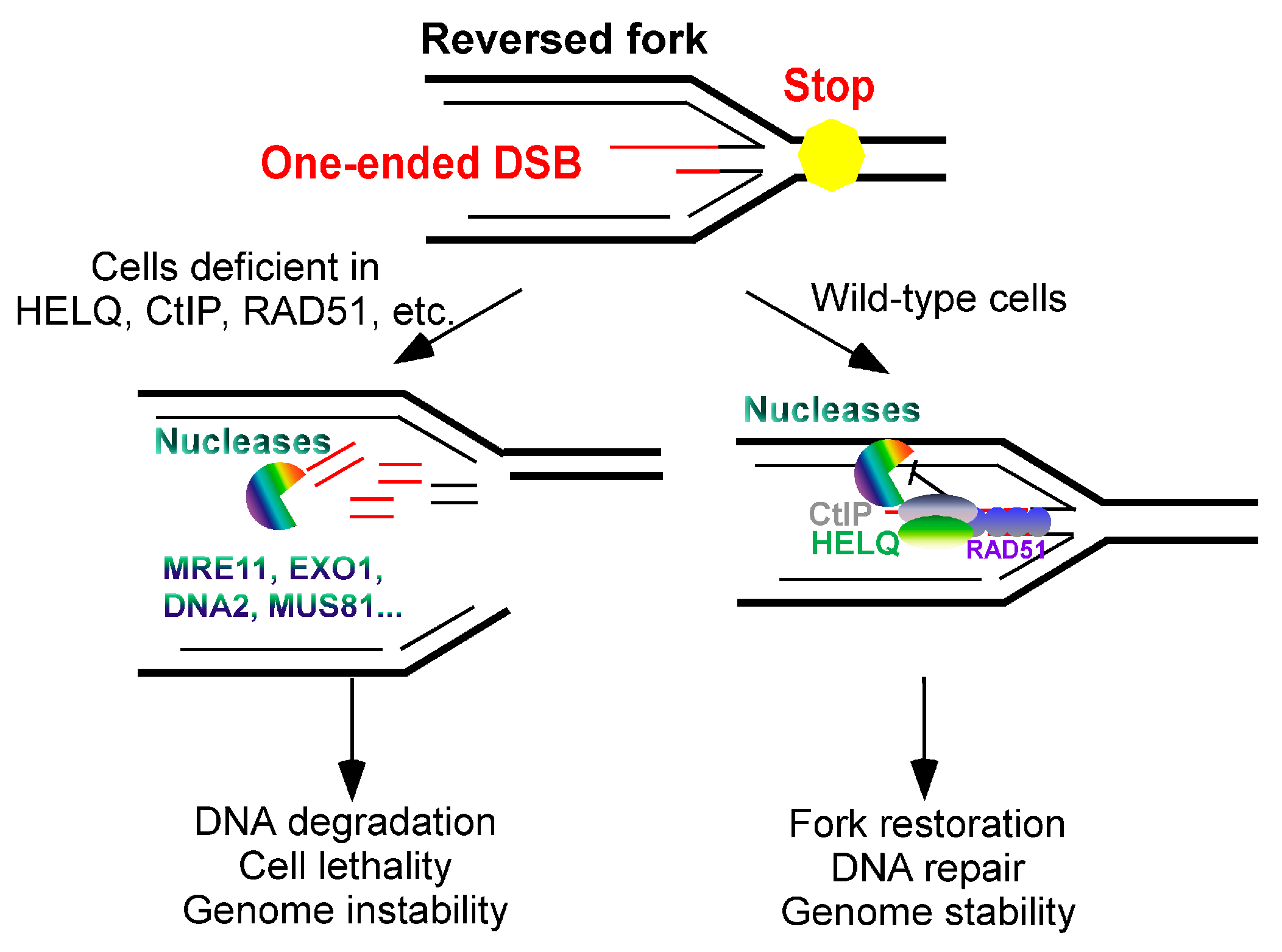
- Reversed fork: Reversed forks, formed at stalled replication forks [29], involve the reconstitution of replication fork DNA. The newly synthesized strands may become uncoupled under replication stress, leading to the formation of a “chicken foot” structure as parent strands anneal [30]. When a reversed fork is inappropriately formed, it creates a one–ended DSB that is vulnerable to nuclease degradation. Intriguingly, it has been reported that the nucleases that resect and degrade stalled replication forks include MRE11, EXO1, and DNA2, as well as the nucleases that promote the resection of DSB ends. This degradation, also known as DNA end resection, can be inhibited by the DSB repair–independent functions of certain common DNA repair proteins. These proteins block the deleterious reversed fork from further degradation [31].
