Omega-3 Polyunsaturated Fatty Acids in Depression
Abstract
1. Introduction
2. Omega-3 Polyunsaturated Fatty Acids
| Omega-3 Fatty Acid Name | Molecular Formula | Chemical Structure | Ref. |
|---|---|---|---|
| Alpha-linolenic acid (ALA) | C18H30O2 | 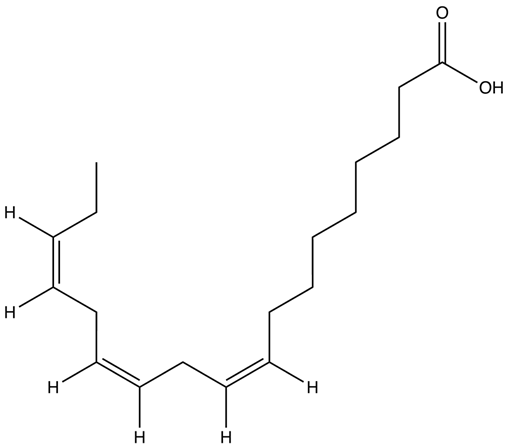 | [42] |
| Eicosapentaenoic acid (EPA) | C20H30O2 |  | [43] |
| Docosahexaenoic acid (DHA) | C22H32O2 |  | [44] |
3. Potential Mechanisms of Action of Omega-3 Polyunsaturated Fatty Acids in Depression
4. Data from Clinical Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Alon, N.; Macrynikola, N.; Jester, D.J.; Keshavan, M.; Reynolds, C.F.; Saxena, S.; Thomas, M.L.; Torous, J.; Jeste, D.V. Social Determinants of Mental Health in Major Depressive Disorder: Umbrella Review of 26 Meta-Analyses and Systematic Reviews. Psychiatry Res. 2024, 335, 115854. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, 5th ed.; Text Revision DSM-5-TD; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Handy, A.; Mangal, R.; Stead, T.S.; Coffee, R.L.; Ganti, L. Prevalence and Impact of Diagnosed and Undiagnosed Depression in the United States. Cureus 2022, 14, e28011. [Google Scholar] [CrossRef]
- Tsugiyama, L.E.; Moraes, R.C.M.; Moraes, Y.A.C.; Francis-Oliveira, J. Promising New Pharmacological Targets for Depression: The Search for Efficacy. Drug Discov. Today 2023, 28, 103804. [Google Scholar] [CrossRef]
- Oliveira-Maia, A.J.; Bobrowska, A.; Constant, E.; Ito, T.; Kambarov, Y.; Luedke, H.; Mulhern-Haughey, S.; von Holt, C. Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022. Adv. Ther. 2024, 41, 34–64. [Google Scholar] [CrossRef]
- Rafeyan, R.; Papakostas, G.I.; Jackson, W.C.; Trivedi, M.H. Inadequate Response to Treatment in Major Depressive Disorder: Augmentation and Adjunctive Strategies. J. Clin. Psychiatry 2020, 81, OT19037BR3. [Google Scholar] [CrossRef]
- González de León, B.; Abt-Sacks, A.; Acosta Artiles, F.J.; del Pino-Sedeño, T.; Ramos-García, V.; Rodríguez Álvarez, C.; Bejarano-Quisoboni, D.; Trujillo-Martín, M.M. Barriers and Facilitating Factors of Adherence to Antidepressant Treatments: An Exploratory Qualitative Study with Patients and Psychiatrists. Int. J. Environ. Res. Public Health 2022, 19, 16788. [Google Scholar] [CrossRef]
- Karrouri, R.; Hammani, Z.; Otheman, Y.; Benjelloun, R. Major Depressive Disorder: Validated Treatments and Future Challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef]
- Hardeveld, F.; Spijker, J.; De Graaf, R.; Nolen, W.A.; Beekman, A.T.F. Prevalence and Predictors of Recurrence of Major Depressive Disorder in the Adult Population. Acta Psychiatr. Scand. 2010, 122, 184–191. [Google Scholar] [CrossRef]
- University of Washington Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). 2021. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 29 July 2024).
- Mills, J.G.; Thomas, S.J.; Larkin, T.A.; Deng, C. Overeating and Food Addiction in Major Depressive Disorder: Links to Peripheral Dopamine. Appetite 2020, 148, 104586. [Google Scholar] [CrossRef]
- Lachance, L.; Ramsey, D. Food, Mood, and Brain Health: Implications for the Modern Clinician. Mo. Med. 2015, 112, 111–115. [Google Scholar]
- Micek, A.; Jurek, J.; Owczarek, M.; Guerrera, I.; Torrisi, S.A.; Castellano, S.; Grosso, G.; Alshatwi, A.A.; Godos, J. Polyphenol-Rich Beverages and Mental Health Outcomes. Antioxidants 2023, 12, 272. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and Cognitive Health: A Life Course Approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Ekstrand, B.; Scheers, N.; Rasmussen, M.K.; Young, J.F.; Ross, A.B.; Landberg, R. Brain Foods—The Role of Diet in Brain Performance and Health. Nutr. Rev. 2021, 79, 693–708. [Google Scholar] [CrossRef]
- Lefèvre-Arbogast, S.; Thomas, A.; Samieri, C. Dietary Factors and Brain Health. Curr. Opin. Lipidol. 2022, 33, 25–30. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. The Use of Diet for Preventing and Treating Depression in Young Men: Current Evidence and Existing Challenges. Br. J. Nutr. 2024, 131, 214–218. [Google Scholar] [CrossRef]
- Grajek, M.; Krupa-Kotara, K.; Białek-Dratwa, A.; Sobczyk, K.; Grot, M.; Kowalski, O.; Staśkiewicz, W. Nutrition and Mental Health: A Review of Current Knowledge about the Impact of Diet on Mental Health. Front. Nutr. 2022, 9, 943998. [Google Scholar] [CrossRef]
- Morgese, M.G.; Schiavone, S.; Maffione, A.B.; Tucci, P.; Trabace, L. Depressive-like Phenotype Evoked by Lifelong Nutritional Omega-3 Deficiency in Female Rats: Crosstalk among Kynurenine, Toll-like Receptors and Amyloid Beta Oligomers. Brain Behav. Immun. 2020, 87, 444–454. [Google Scholar] [CrossRef]
- Hennebelle, M.; Balasse, L.; Latour, A.; Champeil-Potokar, G.; Denis, S.; Lavialle, M.; Gisquet-Verrier, P.; Denis, I.; Vancassel, S. Influence of Omega-3 Fatty Acid Status on the Way Rats Adapt to Chronic Restraint Stress. PLoS ONE 2012, 7, e42142. [Google Scholar] [CrossRef]
- McGrath-Hanna, N.K.; Greene, D.M.; Tavernier, R.J.; Bult-Ito, A. Diet and Mental Health in the Arctic: Is Diet an Important Risk Factor for Mental Health in Circumpolar Peoples? A Review. Int. J. Circumpolar Health 2003, 62, 228–241. [Google Scholar] [CrossRef]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B.W.J.H. Bidirectional Longitudinal Associations of Omega-3 Polyunsaturated Fatty Acid Plasma Levels with Depressive Disorders. J. Psychiatr. Res. 2020, 124, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Oriolo, G.; Kemper, J.; Begarie, D.; Dexpert, S.; Sauvant, J.; Leboyer, M.; Aouizerate, B.; Martin-Santos, R.; et al. Low Omega-3 Polyunsaturated Fatty Acids Predict Reduced Response to Standard Antidepressants in Patients with Major Depressive Disorder. Depress. Anxiety 2022, 39, 407–418. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Hahn, C.G.; Jandacek, R.; Rider, T.; Tso, P.; Stanford, K.E.; Richtand, N.M. Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biol. Psychiatry 2007, 62, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Pan, D.; Xu, D.; Lu, Y.; Yin, S.; Wang, S.; Xia, H.; Liao, W.; Sun, G. Effect of High Ratio of N-6/n-3 PUFAs on Depression: A Meta-Analysis of Prospective Studies. Front. Nutr. 2022, 9, 889576. [Google Scholar] [CrossRef] [PubMed]
- da Silva Sabião, T.; de Oliveira, F.C.; Bressan, J.; Pimenta, A.M.; Hermsdorff, H.H.M.; de Oliveira, F.L.P.; de Deus Mendonça, R.; Carraro, J.C.C. Fatty Acid Intake and Prevalence of Depression among Brazilian Graduates and Postgraduates (CUME Study). J. Affect. Disord. 2024, 346, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.B.; Lawson, S.; Sanigorski, A.; Sinclair, A.J. Arachidonic Acid to Eicosapentaenoic Acid Ratio in Blood Correlates Positively with Clinical Symptoms of Depression. Lipids 1996, 31, S157–S161. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, S.R.; Christophe, A.B.; Maes, M. In Humans, the Seasonal Variation in Poly-Unsaturated Fatty Acids Is Related to the Seasonal Variation in Violent Suicide and Serotonergic Markers of Violent Suicide. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 13–18. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, K.P.; Cribb, L.; Firth, J.; Karmacoska, D.; Mischoulon, D.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; Oliver, G.; et al. EPA and DHA as Markers of Nutraceutical Treatment Response in Major Depressive Disorder. Eur. J. Nutr. 2020, 59, 2439–2447. [Google Scholar] [CrossRef]
