The Impact of Extracellular Histones and Absence of Toll-like Receptors on Cardiac Functional and Electrical Disturbances in Mouse Hearts
Abstract
:1. Introduction
2. Results
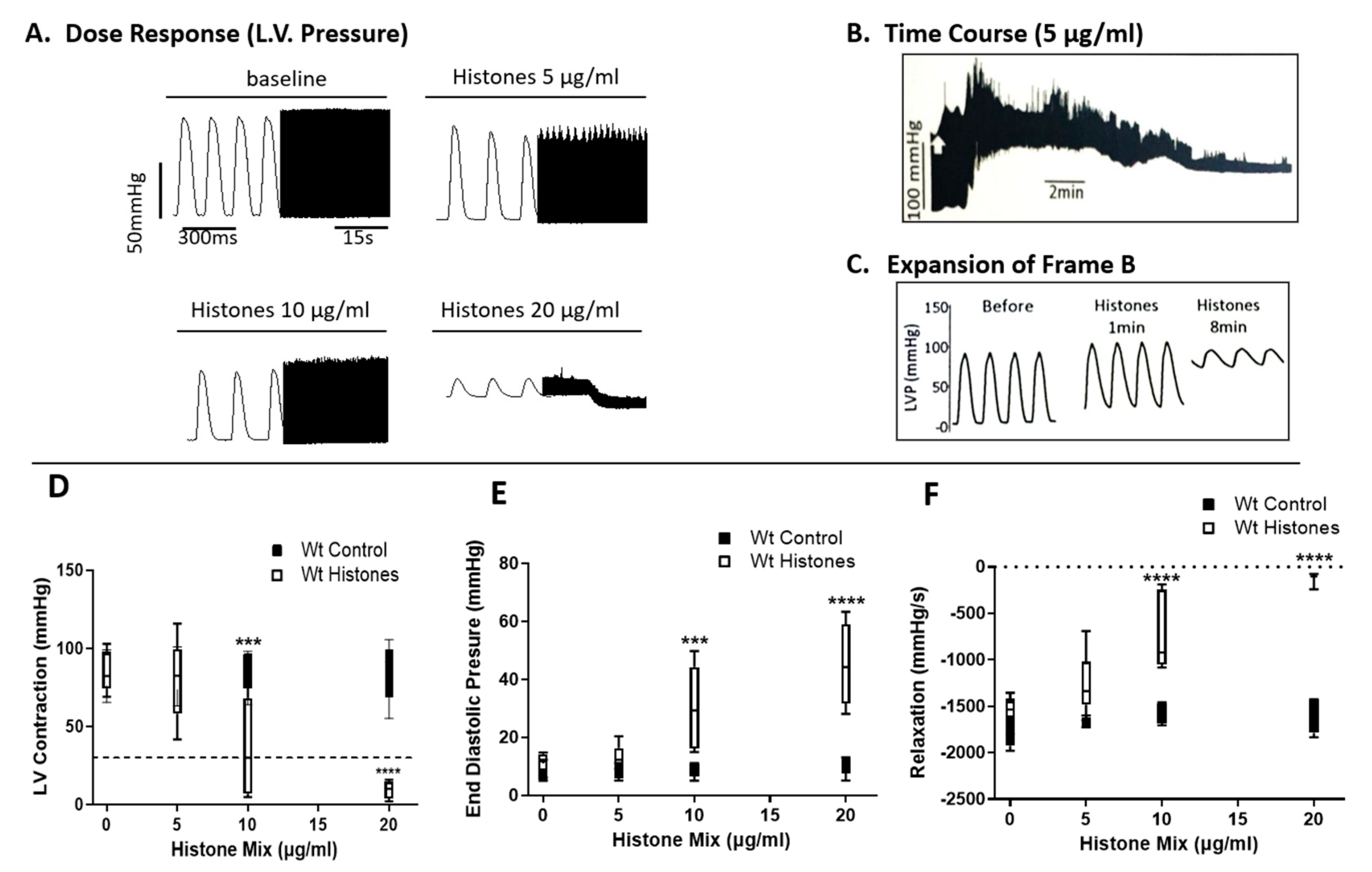
2.1. Functional Abnormalities in Wt Mouse Hearts Perfused Ex Vivo with Histone Mix
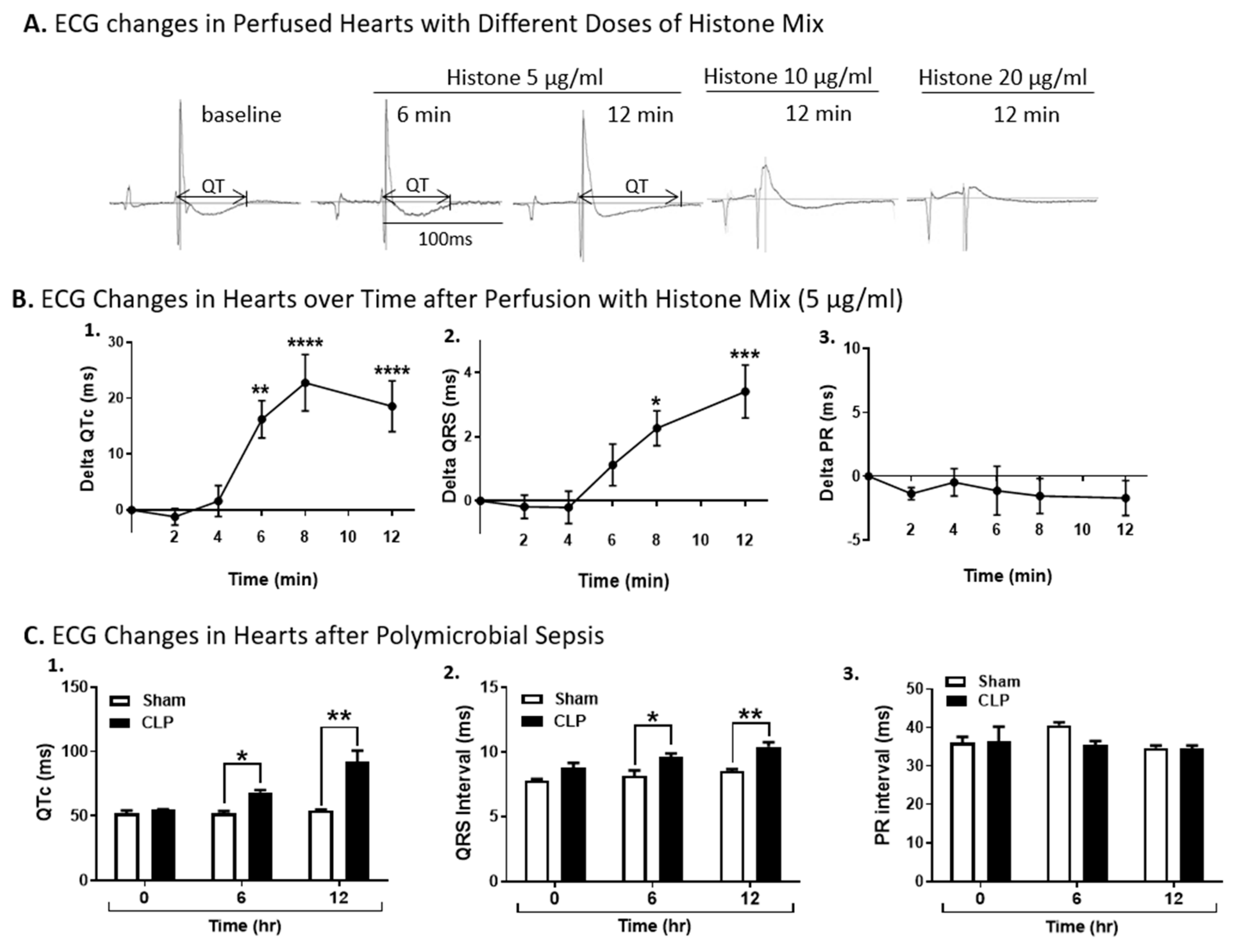
2.2. ECG Changes in Wt Hearts Perfused with Histones or After Induction of Polymicrobial Sepsis by CLP
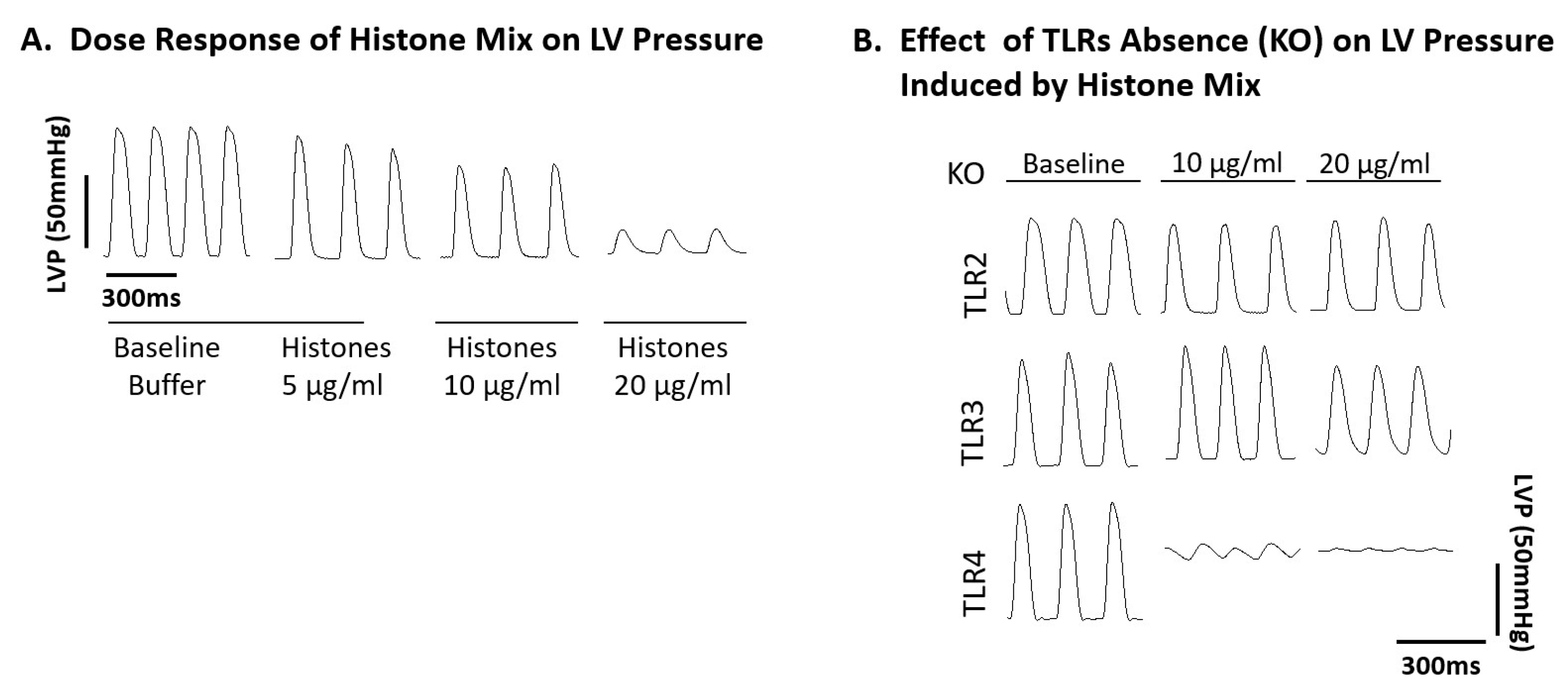
2.3. Ability of Histone Mix to Cause Cessation of Beating Mouse Hearts as a Function of Histone Concentration and Effect of TLR Absence
2.4. Functional Abnormalities in Wt Mouse Hearts Perfused with Histone Mix and Effects of TLR Absence on Functional Responses of Mouse Hearts to Histone Mix
2.5. Effect of TLR Absence on Delta QTc Prolongation in Murine Hearts Perfused with Histone Mix
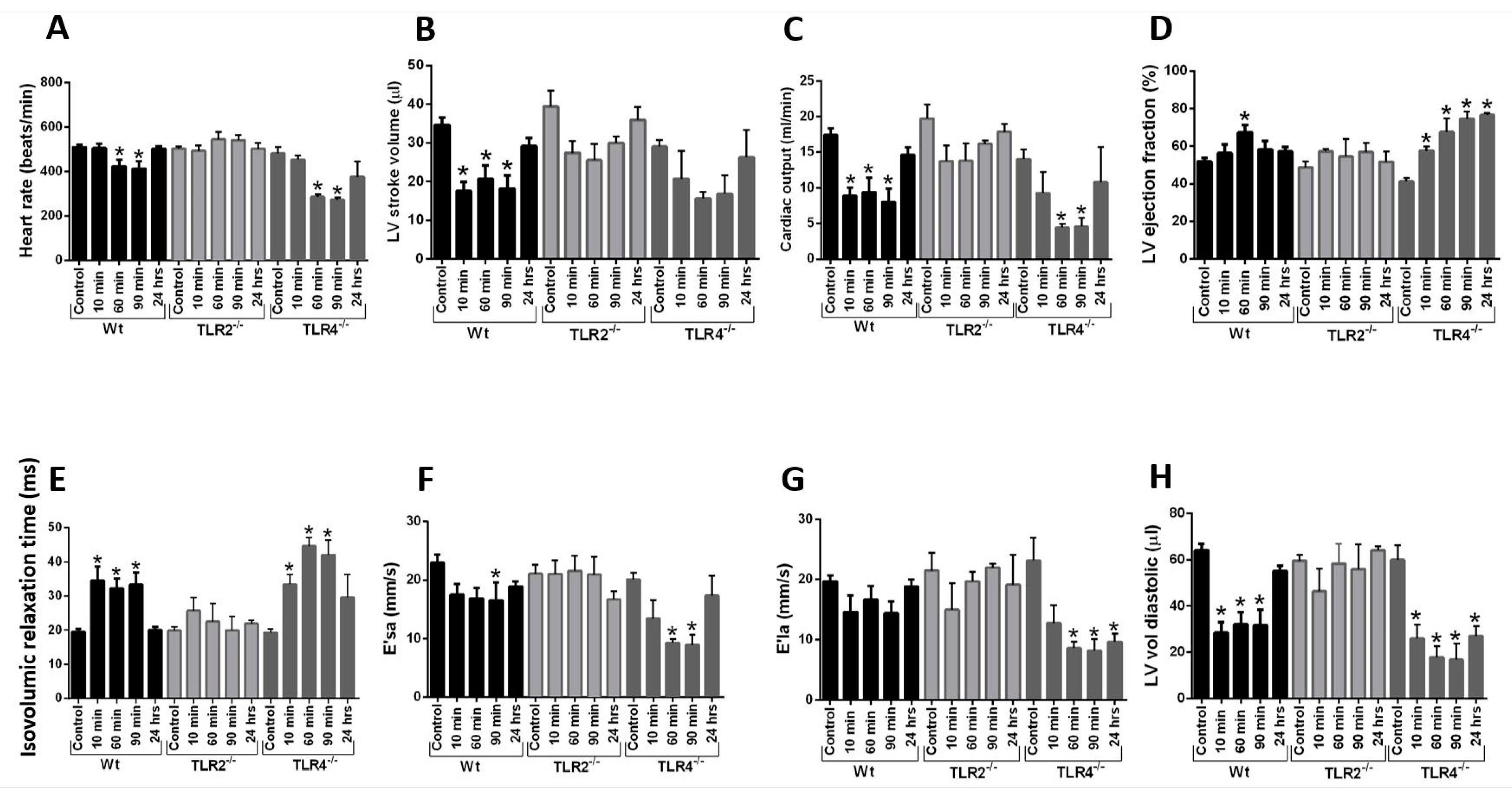
2.6. Echo-Doppler Parameters Measurement after Intravenous Infusion of Histone Mix
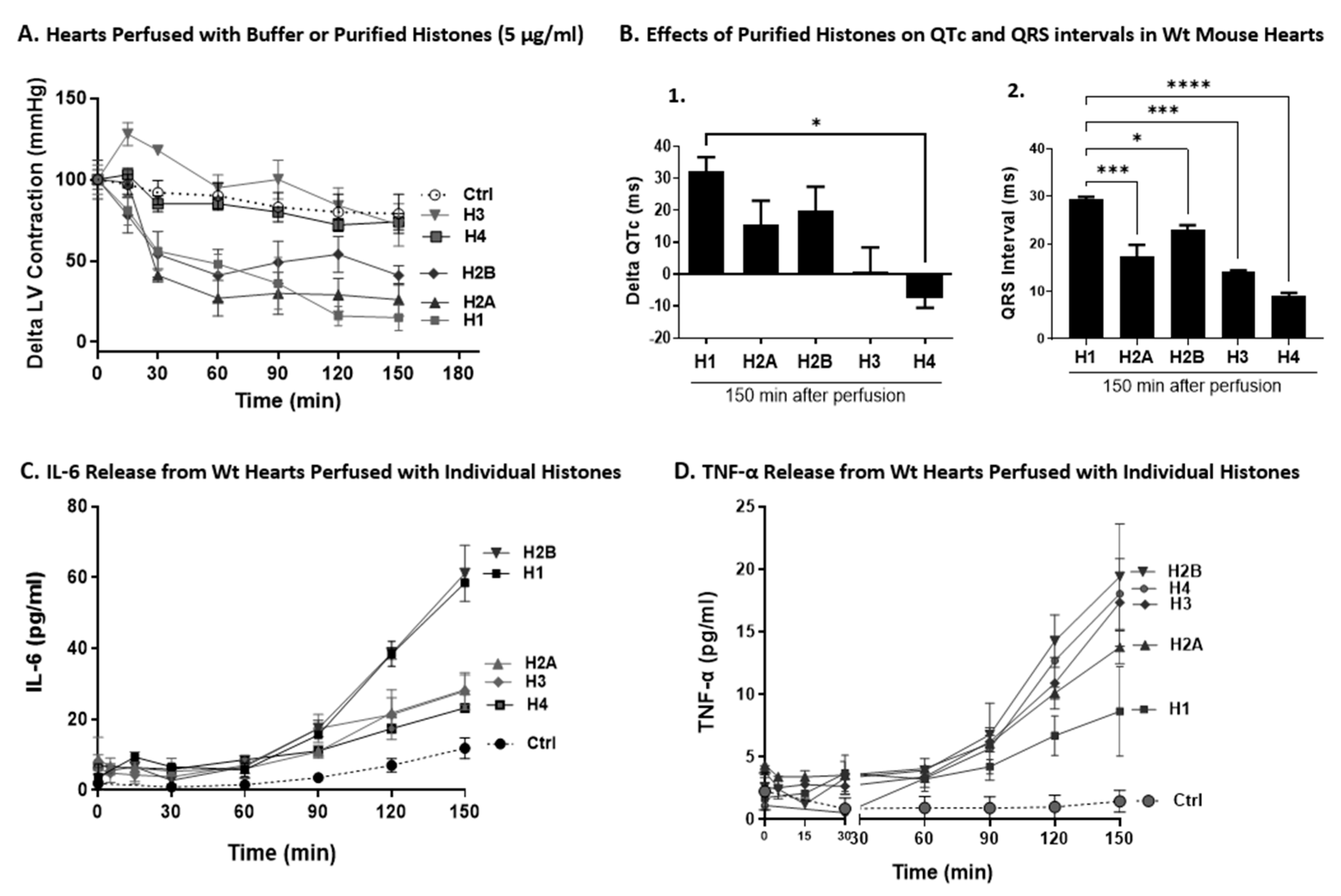
2.7. Effects of Individual Histones on LV Function, ECG Changes, and Cytokine Release on Perfused Hearts
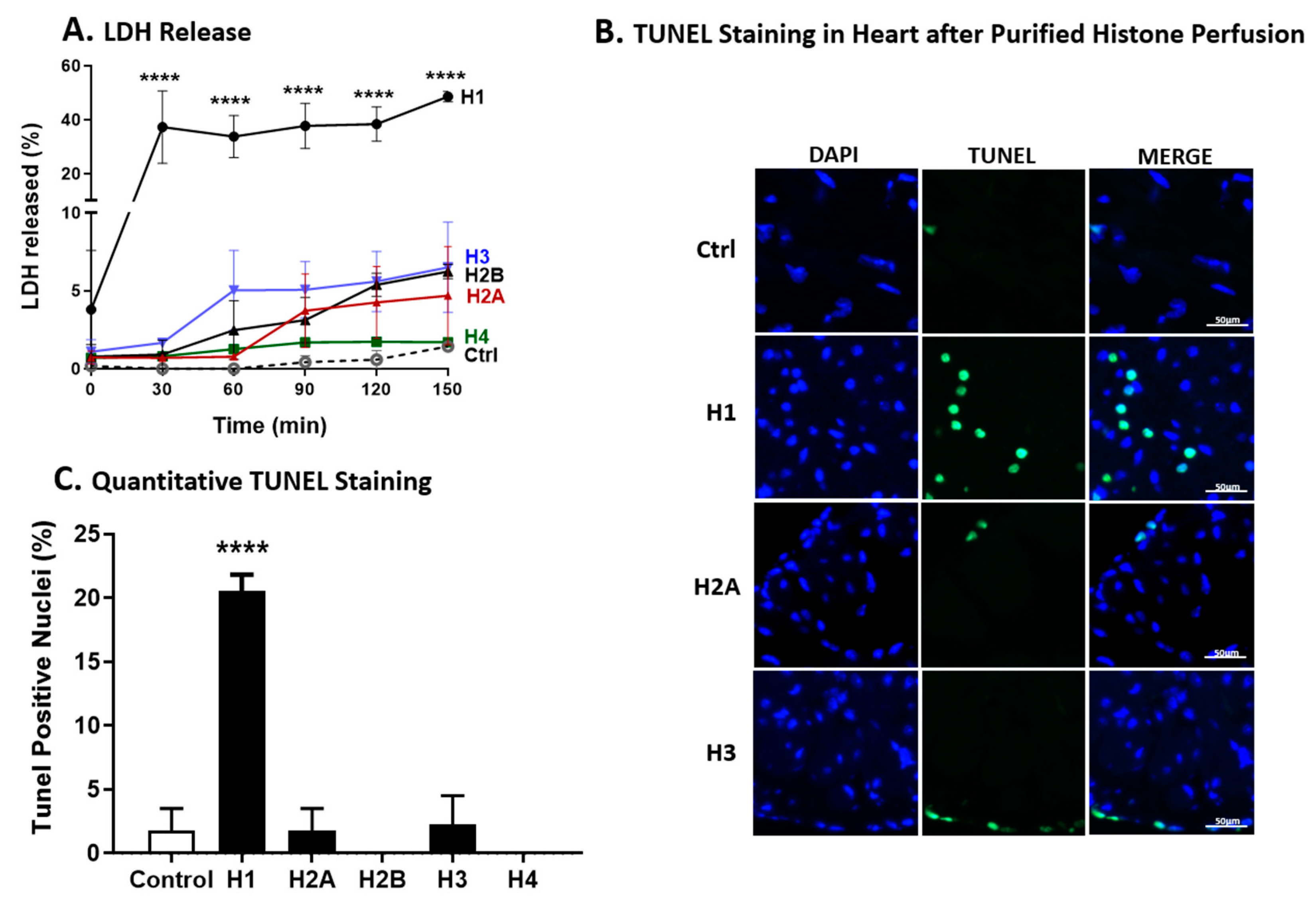
2.8. LDH Release and TUNEL Staining of Wt Hearts after Perfusion with Individual Histones
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Whole Heart Perfusions, Functional LVP and Electrical (ECG) Measurements
4.3. Protocols for Wt Mouse Heart Perfusion with Histone Mixture or Individual Histones
4.4. Cytokine ELISA
4.5. LDH Cytotoxicity Assay and TUNEL Staining
4.6. Echo-Doppler Functional Studies on TLR2, TLR3, and TLR4 KO Mice
4.7. Reagents
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merx, M.W.; Weber, C. Sepsis and the heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, J.E.; Parker, M.M.; Natanson, C.; Suffredini, A.F.; Danner, R.L.; Cunnion, R.E.; Ognibene, F.P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 