4.2. HELQ Promotes End Resection in Resection–Dependent DSB Repair Pathways
4.3. HELQ Inhibits Nascent DNA Degradation (End Resection) in Reversed Forks
4.4. The Function of HELQ in Recombination
4.5. HELQ Function in BIR Repair
5. The Role of HELQ in Human Diseases
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, X.; Zhao, L.; Li, X. HELQ in cancer and reproduction. Neoplasma 2016, 63, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.B.; Sakaguchi, K.; Harris, P.V. mus308 mutants of Drosophila exhibit hypersensitivity to DNA cross-linking agents and are defective in a deoxyribonuclease. Genetics 1990, 125, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.V.; Mazina, O.M.; Leonhardt, E.A.; Case, R.B.; Boyd, J.B.; Burtis, K.C. Molecular cloning of Drosophila mus308, a gene involved in DNA cross-link repair with homology to prokaryotic DNA polymerase I genes. Mol. Cell Biol. 1996, 16, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Wood, R.D. A human DNA helicase homologous to the DNA cross-link sensitivity protein Mus308. J. Biol. Chem. 2002, 277, 8716–8723. [Google Scholar] [CrossRef] [PubMed]
- Fujikane, R.; Komori, K.; Shinagawa, H.; Ishino, Y. Identification of a novel helicase activity unwinding branched DNAs from the hyperthermophilic archaeon, Pyrococcus furiosus. J. Biol. Chem. 2005, 280, 12351–12358. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.P.; Bolt, E.L. Archaeal Hel308 helicase targets replication forks in vivo and in vitro and unwinds lagging strands. Nucleic Acids Res. 2005, 33, 3678–3690. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.D.; Johnson, K.A.; Liu, H.; McRobbie, A.M.; McMahon, S.; Oke, M.; Carter, L.; Naismith, J.H.; White, M.F. Structure of the DNA repair helicase hel308 reveals DNA binding and autoinhibitory domains. J. Biol. Chem. 2008, 283, 5118–5126. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Buechelmaier, E.; Belan, O.; Newton, M.; Vancevska, A.; Kaczmarczyk, A.; Takaki, T.; Rueda, D.S.; Powell, S.N.; Boulton, S.J. HELQ is a dual-function DSB repair enzyme modulated by RPA and RAD51. Nature 2022, 601, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Hou, K.P.; Li, Y.H.; Hao, S.L.; Liu, Y.; Na, Y.A.; Li, C.; Cui, J.; Xu, X.Z.; Wu, X.H.; et al. Human HELQ regulates DNA end resection at DNA double-strand breaks and stalled replication forks. Nucleic Acids Res. 2023, 51, 12207–12223. [Google Scholar] [CrossRef]
- Lever, R.J.; Simmons, E.; Gamble-Milner, R.; Buckley, R.J.; Harrison, C.; Parkes, A.J.; Mitchell, L.; Gausden, J.A.; Skulj, S.; Bertosa, B.; et al. Archaeal Hel308 suppresses recombination through a catalytic switch that controls DNA annealing. Nucleic Acids Res. 2023, 51, 8563–8574. [Google Scholar] [CrossRef]
- Tang, N.; Wen, W.; Liu, Z.; Xiong, X.; Wu, Y. HELQ as a DNA helicase: Its novel role in normal cell function and tumorigenesis (Review). Oncol. Rep. 2023, 50, 220. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; Madhavan, M.V.; Mirchandani, K.D.; McCaffrey, R.M.; Vinciguerra, P.; D’Andrea, A.D. DNA polymerase POLN participates in cross-link repair and homologous recombination. Mol. Cell Biol. 2010, 30, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Buttner, K.; Nehring, S.; Hopfner, K.P. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 2007, 14, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Oka, H.; Mayanagi, K.; Shirai, T.; Matoba, K.; Fujikane, R.; Ishino, Y.; Morikawa, K. Atomic structures and functional implications of the archaeal RecQ-like helicase Hjm. BMC Struct. Biol. 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Northall, S.J.; Buckley, R.; Jones, N.; Penedo, J.C.; Soultanas, P.; Bolt, E.L. DNA binding and unwinding by Hel308 helicase requires dual functions of a winged helix domain. DNA Repair 2017, 57, 125–132. [Google Scholar] [CrossRef]