- Antao, H.S.; Sacadura-Leite, E.; Bandarra, N.M.; Figueira, M.L. Omega-3 Index as Risk Factor in Psychiatric Diseases: A Narrative Review. Front. Psychiatry 2023, 14, 1200403. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Conjugated Fatty Acids in Food and Their Health Benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Kurt, E.; LBassi, T.; Sa, L.; Xie, D. Biotechnological Production of Omega-3 Fatty Acids: Current Status and Future Perspectives. Front. Microbiol. 2023, 14, 1280296. [Google Scholar] [CrossRef] [PubMed]
- Lupette, J.; Benning, C. Human Health Benefits of Very-Long-Chain Polyunsaturated Fatty Acids from Microalgae. Biochimie 2020, 178, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, M. Essential Fatty Acids, DHA Human Brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Omega-3 Fatty Acids. In Encyclopedia of Dietary Supplements; Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D., Eds.; Informa Healthcare: London, UK; New York, NY, USA, 2010; pp. 577–586. [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5280934, Linolenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Linolenic-Acid (accessed on 9 February 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 446284, Icosapent. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Icosapent (accessed on 9 February 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 445580, Doconexent. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Doconexent (accessed on 9 February 2024).
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The Review of Alpha-Linolenic Acid: Sources, Metabolism, and Pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- USDA. USDA National Nutrient Database for Standard Reference, Release 28; Version Current: September 2015, Slightly Revised May 2016; USDA: Washington, DC, USA, 2015.
- Chang, J.P.C.; Su, K.P. Nutritional Neuroscience as Mainstream of Psychiatry: The Evidence-Based Treatment Guidelines for Using Omega-3 Fatty Acids as a New Treatment for Psychiatric Disorders in Children and Adolescents. Clin. Psychopharmacol. Neurosci. 2020, 18, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Simopoulous, A.P. Summary of the NATO Advanced Research Workshop on Dietary Ω3 and Ω6 Fatty Acids: Biological Effects and Nutritional Essentiality. J. Nutr. 1989, 119, 521–528. [Google Scholar] [CrossRef] [PubMed]
- SCF (Scientific Committee for Food) Nutrient and Energy Intakes for the European Community. Reports of the Scientific Committee for Food (Thirty-First Series); European Commission: Luxembourg, 1993. [Google Scholar]
- Cunnane, S.; Drevon, C.A.; Harris, B.; Sinclair, A. Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. ISSFAL Newsl. 2004, 11, 12–25. [Google Scholar]
- Koletzko, B.; Cetin, I.; Thomas Brenna, J.; Alvino, G.; von Berlepsch, J.; Biesalski, H.K.; Clandinin, T.; Debertin, H.; Decsi, T.; Demmelmair, H.; et al. Dietary Fat Intakes for Pregnant and Lactating Women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Labelling Reference Intake Values for N-3 and n-6 Polyunsaturated Fatty Acids. EFSA J. 2009, 1176, 1–11. [Google Scholar] [CrossRef]
- Nudler, E.; Gottesman, M.E. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation, 28 January–1 February 2002; WHO Technical Report Series 916; WHO: Geneva, Switzerland, 2002; pp. 1–149. [Google Scholar]
- FAO Joint. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The Roles of Long-Chain Polyunsaturated Fatty Acids in Pregnancy, Lactation and Infancy: Review of Current Knowledge and Consensus Recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation. 10 Global Nutritional Recommendations to Improve Digestive Health. Available online: http://www.worldgastroenterology.org/assets/downloads/pdf/wdhd/2008/events/wdhd08_cartel_10_global_nutrition.pdf (accessed on 3 August 2008).
- Kamran, M.; Bibi, F.; Rehman, A.U.; Morris, D.W. Major Depressive Disorder: Existing Hypotheses about Pathophysiological Mechanisms and New Genetic Findings. Genes 2022, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Chakrapani, S.; Eskander, N.; De Los Santos, L.A.; Omisore, B.A.; Mostafa, J.A. Neuroplasticity and the Biological Role of Brain Derived Neurotrophic Factor in the Pathophysiology and Management of Depression. Cureus 2020, 12, e11396. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiong, J.Y.; Chai, Y.Q.; Huang, L.; Tang, Z.Y.; Zhang, X.F.; Liu, B.; Zhang, J.T. Possible Antidepressant Mechanisms of Omega-3 Polyunsaturated Fatty Acids Acting on the Central Nervous System. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids Protect against Inflammation through Production of LOX and CYP450 Lipid Mediators: Relevance for Major Depression and for Human Hippocampal Neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Pothineni, N.V.; Singhal, M.; Paydak, H.; Saldeen, T.; Mehta, J.L. Fish, Fish Oils and Cardioprotection: Promise or Fish Tale? Int. J. Mol. Sci. 2018, 19, 3703. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective Mechanism of Omega-3 Polyunsaturated Fatty Acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Fukuda, D.; Aihara, K.I.; Akaike, M.; Shimabukuro, M.; Sata, M. N-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Kendall, A.C.; Dennis, E.A.; Nicolaou, A. Targeted Lipidomic Strategies for Oxygenated Metabolites of Polyunsaturated Fatty Acids. Biochim. Biophys. Acta 2015, 1851, 456–468. [Google Scholar] [CrossRef]
- Engels, F.; Willems, H.; Nijkamp, F.P. Cyclooxygenase-Catalyzed Formation of 9-Hydroxylinoleic Acid by Guinea Pig Alveolar Macrophages under Non-Stimulated Conditions. FEBS Lett. 1986, 209, 249–253. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment That Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Lagarde, M.; Liu, M.; Véricel, E.; Calzada, C.; Chen, P.; Driss, F.; Guichardant, M. Docosahexaenoic Acid, Protectin Synthesis: Relevance against Atherothrombogenesis. Proc. Nutr. Soc. 2014, 73, 186–189. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Prior, P.L.; Galduróz, J.C.F. (N-3) Fatty Acids: Molecular Role and Clinical Uses in Psychiatric Disorders. Adv. Nutr. 2012, 3, 257–265. [Google Scholar] [CrossRef]