1990, 113, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Cunnion, R.E.; Schaer, G.L.; Parker, M.M.; Natanson, C.; Parrillo, J.E. The coronary circulation in human septic shock. Circulation 1986, 73, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, A.; Mukhtar, A.; Nassar, H. Perfusion indices revisited. J. Intensive Care 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.L. Myocardial function in sepsis and endotoxin shock. Am. J. Physiol. 1989, 257 Pt 2, R1265–R1281. [Google Scholar] [CrossRef]
- Kurt, A.N.; Aygun, A.D.; Godekmerdan, A.; Kurt, A.; Dogan, Y.; Yilmaz, E. Serum IL-1beta, IL-6, IL-8, and TNF-alpha levels in early diagnosis and management of neonatal sepsis. Mediat. Inflamm. 2007, 2007, 31397. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Nasu, M. A review of sepsis-induced cardiomyopathy. J. Intensive Care 2015, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Daix, T.; Mathonnet, A.; Brakenridge, S.; Dequin, P.F.; Mira, J.P.; Berbille, F.; Morre, M.; Jeannet, R.; Blood, T.; Unsinger, J.; et al. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: A double-blind, randomized, placebo-controlled trial. Ann. Intensive Care 2023, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Tang, N.; Li, L.; Zou, G.; Xu, Y.; Liu, Z. Diagnostic value of combined detection of IL-1beta, IL-6, and TNF-alpha for sepsis-induced cardiomyopathy. Med. Clin. 2022, 158, 413–417. [Google Scholar] [CrossRef]
- Casey, L.C.; Balk, R.A.; Bone, R.C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 1993, 119, 771–778. [Google Scholar] [CrossRef]
- Kalbitz, M.; Grailer, J.J.; Fattahi, F.; Jajou, L.; Herron, T.J.; Campbell, K.F.; Zetoune, F.S.; Bosmann, M.; Sarma, J.V.; Huber-Lang, M.; et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 2015, 29, 2185–2193. [Google Scholar] [CrossRef]