- Flechsig, H.; Popp, D.; Mikhailov, A.S. In silico investigation of conformational motions in superfamily 2 helicase proteins. PLoS ONE 2011, 6, e21809. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Northall, S.J.; Ptchelkine, D.; Lever, R.; Cubbon, A.; Betts, H.; Taresco, V.; Cooper, C.D.O.; McHugh, P.J.; Soultanas, P.; et al. The HelQ human DNA repair helicase utilizes a PWI-like domain for DNA loading through interaction with RPA, triggering DNA unwinding by the HelQ helicase core. NAR Cancer 2021, 3, zcaa043. [Google Scholar] [CrossRef] [PubMed]
- Tafel, A.A.; Wu, L.; McHugh, P.J. Human HEL308 localizes to damaged replication forks and unwinds lagging strand structures. J. Biol. Chem. 2011, 286, 15832–15840. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.M.; Laszlo, A.H.; Nova, I.C.; Brinkerhoff, H.; Noakes, M.T.; Baker, K.S.; Bowman, J.L.; Higinbotham, H.R.; Mount, J.W.; Gundlach, J.H. Determining the effects of DNA sequence on Hel308 helicase translocation along single-stranded DNA using nanopore tweezers. Nucleic Acids Res. 2019, 47, 2506–2513. [Google Scholar] [CrossRef]
- Brinkerhoff, H.; Kang, A.S.W.; Liu, J.; Aksimentiev, A.; Dekker, C. Multiple rereads of single proteins at single-amino acid resolution using nanopores. Science 2021, 374, 1509–1513. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Hou, G.; Ma, X.; Sheng, D.; Ni, J.; Shen, Y. Hjm/Hel308A DNA helicase from Sulfolobus tokodaii promotes replication fork regression and interacts with Hjc endonuclease in vitro. J. Bacteriol. 2008, 190, 3006–3017. [Google Scholar] [CrossRef]
- He, L.; Lever, R.; Cubbon, A.; Tehseen, M.; Jenkins, T.; Nottingham, A.O.; Horton, A.; Betts, H.; Fisher, M.; Hamdan, S.M.; et al. Interaction of human HelQ with DNA polymerase delta halts DNA synthesis and stimulates DNA single-strand annealing. Nucleic Acids Res. 2023, 51, 1740–1749. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Kockler, Z.W.; Osia, B.; Lee, R.; Musmaker, K.; Malkova, A. Repair of DNA Breaks by Break-Induced Replication. Annu. Rev. Biochem. 2021, 90, 165–191. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X. Microhomology-mediated end joining: New players join the team. Cell Biosci. 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Minocherhomji, S.; Ying, S.; Bjerregaard, V.A.; Bursomanno, S.; Aleliunaite, A.; Wu, W.; Mankouri, H.W.; Shen, H.; Liu, Y.; Hickson, I.D. Replication stress activates DNA repair synthesis in mitosis. Nature 2015, 528, 286–290. [Google Scholar] [CrossRef]
- Berti, M.; Cortez, D.; Lopes, M. The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 2020, 21, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Quinet, A.; Lemacon, D.; Vindigni, A. Replication Fork Reversal: Players and Guardians. Mol. Cell 2017, 68, 830–833. [Google Scholar] [CrossRef]
- Cejka, P.; Symington, L.S. DNA End Resection: Mechanism and Control. Annu. Rev. Genet. 2021, 55, 285–307. [Google Scholar] [CrossRef]
- Cejka, P. DNA End Resection: Nucleases Team Up with the Right Partners to Initiate Homologous Recombination. J. Biol. Chem. 2015, 290, 22931–22938. [Google Scholar] [CrossRef] [PubMed]
- Symington, L.S. Mechanism and regulation of DNA end resection in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 195–212. [Google Scholar] [CrossRef]
- Ranjha, L.; Howard, S.M.; Cejka, P. Main steps in DNA double-strand break repair: An introduction to homologous recombination and related processes. Chromosoma 2018, 127, 187–214. [Google Scholar] [CrossRef]
- Zhao, F.; Kim, W.; Kloeber, J.A.; Lou, Z. DNA end resection and its role in DNA replication and DSB repair choice in mammalian cells. Exp. Mol. Med. 2020, 52, 1705–1714. [Google Scholar] [CrossRef]
- Symington, L.S.; Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 2011, 45, 247–271. [Google Scholar] [CrossRef]