- Kuperstein, F.; Eilam, R.; Yavin, E. Altered Expression of Key Dopaminergic Regulatory Proteins in the Postnatal Brain Following Perinatal N-3 Fatty Acid Dietary Deficiency. J. Neurochem. 2008, 106, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-Effects on Glucocorticoid Receptor Function: Relevance to Glucocorticoid Resistance and the Pathophysiology and Treatment of Major Depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Escoll, P.; Ranz, I.; Muñoz-Antón, N.; Van-Den-Rym, A.; Alvarez-Mon, M.; Martínez-Alonso, C.; Sanz, E.; De-La-Hera, A. Sustained Interleukin-1β Exposure Modulates Multiple Steps in Glucocorticoid Receptor Signaling, Promoting Split-Resistance to the Transactivation of Prominent Anti-Inflammatory Genes by Glucocorticoids. Mediat. Inflamm. 2015, 2015, 347965. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Ruhé, H.G.; Assies, J.; Lok, A.; Koeter, M.W.J.; Visser, I.; Bockting, C.L.H.; Schene, A.H. Relationship between the Hypothalamic-Pituitary-Adrenal-Axis and Fatty Acid Metabolism in Recurrent Depression. Psychoneuroendocrinology 2013, 38, 1607–1617. [Google Scholar] [CrossRef]
- Delarue, J.; Matzinger, O.; Binnert, C.; Schneiter, P.; Chioléro, R.; Tappy, L. Fish Oil Prevents the Adrenal Activation Elicited by Mental Stress in Healthy Men. Diabetes Metab. 2003, 29, 289–295. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Bissette, G.; Umhau, J.C.; George, D.T. Omega-3 Status and Cerebrospinal Fluid Corticotrophin Releasing Hormone in Perpetrators of Domestic Violence. Biol. Psychiatry 2004, 56, 895–897. [Google Scholar] [CrossRef]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B.W.J.H. Omega-3 Polyunsaturated Fatty Acid Levels and Dysregulations in Biological Stress Systems. Psychoneuroendocrinology 2018, 97, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.M.; Pennington, E.R.; Green, W.D.; Beck, M.A.; Brown, D.A.; Shaikh, S.R. Mechanisms Bywhich Dietary Fatty Acids Regulate Mitochondrial Structure-Function in Health and Disease. Adv. Nutr. 2018, 9, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Maciel, A.L.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; dos Santos, T.R.; Darabas, A.C.; Parzianello, M.; Matos, D.; Abatti, M.; et al. ω-3 and Folic Acid Act against Depressive-like Behavior and Oxidative Damage in the Brain of Rats Subjected to Early- or Late-Life Stress. Nutrition 2018, 53, 120–133. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 Fatty Acids Supplementation and Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2016, 15, 534–542. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Cutuli, D.; Landolfo, E.; Nobili, A.; De Bartolo, P.; Sacchetti, S.; Chirico, D.; Marini, F.; Pieroni, L.; Ronci, M.; D’Amelio, M.; et al. Behavioral, Neuromorphological, and Neurobiochemical Effects Induced by Omega-3 Fatty Acids Following Basal Forebrain Cholinergic Depletion in Aged Mice. Alzheimer’s Res. Ther. 2020, 12, 150. [Google Scholar] [CrossRef]
- Yu, J.Z.; Wang, J.; Sheridan, S.D.; Perlis, R.H.; Rasenick, M.M. N-3 Polyunsaturated Fatty Acids Promote Astrocyte Differentiation and Neurotrophin Production Independent of CAMP in Patient-Derived Neural Stem Cells. Mol. Psychiatry 2021, 26, 4605–4615. [Google Scholar] [CrossRef]
- Shirooie, S.; Nabavi, S.F.; Dehpour, A.R.; Belwal, T.; Habtemariam, S.; Argüelles, S.; Sureda, A.; Daglia, M.; Tomczyk, M.; Sobarzo-Sanchez, E.; et al. Targeting MTORs by Omega-3 Fatty Acids: A Possible Novel Therapeutic Strategy for Neurodegeneration? Pharmacol. Res. 2018, 135, 37–48. [Google Scholar] [CrossRef]
- Sidhu, V.K.; Huang, B.X.; Desai, A.; Kevala, K.; Kim, H.Y. Role of DHA in Aging-Related Changes in Mouse Brain Synaptic Plasma Membrane Proteome. Neurobiol. Aging 2016, 41, 73–85. [Google Scholar] [CrossRef]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 Polyunsaturated Fatty Acids and the Treatment of Depression. Crit. Rev. Food Sci. Nutr. 2017, 57, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P. Biological Mechanism of Antidepressant Effect of Omega-3 Fatty Acids: How Does Fish Oil Act as a “Mind-Body Interface”? Neurosignals 2009, 17, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic Acid (DHA): An Ancient Nutrient for the Modern Human Brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of Action of Docosahexaenoic Acid in the Nervous System. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Wassall, S.R.; Stillwell, W. Polyunsaturated Fatty Acid-Cholesterol Interactions: Domain Formation in Membranes. Biochim. Biophys. Acta 2009, 1788, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chalon, S. Omega-3 Fatty Acids and Monoamine Neurotransmission. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Huang, S.Y.; Chiu, C.C.; Shen, W.W. Omega-3 Fatty Acids in Major Depressive Disorder: A Preliminary Double-Blind, Placebo-Controlled Trial. Eur. Neuropsychopharmacol. 2003, 13, 267–271. [Google Scholar] [CrossRef]
- Søgaard, R.; Werge, T.M.; Bertelsen, C.; Lundbye, C.; Madsen, K.L.; Nielsen, C.H.; Lundbæk, J.A. GABA A Receptor Function Is Regulated by Lipid Bilayer Elasticity. Biochemistry 2006, 45, 13118–13129. [Google Scholar] [CrossRef]
- Stachowicz, K. The Role of Polyunsaturated Fatty Acids in Neuronal Signaling in Depression and Cognitive Processes. Arch. Biochem. Biophys. 2023, 737, 109555. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; De Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA Ethanolamides DHEA and EPEA Induce Autophagy through PPARγ Activation in MCF-7 Breast Cancer Cells. J. Cell. Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Ross, S.; Li, X.-C. Molecular Targets of Cannabinoids Associated with Depression. Curr. Med. Chem. 2021, 29, 1827–1850. [Google Scholar] [CrossRef] [PubMed]
- Kotlega, D.; Zembron-Lacny, A.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. The Association of Free Fatty Acids and Eicosanoids with the Severity of Depressive Symptoms in Stroke Patients. Int. J. Mol. Sci. 2020, 21, 5220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hou, B.; Xu, Y.; Zeng, S.; Luo, X.; Zhang, B. Association between Eicosapentaenoic Acid Consumption and the Risk of Depressive Symptoms in US Adults: Analyses from NHANES 2005–2018. J. Affect. Disord. 2024, 354, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, Y.; Lin, S.-Q.; Liu, T.; Liu, C.-A.; Ruan, G.-T.; Ge, Y.-Z.; Xie, H.-L.; Song, M.-M.; Shi, J.-Y.; et al. The Relationship between Different Fatty Acids Intake and the Depressive Symptoms: A Population-Based Study. J. Affect. Disord. 2024, 357, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.H.O.; Jimoh, O.F.; Biswas, P.; O’Brien, A.; Hanson, S.; Abdelhamid, A.S.; Fox, C.; Hooper, L. Omega-3 and Polyunsaturated Fat for Prevention of Depression and Anxiety Symptoms: Systematic Review and Meta-Analysis of Randomised Trials. Br. J. Psychiatry 2021, 218, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Je, Y. Fish Consumption and Depression in Korean Population: The Korea National Health and Nutrition Examination Survey, 2013–2021. J. Affect. Disord. 2024, 359, 255–261. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, Y.; Je, Y. Fish Consumption and Risk of Depression: Epidemiological Evidence from Prospective Studies. Asia Pac. Psychiatry 2018, 10, e12335. [Google Scholar] [CrossRef]
- Pipingas, A.; Cockerell, R.; Grima, N.; Sinclair, A.; Stough, C.; Scholey, A.; Myers, S.; Croft, K.; Sali, A.; Pase, M.P. Randomized Controlled Trial Examining the Effects of Fish Oil and Multivitamin Supplementation on the Incorporation of N-3 and n-6 Fatty Acids into Red Blood Cells. Nutrients 2014, 6, 1956–1970. [Google Scholar] [CrossRef]
