Hints of Biological Activity of Xerosydryle: Preliminary Evidence on the Early Stages of Seedling Development
Abstract
1. Introduction
2. Results
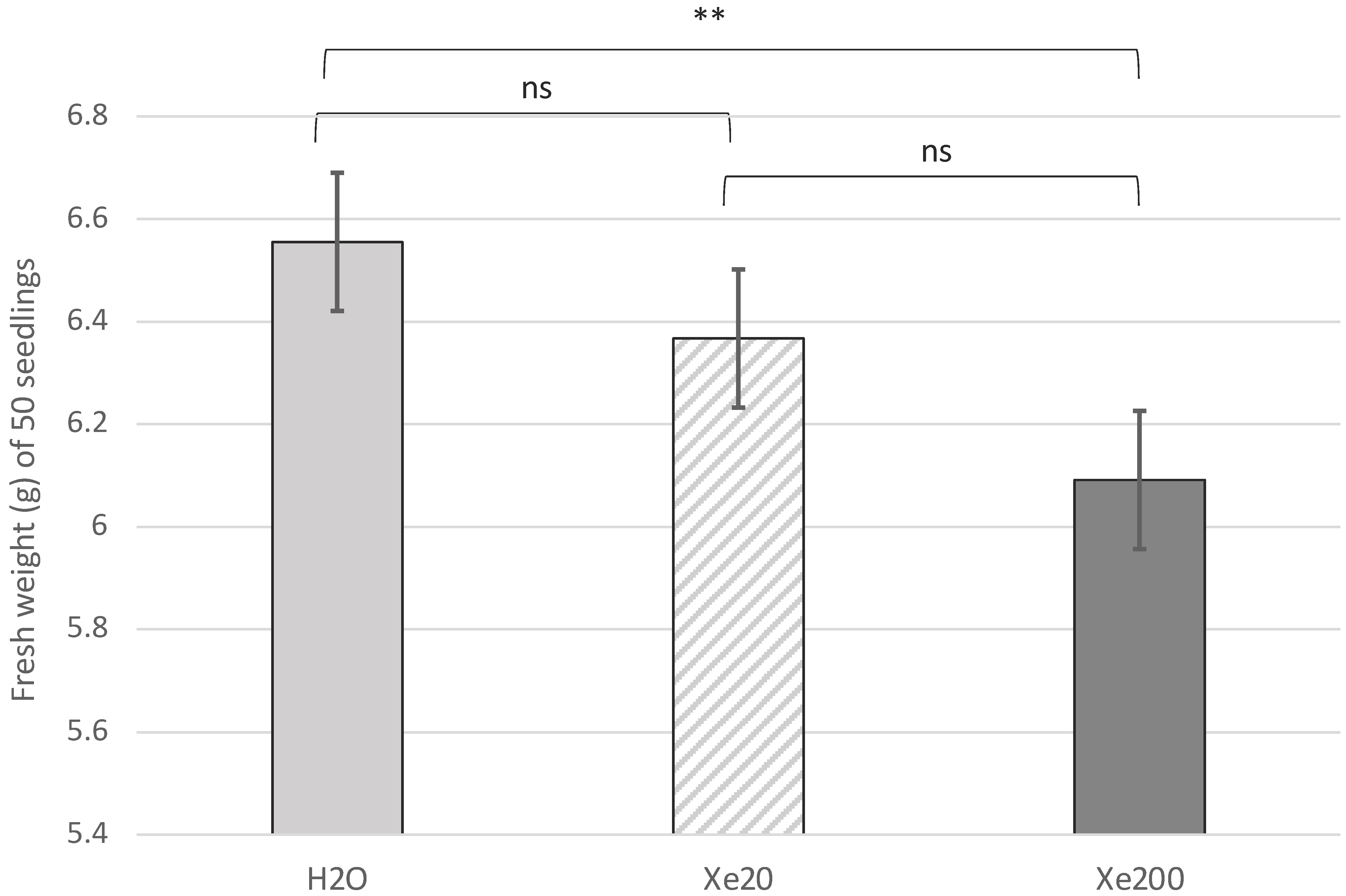