- Longhese, M.P.; Bonetti, D.; Manfrini, N.; Clerici, M. Mechanisms and regulation of DNA end resection. EMBO J. 2010, 29, 2864–2874. [Google Scholar] [CrossRef]
- Mimitou, E.P.; Symington, L.S. DNA end resection--unraveling the tail. DNA Repair 2011, 10, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes. Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.H.; Hiom, K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 2009, 459, 460–463. [Google Scholar] [CrossRef]
- Huertas, P.; Jackson, S.P. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 2009, 284, 9558–9565. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, I.; Howard, S.M.; Kasaciunaite, K.; Pinto, C.; Anand, R.; Seidel, R.; Cejka, P. CtIP promotes the motor activity of DNA2 to accelerate long-range DNA end resection. Proc. Natl. Acad. Sci. USA 2020, 117, 8859–8869. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Chapman, J.R.; Magill, C.; Jackson, S.P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes. Dev. 2008, 22, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Symington, L.S. End resection at double-strand breaks: Mechanism and regulation. Cold Spring Harb. Perspect. Biol. 2014, 6, a016436. [Google Scholar] [CrossRef] [PubMed]
- Sturzenegger, A.; Burdova, K.; Kanagaraj, R.; Levikova, M.; Pinto, C.; Cejka, P.; Janscak, P. DNA2 cooperates with the WRN and BLM RecQ helicases to mediate long-range DNA end resection in human cells. J. Biol. Chem. 2014, 289, 27314–27326. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hua, Y.; Wang, J.; Li, L.; Yuan, J.; Zhang, B.; Wang, Z.; Ji, J.; Kong, D. RNA polymerase III is required for the repair of DNA double-strand breaks by homologous recombination. Cell 2021, 184, 1314–1329 e1310. [Google Scholar] [CrossRef] [PubMed]
- Muzzini, D.M.; Plevani, P.; Boulton, S.J.; Cassata, G.; Marini, F. Caenorhabditis elegans POLQ-1 and HEL-308 function in two distinct DNA interstrand cross-link repair pathways. DNA Repair 2008, 7, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Adelman, C.A.; Lolo, R.L.; Birkbak, N.J.; Murina, O.; Matsuzaki, K.; Horejsi, Z.; Parmar, K.; Borel, V.; Skehel, J.M.; Stamp, G.; et al. HELQ promotes RAD51 paralogue-dependent repair to avert germ cell loss and tumorigenesis. Nature 2013, 502, 381–384. [Google Scholar] [CrossRef]
- Takata, K.; Reh, S.; Tomida, J.; Person, M.D.; Wood, R.D. Human DNA helicase HELQ participates in DNA interstrand crosslink tolerance with ATR and RAD51 paralogs. Nat. Commun. 2013, 4, 2338. [Google Scholar] [CrossRef]
- Luebben, S.W.; Kawabata, T.; Akre, M.K.; Lee, W.L.; Johnson, C.S.; O’Sullivan, M.G.; Shima, N. Helq acts in parallel to Fancc to suppress replication-associated genome instability. Nucleic Acids Res. 2013, 41, 10283–10297. [Google Scholar] [CrossRef]
- Jones, M.J.; Huang, T.T. The Fanconi anemia pathway in replication stress and DNA crosslink repair. Cell Mol. Life Sci. 2012, 69, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Anai, H.; Hanada, K. Mechanisms of interstrand DNA crosslink repair and human disorders. Genes. Environ. 2016, 38, 9. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ji, F.; Helleday, T.; Ying, S. Mechanisms for stalled replication fork stabilization: New targets for synthetic lethality strategies in cancer treatments. EMBO Rep. 2018, 19, e46263. [Google Scholar] [CrossRef] [PubMed]
- Rickman, K.; Smogorzewska, A. Advances in understanding DNA processing and protection at stalled replication forks. J. Cell Biol. 2019, 218, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kondratick, C.M.; Washington, M.T.; Spies, M. Making Choices: DNA Replication Fork Recovery Mechanisms. Semin. Cell Dev. Biol. 2021, 113, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Thakar, T.; Moldovan, G.L. The emerging determinants of replication fork stability. Nucleic Acids Res. 2021, 49, 7224–7238. [Google Scholar] [CrossRef] [PubMed]