- Kalbitz, M.; Fattahi, F.; Grailer, J.J.; Jajou, L.; Malan, E.A.; Zetoune, F.S.; Huber-Lang, M.; Russell, M.W.; Ward, P.A. Complement-induced activation of the cardiac NLRP3 inflammasome in sepsis. FASEB J. 2016, 30, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, Y.; Ito, T.; Nakahara, M.; Yamaguchi, K.; Yasuda, T. Sepsis-induced myocardial dysfunction: Pathophysiology and management. J. Intensive Care 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Chaput, C.; Zychlinsky, A. Sepsis: The dark side of histones. Nat. Med. 2009, 15, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, M.; Ito, T.; Kawahara, K.; Yamamoto, M.; Nagasato, T.; Shrestha, B.; Yamada, S.; Miyauchi, T.; Higuchi, K.; Takenaka, T.; et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE 2013, 8, e75961. [Google Scholar] [CrossRef] [PubMed]
- Ekaney, M.L.; Otto, G.P.; Sossdorf, M.; Sponholz, C.; Boehringer, M.; Loesche, W.; Rittirsch, D.; Wilharm, A.; Kurzai, O.; Bauer, M.; et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 2014, 18, 543. [Google Scholar] [CrossRef]
- Grailer, J.J.; Fattahi, F.; Dick, R.S.; Zetoune, F.S.; Ward, P.A. Cutting edge: Critical role for C5aRs in the development of septic lymphopenia in mice. J. Immunol. 2015, 194, 868–872. [Google Scholar] [CrossRef]
- Bosmann, M.; Grailer, J.J.; Ruemmler, R.; Russkamp, N.F.; Zetoune, F.S.; Sarma, J.V.; Standiford, T.J.; Ward, P.A. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013, 27, 5010–5021. [Google Scholar] [CrossRef]
- Fattahi, F.; Grailer, J.J.; Jajou, L.; Zetoune, F.S.; Andjelkovic, A.V.; Ward, P.A. Organ distribution of histones after intravenous infusion of FITC histones or after sepsis. Immunol. Res. 2015, 61, 177–186. [Google Scholar] [CrossRef]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Monestier, M.; Esmon, N.L.; Esmon, C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011, 187, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Scherbaum, C.R.; Darisipudi, M.N.; Mulay, S.R.; Hagele, H.; Lichtnekert, J.; Hagemann, J.H.; Rupanagudi, K.V.; Ryu, M.; Schwarzenberger, C.; et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 