- Okereke, O.I.; Vyas, C.M.; Mischoulon, D.; Chang, G.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; Lee, I.M.; et al. Effect of Long-Term Supplementation with Marine Omega-3 Fatty Acids vs. Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA 2021, 326, 2385–2394. [Google Scholar] [CrossRef]
- Vyas, C.M.; Mischoulon, D.; Chang, G.; Cook, N.R.; Weinberg, A.; Copeland, T.; Kang, J.H.; Bubes, V.; Friedenberg, G.; LeBoff, M.S.; et al. Effects of Vitamin D3 and Marine Omega-3 Fatty Acids Supplementation on Indicated and Selective Prevention of Depression in Older Adults: Results From the Clinical Center Sub-Cohort of the VITamin D and OmegA-3 TriaL (VITAL). J. Clin. Psychiatry 2023, 84, 22m14629. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, F.; Sun, Y.; Wang, Z.; Li, Q.; Wang, H.; Lu, Y. N-3 Polyunsaturated Fatty Acids in Elderly with Mild Cognitive Impairment: A Systemic Review and Meta-Analysis. J. Alzheimer’s Dis. 2024, 99, S81–S95. [Google Scholar] [CrossRef] [PubMed]
- Mengelberg, A.; Leathem, J.; Podd, J.; Hill, S.; Conlon, C. The Effects of Docosahexaenoic Acid Supplementation on Cognition and Well-Being in Mild Cognitive Impairment: A 12-Month Randomised Controlled Trial. Int. J. Geriatr. Psychiatry 2022, 37, 10.1002/gps.5707. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.A.; Kiecolt-Glaser, J.K.; Malarkey, W.B.; Belury, M.A. Omega-3 Fatty Acids Reduce Depressive Symptoms Only Among the Socially Stressed: A Corollary of the Social Signal Transduction Theory of Depression. Health Psychol. 2023, 42, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Bharti, V.; Bhardwaj, A.; Hood, K.; Elias, D.A.; Metcalfe, A.W.S.; Kim, J.S. A Systematic Review and Meta-Analysis of Lipid Metabolomic Signatures of Major Depressive Disorder. J. Psychiatr. Res. 2021, 139, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Noguchi, H.; Hamazaki, K.; Sekiguchi, M.; Kinoshita, T.; Katsumata, N.; Narisawa, T.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Association between blood polyunsaturated fatty acid levels and depressive symptoms in breast cancer survivors. Prostaglandins Leukot. Essent. Fat. Acids 2018, 139, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Feng, J.S.; Yang, Z.; Huang, Q.T.; Lin, J.D.; Yang, B.; Su, K.P.; Pan, J.Y. High-Dose Omega-3 Polyunsaturated Fatty Acid Supplementation Might Be More Superior than Low-Dose for Major Depressive Disorder in Early Therapy Period: A Network Meta-Analysis. BMC Psychiatry 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.G.; Bo, A.; Wu, S.J.; Gai, Q.Y.; Chi, I. Omega-3 Polyunsaturated Fatty Acids and Reduction of Depressive Symptoms in Older Adults: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2018, 241, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of Omega-3 Highly Unsaturated Fatty Acids in the Treatment of Depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef]
- Guu, T.W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.H.; Nguyen, N.T.K.; Huang, S.Y. New Insights into Depressive Disorder with Respect to Low-Grade Inflammation and Fish Oil Intake. J. Oleo Sci. 2021, 70, 1539–1550. [Google Scholar] [CrossRef]
- Freund Levi, Y.; Vedin, I.; Cederholm, T.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Hjorth, E.; Schultzberg, M.; Vessby, B.; Wahlund, L.O.; et al. Transfer of Omega-3 Fatty Acids across the Blood-Brain Barrier after Dietary Supplementation with a Docosahexaenoic Acid-Rich Omega-3 Fatty Acid Preparation in Patients with Alzheimer’s Disease: The OmegAD Study. J. Intern. Med. 2014, 275, 428–436. [Google Scholar] [CrossRef]
- McNamara, R.K. Evaluation of Docosahexaenoic Acid Deficiency as a Preventable Risk Factor for Recurrent Affective Disorders: Current Status, Future Directions, and Dietary Recommendations. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Kelaiditis, C.F.; Gibson, E.L.; Dyall, S.C. Effects of Long-Chain Omega-3 Polyunsaturated Fatty Acids on Reducing Anxiety and/or Depression in Adults; A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Prostaglandins Leukot. Essent. Fat. Acids 2023, 192, 102572. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Álvarez-Pérez, J.; Toledo, E.; Salas-Salvadó, J.; Ortega-Azorín, C.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; Romaguera, D.; Pérez-López, J.; et al. Seafood Consumption, Omega-3 Fatty Acids Intake, and Life-Time Prevalence of Depression in the PREDIMED-plus Trial. Nutrients 2018, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; von der Haar, A.; Baalmann, A.K.; Wellbrock, M.; Heise, T.L.; Rach, S. Effects of N-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-analysis. Nutrients 2021, 13, 1070. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Ting, B.; Chen, D.T.L.; Hsu, W.T.; Lin, S.C.; Kuo, C.Y.; Wang, M.F. Omega-3 Fatty Acids for Depression in the Elderly and Patients with Dementia: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 536. [Google Scholar] [CrossRef]
- Mischoulon, D.; Best-Popescu, C.; Laposata, M.; Merens, W.; Murakami, J.L.; Wu, S.L.; Papakostas, G.I.; Dording, C.M.; Sonawalla, S.B.; Nierenberg, A.A.; et al. A Double-Blind Dose-Finding Pilot Study of Docosahexaenoic Acid (DHA) for Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008, 18, 639–645. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A Randomised Cross-over Trial in Healthy Adults Indicating Improved Absorption of Omega-3 Fatty Acids by Pre-Emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Redmon, J.B.; Wimmergren, N.; Donadio, J.V.; Bibus, D.M. Enhanced Absorption of N-3 Fatty Acids from Emulsified Compared with Encapsulated Fish Oil. J. Am. Diet. Assoc. 2009, 109, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Tayama, J.; Ogawa, S.; Nakaya, N.; Sone, T.; Hamaguchi, T.; Takeoka, A.; Hamazaki, K.; Okamura, H.; Yajima, J.; Kobayashi, M.; et al. Omega-3 Polyunsaturated Fatty Acids and Psychological Intervention for Workers with Mild to Moderate Depression: A Double-Blind Randomized Controlled Trial. J. Affect. Disord. 2019, 245, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Voyias, P.D.; Sallis, H.M.; Dawson, S.; Ness, A.R.; Churchill, R.; Perry, R. Omega-3 Fatty Acids for Depression in Adults. Cochrane Database Syst. Rev. 2021, 11, CD004692. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Mann, S.K.; Malhi, N.K.; Marwaha, R. The Role of Omega-3 Fatty Acids in the Treatment of Depression in Children and Adolescents: A Literature Review. Cureus 2023, 15, e44584. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-Analysis and Meta-Regression of Omega-3 Polyunsaturated Fatty Acid Supplementation for Major Depressive Disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Sakuma, K.; Okuya, M.; Ikeda, M.; Iwata, N. Omega-3 Fatty Acids for Treating Residual Depressive Symptoms in Adult Patients with Bipolar Disorder: A Systematic Review and Meta-Analysis of Double-Blind Randomized, Placebo-Controlled Trials. Bipolar Disord. 