- Kamp, J.A.; Lemmens, B.; Romeijn, R.J.; Changoer, S.C.; van Schendel, R.; Tijsterman, M. Helicase Q promotes homology-driven DNA double-strand break repair and prevents tandem duplications. Nat. Commun. 2021, 12, 7126. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Mol. Cell 2016, 64, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, S.; Zhuang, L.; Li, W.; Qin, Y.; Chen, Z.J. The screening of HELQ gene in Chinese patients with premature ovarian failure. Reprod. Biomed. Online 2015, 31, 573–576. [Google Scholar] [CrossRef][Green Version]
- Traband, E.L.; Hammerlund, S.R.; Shameem, M.; Narayan, A.; Ramana, S.; Tella, A.; Sobeck, A.; Shima, N. Mitotic DNA Synthesis in Untransformed Human Cells Preserves Common Fragile Site Stability via a FANCD2-Driven Mechanism That Requires HELQ. J. Mol. Biol. 2023, 435, 168294. [Google Scholar] [CrossRef]
- Li, Y.P.; Yang, J.J.; Xu, H.; Guo, E.Y.; Yu, Y. Structure-function analysis of DNA helicase HELQ: A new diagnostic marker in ovarian cancer. Oncol. Lett. 2016, 12, 4439–4444. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, L.M.; Kinnunen, L.; Kiiski, J.I.; Khan, S.; Blomqvist, C.; Aittomaki, K.; Nevanlinna, H. Screening of HELQ in breast and ovarian cancer families. Fam. Cancer 2016, 15, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhu, J.Y.; Liu, Y.B.; Fu, K.; Tian, Y.; Li, P.Y.; Yang, W.Q.; Yang, S.Y.; Yin, J.Y.; Yin, G.; et al. Helicase POLQ-like (HELQ) as a novel indicator of platinum-based chemoresistance for epithelial ovarian cancer. Gynecol. Oncol. 2018, 149, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Tian, Y. Expressions of HELQ and RAD51C in endometrial stromal sarcoma and their clinical significance. Nan Fang. Yi Ke Da Xue Xue Bao 2020, 40, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yang, S.; Lei, M.; He, Q.; Wu, L.; Zhang, Y. DNA Repair Protein HELQ and XAB2 as Chemoresponse and Prognosis Biomarkers in Ascites Tumor Cells of High-Grade Serous Ovarian Cancer. J. Oncol. 2022, 2022, 7521934. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Marsit, C.J.; Houseman, E.A.; Butler, R.; Nelson, H.H.; McClean, M.D.; Kelsey, K.T. Gene-environment interactions of novel variants associated with head and neck cancer. Head. Neck 2012, 34, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.N.; Zhou, Y.F.; Peng, A.F.; Long, X.H.; Chen, X.Y.; Liu, Z.L.; Xia, H. HELQ reverses the malignant phenotype of osteosarcoma cells via CHK1-RAD51 signaling pathway. Oncol. Rep. 2017, 37, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Gao, Y.Y.; Ju, Q.Q.; Zhang, C.X.; Gong, M.; Li, Z.L. HELQ and EGR3 expression correlate with IGHV mutation status and prognosis in chronic lymphocytic leukemia. J. Transl. Med. 2021, 19, 42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Hou, K.; Liu, Y.; Na, Y.; Li, C.; Luo, H.; Wang, H. Helicase HELQ: Molecular Characters Fit for DSB Repair Function. Int. J. Mol. Sci. 2024, 25, 8634. https://doi.org/10.3390/ijms25168634
Zhao Y, Hou K, Liu Y, Na Y, Li C, Luo H, Wang H. Helicase HELQ: Molecular Characters Fit for DSB Repair Function. International Journal of Molecular Sciences. 2024; 25(16):8634. https://doi.org/10.3390/ijms25168634
Chicago/Turabian StyleZhao, Yuqin, Kaiping Hou, Yu Liu, Yinan Na, Chao Li, Haoyuan Luo, and Hailong Wang. 2024. "Helicase HELQ: Molecular Characters Fit for DSB Repair Function" International Journal of Molecular Sciences 25, no. 16: 8634. https://doi.org/10.3390/ijms25168634
APA StyleZhao, Y., Hou, K., Liu, Y., Na, Y., Li, C., Luo, H., & Wang, H. (2024). Helicase HELQ: Molecular Characters Fit for DSB Repair Function. International Journal of Molecular Sciences, 25(16), 8634. https://doi.org/10.3390/ijms25168634