2012, 23, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gimenez, J.L.; Roma-Mateo, C.; Carbonell, N.; Palacios, L.; Peiro-Chova, L.; Garcia-Lopez, E.; Garcia-Simon, M.; Lahuerta, R.; Gimenez-Garzo, C.; Berenguer-Pascual, E.; et al. A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci. Rep. 2017, 7, 10643. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, S.; Annicchiarico, L.; Corsini, F.; Zigiotto, L.; Herbet, G.; Moritz-Gasser, S.; Dalpiaz, C.; Vitali, L.; Tate, M.; De Benedictis, A.; et al. Planning Brain Tumor Resection Using a Probabilistic Atlas of Cortical and Subcortical Structures Critical for Functional Processing: A Proof of Concept. Oper. Neurosurg. 2021, 20, E175–E183. [Google Scholar] [CrossRef] [PubMed]
- Lagedal, R.; Eriksson, O.; Sorman, A.; Huckriede, J.B.; Kristensen, B.; Franzen, S.; Larsson, A.; Bergqvist, A.; Alving, K.; Forslund, A.; et al. Impaired Antibody Response Is Associated with Histone-Release, Organ Dysfunction and Mortality in Critically Ill COVID-19 Patients. J. Clin. Med. 2022, 11, 3419. [Google Scholar] [CrossRef]
- Annane, D.; Sebille, V.; Duboc, D.; Le Heuzey, J.Y.; Sadoul, N.; Bouvier, E.; Bellissant, E. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am. J. Respir. Crit. Care Med. 2008, 178, 20–25. [Google Scholar] [CrossRef]
- Hunter, J.D.; Doddi, M. Sepsis and the heart. Br. J. Anaesth. 2010, 104, 3–11. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Caille, V.; Charron, C.; Belliard, G.; Page, B.; Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 2008, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.B.; Ozment, T.R.; Wondergem, R.; Li, C.; Williams, D.L. Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis. Shock 2015, 43, 185–191. [Google Scholar] [CrossRef]