2021, 23, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Chambergo-Michilot, D.; Brañez-Condorena, A.; Falvy-Bockos, I.; Pacheco-Mendoza, J.; Benites-Zapata, V.A. Efficacy of Omega-3 Supplementation on Sertraline Continuous Therapy to Reduce Depression or Anxiety Symptoms: A Systematic Review and Meta-Analysis. Psychiatry Res. 2021, 296, 113652. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.; Cardoos, A.; Walker, R.; Mischoulon, D. Inflammation as a Predictive Biomarker for Response to Omega-3 Fatty Acids in Major Depressive Disorder: A Proof-of-Concept Study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Su, K.P. Personalized Medicine with Omega-3 Fatty Acids for Depression in Children and Pregnant Women and Depression Associated with Inflammation. J. Clin. Psychiatry 2015, 76, e1476–e1477. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Chang, J.P.C.; Chang, H.C.; Pariante, C.M. Omega-3 Fatty Acids in the Prevention of Interferon- Alpha-Induced Depression: Results from a Randomized, Controlled Trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chen, W.J.; Chang, J.P.C.; Guu, T.W.; Hsin, M.C.; Huang, C.K.; Mischoulon, D.; Capuron, L.; Su, K.P. Personalized Medicine of Omega-3 Fatty Acids in Depression Treatment in Obese and Metabolically Dysregulated Patients. J. Pers. Med. 2023, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, S.A.; Mostafavi, S.A.; Akhondzadeh, S.; Mohammadi, M.R.; Hosseini, S.; Eshraghian, M.R.; Chamari, M. Omega-3 Supplementation Effects on Body Weight and Depression among Dieter Women with Co-Morbidity of Depression and Obesity Compared with the Placebo: A Randomized Clinical Trial. Clin. Nutr. ESPEN 2018, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician Guidelines for the Treatment of Psychiatric Disorders with Nutraceuticals and Phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, L.; Czarny, P.L.; Ziółkowska, S.; Białek, K.; Szemraj, J.; Gałecki, P.; Su, K.P.; Śliwiński, T. How Fish Consumption Prevents the Development of Major Depressive Disorder? A Comprehensive Review of the Interplay between n-3 PUFAs, LTP and BDNF. Prog. Lipid Res. 2023, 92, 101254. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Freed, R.D.; Alonso, C.M.; Senger, S.; Stadterman, J.; Davison, B.A.; Klein, R.G. A Double-Blind Placebo-Controlled Trial of Omega-3 Fatty Acids as a Monotherapy for Adolescent Depression. J. Clin. Psychiatry 2018, 79, 17m11596. [Google Scholar] [CrossRef] [PubMed]
- Jahangard, L.; Sadeghi, A.; Ahmadpanah, M.; Holsboer-Trachsler, E.; Sadeghi Bahmani, D.; Haghighi, M.; Brand, S. Influence of Adjuvant Omega-3-Polyunsaturated Fatty Acids on Depression, Sleep, and Emotion Regulation among Outpatients with Major Depressive Disorders—Results from a Double-Blind, Randomized and Placebo-Controlled Clinical Trial. J. Psychiatr. Res. 2018, 107, 48–56. [Google Scholar] [CrossRef]
- Jiang, W.; Whellan, D.J.; Adams, K.F.; Babyak, M.A.; Boyle, S.H.; Wilson, J.L.; Patel, C.B.; Rogers, J.G.; Harris, W.S.; O’Connor, C.M. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. 2018, 6, 833–843. [Google Scholar] [CrossRef]
- Yang, B.; Lin, L.; Bazinet, R.P.; Chien, Y.C.; Chang, J.P.C.; Satyanarayanan, S.K.; Su, H.; Su, K.P. Clinical Efficacy and Biological Regulations of ω-3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 215–224. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Harris, W.S. A Randomized Placebo-Controlled Trial of Omega-3 and Sertraline in Depressed Patients with or at Risk for Coronary Heart Disease. J. Clin. Psychiatry 2019, 80, 19m12742. [Google Scholar] [CrossRef] [PubMed]
- Katrenčíková, B.; Vaváková, M.; Waczulíková, I.; Oravec, S.; Garaiova, I.; Nagyová, Z.; Hlaváčová, N.; Ďuračková, Z.; Trebatická, J. Lipid Profile, Lipoprotein Subfractions, and Fluidity of Membranes in Children and Adolescents with Depressive Disorder: Effect of Omega-3 Fatty Acids in a Double-blind Randomized Controlled Study. Biomolecules 2020, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Strawn, J.R.; Tallman, M.J.; Welge, J.A.; Patino, L.R.; Blom, T.J.; Delbello, M.P. Effects of Fish Oil Monotherapy on Depression and Prefrontal Neurochemistry in Adolescents at High Risk for Bipolar i Disorder: A 12-Week Placebo-Controlled Proton Magnetic Resonance Spectroscopy Trial. J. Child. Adolesc. Psychopharmacol. 2020, 30, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.C.; Chang, S.S.; Yang, H.T.; Chen, H.T.; Chien, Y.C.; Yang, B.; Su, H.; Su, K.P. Omega-3 Polyunsaturated Fatty Acids in Cardiovascular Diseases Comorbid Major Depressive Disorder—Results from a Randomized Controlled Trial. Brain Behav. Immun. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Omega-3 Fatty-Acids Modulate Symptoms of Depressive Disorder, Serum Levels of Omega-3 Fatty Acids and Omega-6/Omega-3 Ratio in Children. A Randomized, Double-Blind and Controlled Trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Crowe, T.M.; Wong, A.; et al. Omega-3 Fatty Acids for Major Depressive Disorder With High Inflammation: A Randomized Dose-Finding Clinical Trial. J. Clin. Psychiatry 2022, 83, 21m14074. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Liu, M.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Felger, J.C.; Ziegler, T.R.; Fava, M.; Mischoulon, D.; Rapaport, M.H. Clinical Response to EPA Supplementation in Patients with Major Depressive Disorder Is Associated with Higher Plasma Concentrations of Pro-Resolving Lipid Mediators. Neuropsychopharmacology 2023, 48, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Piperoglou, M.; Hopwood, M.; Norman, T.R. Adjunctive Docosahexaenoic Acid in Residual Symptoms of Depression and Anxiety. J. Clin. Psychopharmacol. 2023, 43, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Rice, S.; Davey, C.G.; Quinn, A.L.; Hermens, D.F.; Zmicerevska, N.; Nichles, A.; Hickie, I.; Incerti, L.; Weller, A.; et al. The Addition of Fish Oil to Cognitive Behavioral Case Management for Youth Depression: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial. Biol. Psychiatry 2024, 95, 426–433. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Hu, X.; Zhang, Y.; Wang, D.; Gao, Y.; Wang, J.; Wang, Q.; Song, C.; Huang, S.; et al. Omega-3 Supplementation Improves Depressive Symptoms, Cognitive Function and Niacin Skin Flushing Response in Adolescent Depression: A Randomized Controlled Clinical Trial. J. Affect. Disord. 2024, 345, 394–403. [Google Scholar] [CrossRef]
- Suneson, K.; Söderberg Veibäck, G.; Lindahl, J.; Tjernberg, J.; Ståhl, D.; Ventorp, S.; Ängeby, F.; Lundblad, K.; Wolkowitz, O.M.; Lindqvist, D. Omega-3 Fatty Acids for Inflamed Depression—A Match/Mismatch Study. Brain Behav. Immun. 2024, 118, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, Y.; Fujita, D.; Daimon, A.; Nunode, M.; Sawada, M.; Sano, T.; Ohmichi, M. Possible Prevention of Post-Partum Depression by Intake of Omega-3 Polyunsaturated Fatty Acids and Its Relationship with Interleukin 6. J. Obstet. Gynaecol. Res. 2021, 47, 1371–1379. [Google Scholar] [CrossRef]
- Da Rocha, C.M.M.; Kac, G. High Dietary Ratio of Omega-6 to Omega-3 Polyunsaturated Acids during Pregnancy and Prevalence of Post-Partum Depression. Matern. Child. Nutr. 2012, 8, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Nishi, D.; Su, K.P.; Usuda, K.; Pei-Chen Chang, J.; Chiang, Y.J.; Chen, H.T.; Chien, Y.C.; Guu, T.W.; Okazaki, E.; Hamazaki, K.; et al. The Efficacy of Omega-3 Fatty Acids for Depressive Symptoms among Pregnant Women in Japan and Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial (SYNCHRO; NCT01948596). Psychother. Psychosom. 2019, 88, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Yoshihara, H.; Hoshi, Y.; Hamazaki, K.; Moriguchi, T. Effects of Varied Omega-3 Fatty Acid Supplementation on Postpartum Mental Health and the Association between Prenatal Erythrocyte Omega-3 Fatty Acid Levels and Postpartum Mental Health. Nutrients 2023, 15, 4388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zou, Y.; Li, S.M.; Wang, L.; Sun, Y.H.; Shi, L.; Lu, L.; Bao, Y.P.; Li, S.X. The Efficacy and Safety of Omega-3 Fatty Acids on Depressive Symptoms in Perinatal Women: A Meta-Analysis of Randomized Placebo-Controlled Trials. Transl. Psychiatry 2020, 10, 193. [Google Scholar] [CrossRef]
- Masot, O.; Ochoa Herrera, J.J.; Paraíso Pueyo, E.; Roca, J.; Miranda, J.; Lavedán Santamaría, A. The Impact of Docosahexaenoic Acid on Maternal Mental Health: Scoping Review. Nutr. Hosp. 2023, 40, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Hulkkonen, P.; Kataja, E.L.; Vahlberg, T.; Koivuniemi, E.; Houttu, N.; Pellonperä, O.; Mokkala, K.; Karlsson, H.; Laitinen, K. The Efficacy of Probiotics and/or n-3 Long-Chain Polyunsaturated Fatty Acids Intervention on Maternal Prenatal and Postnatal Depressive and Anxiety Symptoms among Overweight and Obese Women. J. Affect. Disord. 2021, 289, 21–30. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Steijn, K.; Roos, C.; Assies, J.; Bergink, V.; Ruhé, H.G.; Schene, A.H. Omega-3 Fatty Acid Supplementation for Perinatal Depression: A Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r13106. [Google Scholar] [CrossRef]
- Suradom, C.; Suttajit, S.; Oon-arom, A.; Maneeton, B.; Srisurapanont, M. Omega-3 Polyunsaturated Fatty Acid (n-3 PUFA) Supplementation for Prevention and Treatment of Perinatal Depression: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Nord. J. Psychiatry 2021, 75, 239–246. [Google Scholar] [CrossRef]
- Firouzabadi, F.D.; Shab-Bidar, S.; Jayedi, A. The Effects of Omega-3 Polyunsaturated Fatty Acids Supplementation in Pregnancy, Lactation, and Infancy: An Umbrella Review of Meta-Analyses of Randomized Trials. Pharmacol. Res. 2022, 177, 106100. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.M.; dos Santos, L.C. Effect of Antenatal Omega-3 Supplementation on Maternal Depressive Symptoms from Pregnancy to 6 Months Postpartum: A Randomized Double-Blind Placebo-Controlled Trial. Nutr. Neurosci. 2023, 26, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Nishi, D.; Su, K.P.; Usuda, K.; Chang, J.P.C.; Chiang, Y.J.J.; Guu, T.W.; Hamazaki, K.; Nakaya, N.; Sone, T.; Hashimoto, K.; et al. Differences between Japan and Taiwan in the Treatment of Pregnant Women with Depressive Symptoms by Omega-3 Fatty Acids: An Open-Label Pilot Study. Nutr. Neurosci. 2019, 22, 63–71. [Google Scholar] [CrossRef]
- Decandia, D.; Landolfo, E.; Sacchetti, S.; Gelfo, F.; Petrosini, L.; Cutuli, D. N-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review. Nutrients 2022, 14, 1982. [Google Scholar] [CrossRef]
- Iqbal, A.Z.; Wu, S.K.; Zailani, H.; Chiu, W.C.; Liu, W.C.; Su, K.P.; Lee, S. Effects of Omega-3 Polyunsaturated Fatty Acids Intake on Vasomotor Symptoms, Sleep Quality and Depression in Postmenopausal Women: A Systematic Review. Nutrients 2023, 15, 4231. [Google Scholar] [CrossRef]
- Molfino, A.; Gioia, G.; Fanelli, F.R.; Muscaritoli, M. The Role for Dietary Omega-3 Fatty Acids Supplementation in Older Adults. Nutrients 2014, 6, 4058–4073. [Google Scholar] [CrossRef]
- Reily, N.M.; Tang, S.; Negrone, A.; Gan, D.Z.Q.; Sheanoda, V.; Christensen, H. Omega-3 Supplements in the Prevention and Treatment of Youth Depression and Anxiety Symptoms: A Scoping Review. PLoS ONE 2023, 18, e0284057. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol. Assess. 2016, 224, 1–826. [Google Scholar] [CrossRef]
- Tatsuno, I.; Saito, Y.; Kudou, K.; Ootake, J. Long-Term Safety and Efficacy of TAK-085 in Japanese Subjects with Hypertriglyceridemia Undergoing Lifestyle Modification: The Omega-3 Fatty Acids Randomized Long-Term (ORL) Study. J. Clin. Lipidol. 2013, 7, 615–625. [Google Scholar] [CrossRef]
- Chang, C.H.; Tseng, P.T.; Chen, N.Y.; Lin, P.C.; Lin, P.Y.; Chang, J.P.C.; Kuo, F.Y.; Lin, J.; Wu, M.C.; Su, K.P. Safety and Tolerability of Prescription Omega-3 Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prostaglandins Leukot. Essent. Fat. Acids 2018, 129, 1–12. [Google Scholar] [CrossRef]
- Bagge, A.; Schött, U.; Kander, T. High-Dose Omega-3 Fatty Acids Have No Effect on Platelet Aggregation or Coagulation Measured with Static and Flow-Based Aggregation Instruments and Sonoclot; an Observational Study in Healthy Volunteers. Scand. J. Clin. Lab. Investig. 2018, 78, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Sampogna, G.; Toni, C.; Catapano, P.; Della Rocca, B.; Di Vincenzo, M.; Luciano, M.; Fiorillo, A. New Trends in Personalized Treatment of Depression. Curr. Opin. Psychiatry 2024, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Saji Parel, N.; Krishna, P.V.; Gupta, A.; Uthayaseelan, K.; Uthayaseelan, K.; Kadari, M.; Subhan, M.; Kasire, S.P. Depression and Vitamin D: A Peculiar Relationship. Cureus 2022, 14, e24363. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.S.; Foshati, S.; Haddadian-Khouzani, S.; Rouhani, M.H. The Role of Selenium in Depression: A Systematic Review and Meta-Analysis of Human Observational and Interventional Studies. Sci. Rep. 2022, 12, 1045. [Google Scholar] [CrossRef]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of Marine Omega-3 Supplements and Human Health. BioMed. Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef]
- Heller, M.; Gemming, L.; Tung, C.; Grant, R. Oxidation of Fish Oil Supplements in Australia. Int. J. Food Sci. Nutr. 2019, 70, 540–550. [Google Scholar] [CrossRef]
| Food | Measure | g/Measure | |||
|---|---|---|---|---|---|
| ALA | EPA | DHA | |||
| Egg | yolk (dried) | 1 cup | 0.170 | ||
| whole (dried) | 0.169 | ||||
| whole (cooked, hard-boiled) | 0.007 | 0.052 | |||
| Fish | mackerel (salted) | 1.0 piece | 1.295 | 2.372 | |
| salmon | 1.0 filet | 0.583–0.977 | 1.246–1.642 | ||
| herring | 1.300–1.788 | 1.272–1.580 | |||
| mackerel | 0.429–1.006 | 0.615–1.569 | |||
| bluefish | 0.378 | 0.778–0.779 | |||
| trout | 0.161–0.669 | 0.408–0.744 | |||
| sea bass | 0.208 | 0.560–0.562 | |||
| sucker | 0.303 | 0.460 | |||
| pompano | 0.197 | 0.444 | |||
| tilefish | 0.5 filet | 0.258 | 1.100 | ||
| wolffish | 0.468 | 0.482 | |||
| Fish oil | salmon | 1.0 tbsp | 1.771 | 2.480 | |
| sardine | 1.379 | 1.449 | |||
| menhaden | 1.791 | 1.164 | |||
| herring | 0.853 | 0.572 | |||
| cod liver | 0.310 | 0.494 | |||