- Rich, M.M.; McGarvey, M.L.; Teener, J.W.; Frame, L.H. ECG changes during septic shock. Cardiology 2002, 97, 187–196. [Google Scholar] [CrossRef]
- Kalbitz, M.; Fattahi, F.; Herron, T.J.; Grailer, J.J.; Jajou, L.; Lu, H.; Huber-Lang, M.; Zetoune, F.S.; Sarma, J.V.; Day, S.M.; et al. Complement Destabilizes Cardiomyocyte Function In Vivo after Polymicrobial Sepsis and In Vitro. J. Immunol. 2016, 197, 2353–2361. [Google Scholar] [CrossRef]
- Varriale, P.; Ramaprasad, S. Septic cardiomyopathy as a cause of long QT syndrome. J. Electrocardiol. 1995, 28, 327–329. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Jaynes, H.A.; Kingery, J.R.; Mourad, N.A.; Trujillo, T.N.; Overholser, B.R.; Kovacs, R.J. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 479–487. [Google Scholar] [CrossRef]
- Fattahi, F.; Russell, M.W.; Malan, E.A.; Parlett, M.; Abe, E.; Zetoune, F.S.; Ward, P.A. Harmful Roles of TLR3 and TLR9 in Cardiac Dysfunction Developing during Polymicrobial Sepsis. Biomed. Res. Int. 2018, 2018, 4302726. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yu, M.M.; Shou, S.T.; Chai, Y.F. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Maeder, M.; Fehr, T.; Rickli, H.; Ammann, P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006, 129, 1349–1366. [Google Scholar] [CrossRef]
- Rudiger, A.; Singer, M. Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 2007, 35, 1599–1608. [Google Scholar] [CrossRef]
- Fattahi, F.; Grailer, J.J.; Lu, H.; Dick, R.S.; Parlett, M.; Zetoune, F.S.; Nunez, G.; Ward, P.A. Selective Biological Responses of Phagocytes and Lungs to Purified Histones. J. Innate Immun. 2017, 9, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Joulin, O.; Petillot, P.; Labalette, M.; Lancel, S.; Neviere, R. Cytokine profile of human septic shock serum inducing cardiomyocyte contractile dysfunction. Physiol. Res. 2007, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.P.; Gayan-Ramirez, G.; Van den Bergh, A.; Herijgers, P.; Maes, K.; Verbeken, E.; Decramer, M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 2005, 111, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Cain, B.S.; Meldrum, D.R.; Dinarello, C.A.; Meng, X.; Joo, K.S.; Banerjee, A.; Harken, A.H. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit. Care Med. 1999, 27, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, J.E.; Burch, C.; Shelhamer, J.H.; Parker, M.M.; Natanson, C.; Schuette, W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J. Clin. Investig. 1985, 76, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. [Google Scholar] [CrossRef]
- Standiford, T.J.; Strieter, R.M. TNF and IL-1 in sepsis: Good cytokines gone bad. J. Lab. Clin. Med. 1992, 120, 179–180. [Google Scholar]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Prucha, M.; Bellingan, G.; Zazula, R. Sepsis biomarkers. Clin. Chim. Acta 2015, 440, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Atefi, G.; Zetoune, F.S.; Herron, T.J.; Jalife, J.; Bosmann, M.; Al-Aref, R.; Sarma, J.V.; Ward, P.A. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 2011, 25, 2500–2508. [Google Scholar] [CrossRef]
- Gillrie, M.R.; Lee, K.; Gowda, D.C.; Davis, S.P.; Monestier, M.; Cui, L.; Hien, T.T.; Day, N.P.; Ho, M. Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am. J. Pathol. 2012, 180, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Mathur, S.; Wang, Y.; Bateman, R.M.; Walley, K.R. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc. Res. 2006, 72, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, X.; Bao, H.; Mi, S.; Cai, W.; Yan, H.; Wang, Q.; Wang, Z.; Yan, J.; Fan, G.C.; et al. Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PLoS ONE 2012, 7, e40763. [Google Scholar] [CrossRef]