| Meat | lamb | 3.0 oz | 0.053–0.139 | 0.383–1.088 | |
| beef | 0.087–0.130 | 0.570 | |||
| pork | 0.391 | ||||
| turkey | 1.0 breast | 0.017–0.082 | 0.199 | ||
| chicken | 1.0 cup | 0.022–0.042 | 0.112–0.154 | ||
| Mollusks | 3.0 oz | 0.033–0.300 | 0.173–0.430 | ||
| Nuts | walnuts (black, dried) | 1.0 cup | 3.346 | ||
| mixed nuts (dry roasted, with peanuts and salt) | 2.662 | ||||
| pistachio nuts (dry roasted, with or without salt) | 0.261 | ||||
| pine nuts (dried) | 0.151 | ||||
| peanuts (all types, dry-roasted, without salt) | 0.037 | ||||
| Brazil nuts (dried, unblanched) | 0.024 | ||||
| Oil | flaxseed (cold pressed) | 1.0 tbsp | 7.258 | ||
| flaxseed (contains added sliced flaxseed) | 6.703 | ||||
| canola | 1.279 | ||||
| soy (industrial, refined, for woks and light frying) | 0.940 | ||||
| Seeds | chia seeds (dried) | 1.0 oz | 5.055 | ||
| hemp seed (hulled) | 3.0 tbsp | 2.605 | |||
| beans (navy, mature, raw) | 1.0 cup | 1.119 | |||
| beans (cooked, boiled, with salt) | 0.375 | ||||
| beans (cooked, boiled, without salt) | 0.322 | ||||
| pumpkin and squash seed kernels (dried) | 0.155 | ||||
| pumpkin and squash seed kernels (roasted, with or without salt) | 0.131 | ||||
| sunflower seed kernels (oil roasted, with or without salt) | 0.103 | ||||
| Shrimp | 3.0 oz | 0.093–0.115 | 0.105–0.120 | ||
| Type of Depression | Type of Study | Participants | Intervention | Main Outcomes | Ref. |
|---|---|---|---|---|---|
| Major depressive disorder | Randomized, double-blind, placebo-controlled trial | Adolescents (12–19 years old), 51 participants | Intervention group: Capsules containing EPA + DHA in a 2:1 ratio taken for 10 weeks. Each participant started with an initial dose of 1.2 g/day. Doses were raised in increments of 0.6 g/day every 2 weeks (maximum possible dose of EPA + DHA was 3.6 g/day, i.e., 2.4 g/day + 1.2 g/day, respectively). Control group: Placebo capsules (corn and soybean oils) taken for 10 weeks. | Supplementation of omega-3 fatty acids was not superior to placebo in reducing depression severity as measured by the Children’s Depression Rating Scale-Revised or the Beck Depression Inventory-II. | [147] |
| Major depressive disorder | Randomized, double-blind, placebo-controlled clinical trial | Adults, 50 participants | All participants received sertraline (50–200 mg/day). Intervention group: 1 capsule containing 1000 mg of omega-3-polyunsaturated fatty acids taken for 12 weeks. Control group: 1 capsule containing placebo taken for 12 weeks. | Adjuvant treatment with omega-3 polyunsaturated fatty acids improved symptoms of depression (measured by the Beck Depression Inventory and Montgomery–Asberg Depression Rating Scale), sleep disturbances, anxiety, intolerance of uncertainty, regulation of emotions. | [148] |
| Major depressive disorder and chronic heart failure | Randomized, double-blind, placebo-controlled trial | Adults, 108 participants | Intervention groups: 1st group received 4 capsules/day of EPA/DHA at a dose of 500 mg per capsule (EPA/DHA ratio of 2:1) for 12 weeks; 2nd group received 4 capsules/day of almost pure EPA at a dose of 500 mg per capsule for 12 weeks. Control group: 4 capsules/day of placebo (corn oil) taken for 12 weeks. Patients not tolerating 4 capsules of the product took a reduced dose (1 capsule was the minimum dose which allowed to stay in the study). | Supplementation with EPA + DHA or EPA was effective in increasing levels of omega-3 fatty acids in erythrocytes, and the omega-3 index compared to placebo. However, no between-group differences in depression symptoms were observed when measured by the Hamilton Depression Scale and the Beck Depression Inventory-II. | [149] |
| Depression with overweight or obesity | Randomized, double-blind, controlled trial | Adult women, 45 participants | Intervention group: 6 capsules of omega-3/day (each capsule containing 180 mg of EPA + 120 mg of DHA) taken for 12 weeks. Control group: 6 capsules of placebo/day taken for 12 weeks. | Supplementation of omega-3 fatty acids significantly reduced depression versus placebo when measured by the Beck Depression Inventory. | [144] |
| Mild to moderate depression | Randomized, double-blind, controlled trial | Adults, 90 participants | All participants received psychoeducation. Intervention group: 15 capsules/day containing 300 mg of omega-3 fatty acids (i.e., 558 mg of DHA and 1064 mg of EPA/day) taken for 12 weeks. Control group: 15 capsules/day containing placebo taken for 12 weeks. | A combined intervention of omega-3 polyunsaturated fatty acids and psychoeducation can successfully ameliorate symptoms in people with mild to moderate depression when measured by the Beck Depression Inventory-II. However, the combination of omega-3 polyunsaturated fatty acids and psychoeducation was not better than the psychoeducation intervention alone. | [134] |
| Major depressive disorder | Randomized, double-blind, controlled trial | Adults, 88 participants | All participants took 4 identical capsules per day for 12 weeks. Each capsule contained either concentrated EPA (750 mg) or DHA (350 mg). Compared groups: 1st group took 4 capsules with EPA/day (3.0 g of EPA/day); 2nd group took 4 capsules with DHA/day (1.4 g of DHA/day); 3rd group took 2 capsules with EPA plus 2 capsules with DHA (1.5 g and 0.7 g of EPA and DHA/day, respectively). | Monotherapy with EPA or the combination of a higher dose of EPA and a lower dose of DHA resulted in a significantly higher remission rate than monotherapy with DHA, but no differences were observed between treatment with EPA versus EPA + DHA when measured by the Kaplan-Meier estimates of cumulative remission rates. Eicosapentaenoylethanolamide levels in plasma were positively correlated with rates of clinical remission. | [150] |
| Major depressive disorder with or at high risk for coronary heart disease | Randomized, double-blind, controlled trial | Adults, 144 participants | All patients received 50 mg/day of sertraline. Intervention group: 4 capsules with EPA/day (i.e., 2 g of EPA/day) for 10 weeks. Control group: 4 capsules of placebo (corn oil)/day for 10 weeks. | There were no differences between the groups in depression symptoms when measured by the Beck Depression Inventory II and the Hamilton Rating Scale for Depression. There were also no differences when compared the 10-week remission. | [151] |
| Depressive disorder or mixed anxiety and depressive disorder | Randomized, double-blind, controlled trial | Children (7–18 years old), 58 participants | All patients received standard antidepressant therapy. Intervention group: 20 mL of omega-3 fatty acid-rich fish oil emulsion (providing 2400 mg of total omega-3 fatty acids: 1000 mg of EPA and 750 mg of DHA, EPA:DHA ratio = 1.33:1) taken for 12 weeks. Control group: 20 mL of omega-6 fatty acid-rich sunflower oil emulsion taken for 12 weeks. | Improvement of symptoms measured by the Children’s Depression Inventory (CDI) accompanied by an increase in large HDL subfractions and reduction in small HDL subfractions in the group supplemented with omega-3 fatty acids was observed. A negative correlation between CDI score and HDL-cholesterol and the large HDL subfraction, but not LDL-cholesterol subfractions was detected. CDI score was not associated with erythrocyte membrane fluidity. | [152] |