- Mathur, S.; Walley, K.R.; Wang, Y.; Indrambarya, T.; Boyd, J.H. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ. J. 2011, 75, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, N.; Shishido, T.; Takeishi, Y.; Kubota, I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 2004, 110, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, H.; Graveline, A.R.; Buys, E.S.; Schmidt, U.; Bloch, K.D.; Rosenzweig, A.; Chao, W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1900–H1909. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ha, T.; Zhang, X.; Liu, L.; Wang, X.; Kelley, J.; Singh, K.; Kao, R.; Gao, X.; Williams, D.; et al. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit. Care Med. 2012, 40, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Tidswell, M.; Tillis, W.; Larosa, S.P.; Lynn, M.; Wittek, A.E.; Kao, R.; Wheeler, J.; Gogate, J.; Opal, S.M.; Eritoran Sepsis Study, G. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit. Care Med. 2010, 38, 72–83. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- Monestier, M.; Fasy, T.M.; Losman, M.J.; Novick, K.E.; Muller, S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol. Immunol. 1993, 30, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef] [PubMed]
- Sur Chowdhury, C.; Giaglis, S.; Walker, U.A.; Buser, A.; Hahn, S.; Hasler, P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: Analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 2014, 16, R122. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Bromberg, R.; Deane, K.D.; Lahey, L.J.; Derber, L.A.; Chandra, P.E.; Edison, J.D.; Gilliland, W.R.; Tibshirani, R.J.; Norris, J.M.; et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 2012, 7, e35296. [Google Scholar] [CrossRef]
- Costa, O.; Monier, J.C. Antihistone antibodies detected by ELISA and immunoblotting in systemic lupus erythematosus and rheumatoid arthritis. J. Rheumatol. 1986, 13, 722–725. [Google Scholar]
- Bruschi, M.; Petretto, A.; Bertelli, R.; Galetti, M.; Bonanni, A.; Pratesi, F.; Migliorini, P.; Candiano, G.; Vaglio, A.; Ghiggeri, G.M. Post-translational modified proteins are biomarkers of autoimmune-processes: NETosis and the inflammatory-autoimmunity connection. Clin. Chim. Acta 2017, 464, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, S.S.; Ruan, X.; Hoffman, M.K.; Zettel, K.R.; Tracy, A.P.; Schroeder, L.M.; Cai, C.; Hoffman, R.A.; Scott, M.J.; Pape, H.C.; et al. Selective roles for toll-like receptors 2, 4, and 9 in systemic inflammation and immune dysfunction following peripheral tissue injury. J. Trauma. Acute Care Surg. 2013, 74, 1454–1461. [Google Scholar] [CrossRef]
- Fattahi, F.; Grailer, J.J.; Parlett, M.; Lu, H.; Malan, E.A.; Abe, E.; Russell, M.W.; Frydrych, L.M.; Delano, M.J.; Zetoune, F.S.; et al. Requirement of Complement C6 for Intact Innate Immune Responses in Mice. J. Immunol. 2020, 205, 251–260. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loaiza, R.; Fattahi, F.; Kalbitz, M.; Grailer, J.J.; Russell, M.W.; Jalife, J.; Valdivia, H.H.; Zetoune, F.S.; Ward, P.A. The Impact of Extracellular Histones and Absence of Toll-like Receptors on Cardiac Functional and Electrical Disturbances in Mouse Hearts. Int. J. Mol. Sci. 2024, 25, 8653. https://doi.org/10.3390/ijms25168653
Loaiza R, Fattahi F, Kalbitz M, Grailer JJ, Russell MW, Jalife J, Valdivia HH, Zetoune FS, Ward PA. The Impact of Extracellular Histones and Absence of Toll-like Receptors on Cardiac Functional and Electrical Disturbances in Mouse Hearts. International Journal of Molecular Sciences. 2024; 25(16):8653. https://doi.org/10.3390/ijms25168653
Chicago/Turabian StyleLoaiza, Randall, Fatemeh Fattahi, Miriam Kalbitz, Jamison J. Grailer, Mark W. Russell, Jose Jalife, Hector H. Valdivia, Firas S. Zetoune, and Peter A. Ward. 2024. "The Impact of Extracellular Histones and Absence of Toll-like Receptors on Cardiac Functional and Electrical Disturbances in Mouse Hearts" International Journal of Molecular Sciences 25, no. 16: 8653. https://doi.org/10.3390/ijms25168653