| Major depressive disorder or depressive disorder not otherwise specified | Randomized, double-blind, controlled trial | Adolescents (9–21 years old), 42 participants | Intervention group: 3 capsules/day with fish oil taken for 12 weeks (1 capsule contains 450 mg of EPA, 40 mg DHA, and 260 mg of DHA; the total daily dose of EPA + DHA was 2130 mg; EPA/DHA ratio was 1.7:1). Control group: 3 capsules of placebo/day taken for 12 weeks. | Monotherapy with fish oil was not superior to placebo in reducing depressive symptoms in high-risk youths when measured by the Childhood Depression Rating Scale-Revised. | [153] |
| Major depressive disorder comorbid with cardiovascular diseases | Randomized, double-blind, controlled trial | Adults, 59 participants | Intervention group: omega-3 polyunsaturated fatty acids (2 g of EPA/day and 1 g of DHA/day) taken for 12 weeks. Control group: placebo taken for 12 weeks. | Omega-3 polyunsaturated fatty acids showed efficacy in improving core depression symptoms only in patients with severe major depressive disorder, measured by the Hamilton Depression Rating Scale. | [154] |
| Depressive disorder (n = 31) or mixed anxiety and depressive disorder (n = 29) | Randomized, double-blind, controlled trial | Children (7–18 years old), 60 participants | All participants received standard antidepressant therapy. Intervention group: omega-3 fish oil emulsion providing 2400 mg of total omega-3 fatty acids (1000 mg of EPA and 750 mg of DHA, EPA:DHA ratio = 1.33:1) taken for 12 weeks. Control group: omega-6 sunflower oil emulsion containing 2467 mg of omega-6 linoleic acid in triacylglycerol form taken for 12 weeks. | Significant reduction in CDI scores in the group receiving omega-3 fish oil emulsion when compared to the group receiving omega-6 fish oil emulsion. At the baseline, significantly lower concentrations of EPA and DHA levels as well as a higher omega-6/omega-3 ratio were detected in depressed patients. | [155] |
| Major depressive disorder | Randomized, double-blind, controlled trial | Adults, 61 participants | Intervention groups: 1st group took 1 g of EPA/day for 12 weeks; 2nd group took 2 g of EPA/day for 12 weeks; 3rd group took 4 g of EPA/day for 12 weeks. Control group: placebo taken for 12 weeks. | 4 g of EPA reduced depression symptoms measured by the Depressive Symptomatology, Clinician-Rated version. | [156] |
| Major depressive disorder | Randomized, double-blind, controlled trial | Adults, 45 participants | Intervention groups: EPA in the form of capsules containing approximately 590 mg of EPA and 152 mg of DHA. 1st group: 1 g of EPA/day taken for 12 weeks; 2nd group: 2 g of EPA/day taken for 12 weeks; 3rd group: 4 g of EPA/day taken for 12 weeks. Control group: placebo taken for 12 weeks. | EPA given at a dose of 4 g/day reduced clinical symptoms of depression, defined as achieving ≥50% reduction in the Inventory Depressive Symptomatology-30 scores. | [157] |
| Major depressive disorder or bipolar disorder | Randomized, double-blind, controlled, cross-over study | Adults, 42 participants | All patients received either antidepressant medications (SSRIs, desvenlafaxine, agomelatine, mirtazapine, vortioxetine) or medications for bipolar disorder (lithium, lamotrigine, clonazepam). Each capsule contained 130 mg of DHA and 35 mg of EPA. Patients received either 260 mg/day (2 capsules) or 520 mg/day (4 capsules) of DHA. | No change in residual symptoms of depression was detected, measured by the Hamilton Depression Rating Scale. | [158] |
| Major depressive disorder | Randomized, double-blind, controlled trial | Adolescents, young adults (15–25 years old), 233 participants | All participants were subjected to 50-min cognitive behavioral case management sessions every 2 weeks. Intervention group: 4 gelatin capsules with marine fish oil/day (providing 840 mg of EPA, 560 mg of DHA, and 5 mg of vitamin E) taken for 12 weeks. Control group: 4 gelatin capsules with paraffin oil taken for 12 weeks. | No significant effect of the fish oil treatment in major depressive disorder was observed when measured by the Quick Inventory of Depressive Symptomatology, Adolescent Version and the Montgomery–Åsberg Depression Rating Scale. Erythrocyte levels of polyunsaturated fatty acids were not associated with depression severity. | [159] |
| Depressive disorder | Randomized, open-label, controlled trial | Adolescents (12–13 years old), 71 participants | Intervention group: Paxil (paroxetine, 20 mg) + 3 capsules of omega-3-rich fish oil/day (providing 1941 mg of EPA + 759 mg of DHA; EPA:DHA ratio 2.56:1) taken for 12 weeks. Control group: Paxil (paroxetine, 20 mg)/day taken for 12 weeks. | Omega-3 fatty acids given with Paxil were more effective than Paxil alone in reducing depressive symptoms when measured by the Montgomery– Åsberg Depression Rating Scale. The combined treatment also improved cognitive function and memory better than monotherapy. | [160] |
| Major depressive disorder with or without high baseline high-sensitivity C-reactive protein | Match-mismatch trial, patients and raters were blind to baseline high-sensitivity C-reactive protein status | Adults, 101 participants | All subjects took their regular antidepressant drugs + 4 capsules of the study product/day (i.e., 2.2 g of EPA + 400 mg of DHA + 800 mg of other fatty acids + 10–24 mg of tocopherol-rich extracts) for 8 weeks. Subjects were allowed to take nutraceuticals other than omega-3 fatty acids (e.g., supplements with vitamins, zinc, or magnesium). | Omega-3 supplementation had a greater antidepressant effect in patients with moderately elevated high-sensitivity C-reactive protein compared to patients with lower high-sensitivity C-reactive protein when measured by the 17-item Hamilton Depression Rating Scale. | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serefko, A.; Jach, M.E.; Pietraszuk, M.; Świąder, M.; Świąder, K.; Szopa, A. Omega-3 Polyunsaturated Fatty Acids in Depression. Int. J. Mol. Sci. 2024, 25, 8675. https://doi.org/10.3390/ijms25168675
Serefko A, Jach ME, Pietraszuk M, Świąder M, Świąder K, Szopa A. Omega-3 Polyunsaturated Fatty Acids in Depression. International Journal of Molecular Sciences. 2024; 25(16):8675. https://doi.org/10.3390/ijms25168675
Chicago/Turabian StyleSerefko, Anna, Monika Elżbieta Jach, Marlena Pietraszuk, Małgorzata Świąder, Katarzyna Świąder, and Aleksandra Szopa. 2024. "Omega-3 Polyunsaturated Fatty Acids in Depression" International Journal of Molecular Sciences 25, no. 16: 8675. https://doi.org/10.3390/ijms25168675
APA StyleSerefko, A., Jach, M. E., Pietraszuk, M., Świąder, M., Świąder, K., & Szopa, A. (2024). Omega-3 Polyunsaturated Fatty Acids in Depression. International Journal of Molecular Sciences, 25(16), 8675. https://doi.org/10.3390/ijms25168675