2.1. Weight of Seedlings during Germination
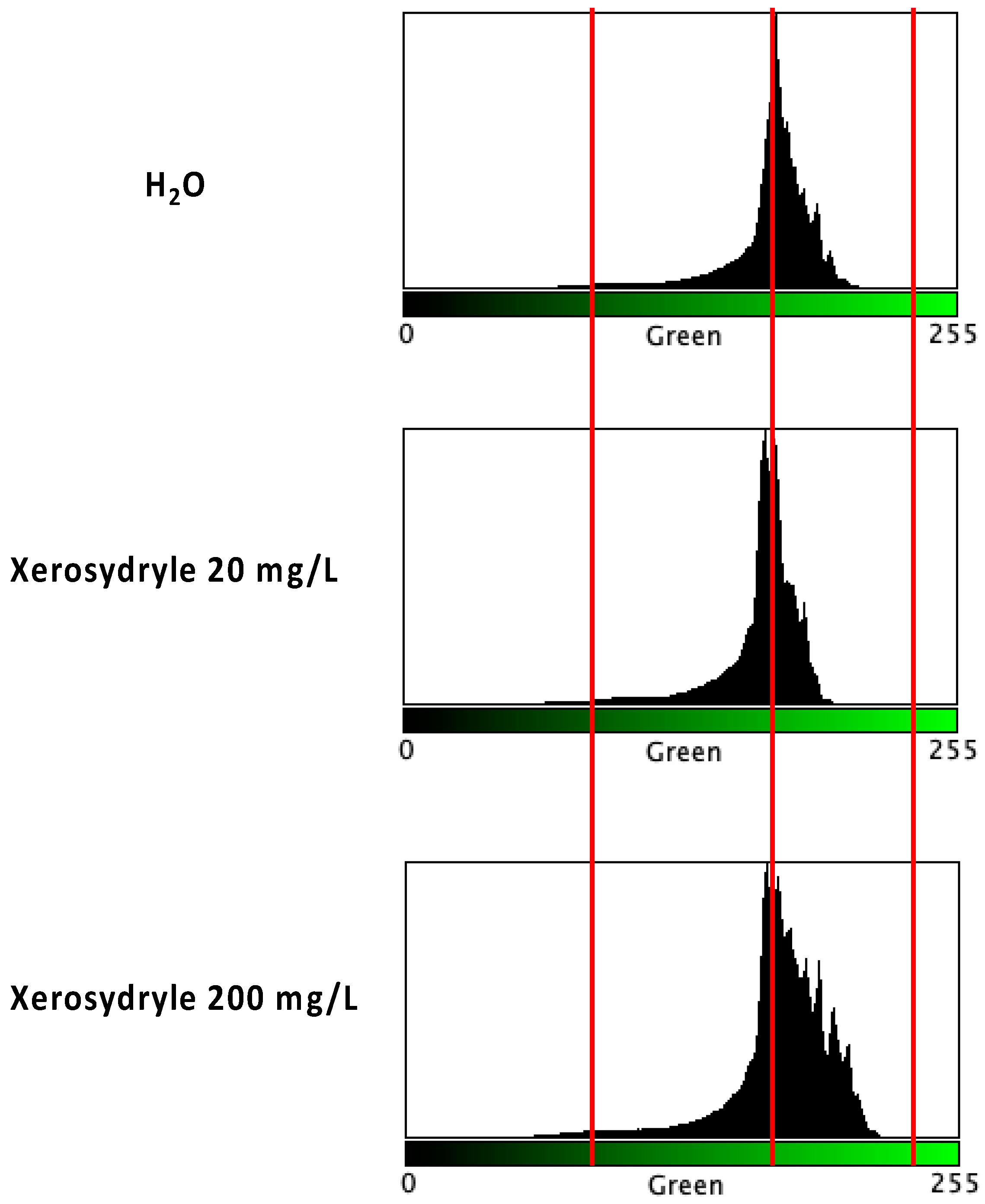
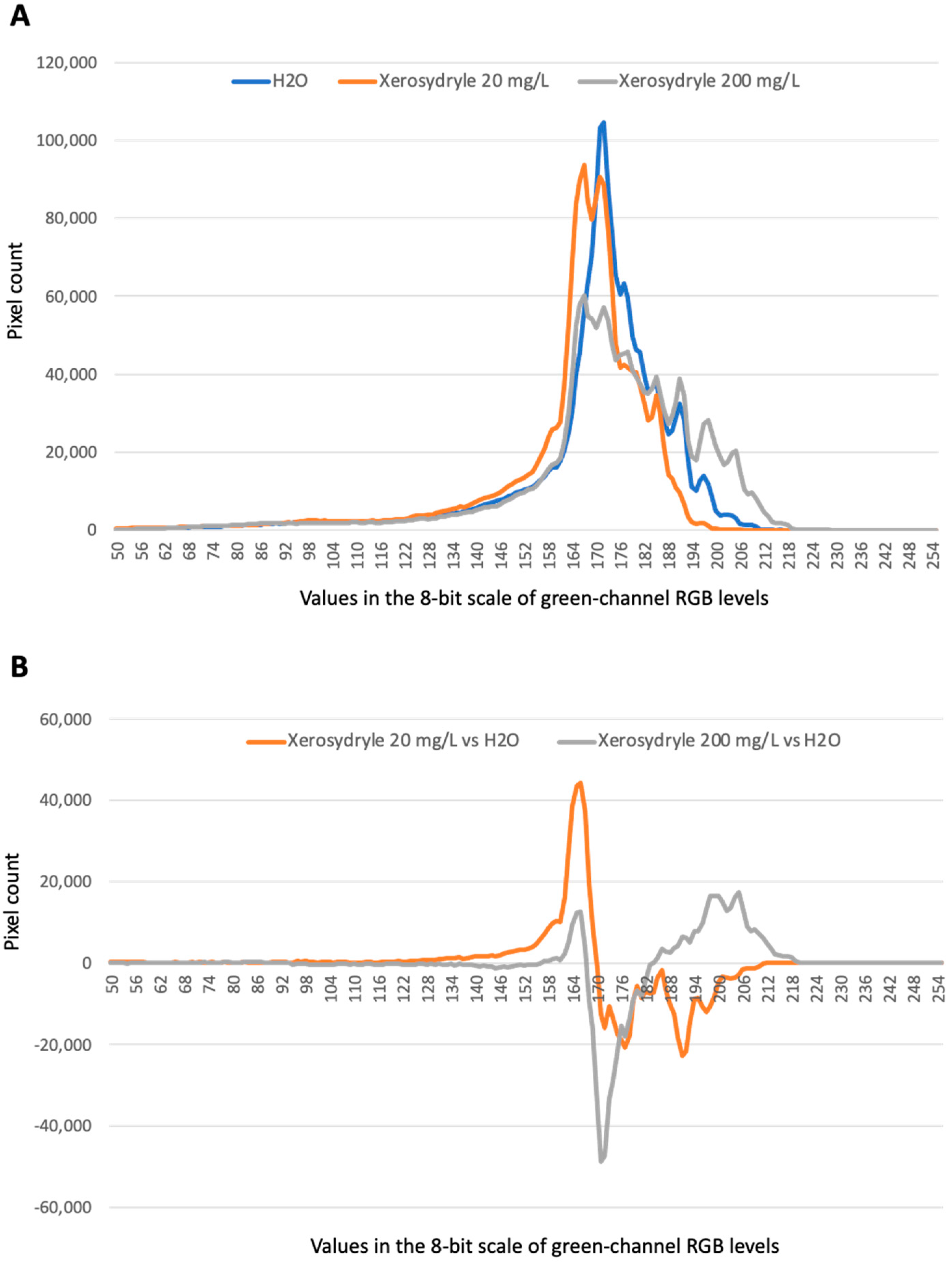
2.2. Chlorophyll Content
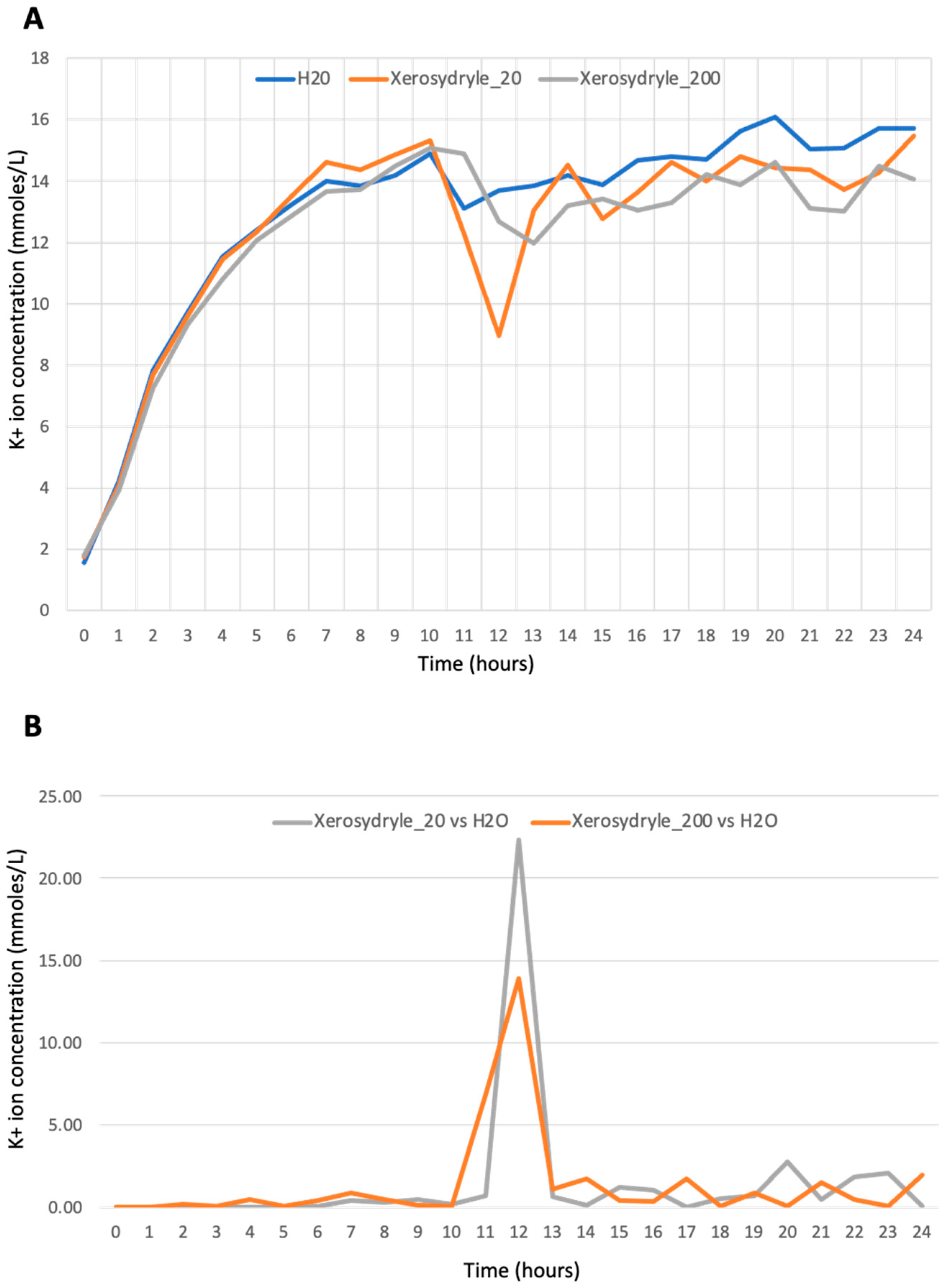
2.3. Effect of Xerosydryle on Potassium Release during Seed Germination
2.4. Transcriptome Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Germination Treatments
4.4. Potassium Reuptake Measurement at Germination
4.5. Measuring Seedlings Weight
4.6. Chlorophyll Content Determination through Image Analysis of Germinating Seedlings
4.7. Transcriptome and Differential Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
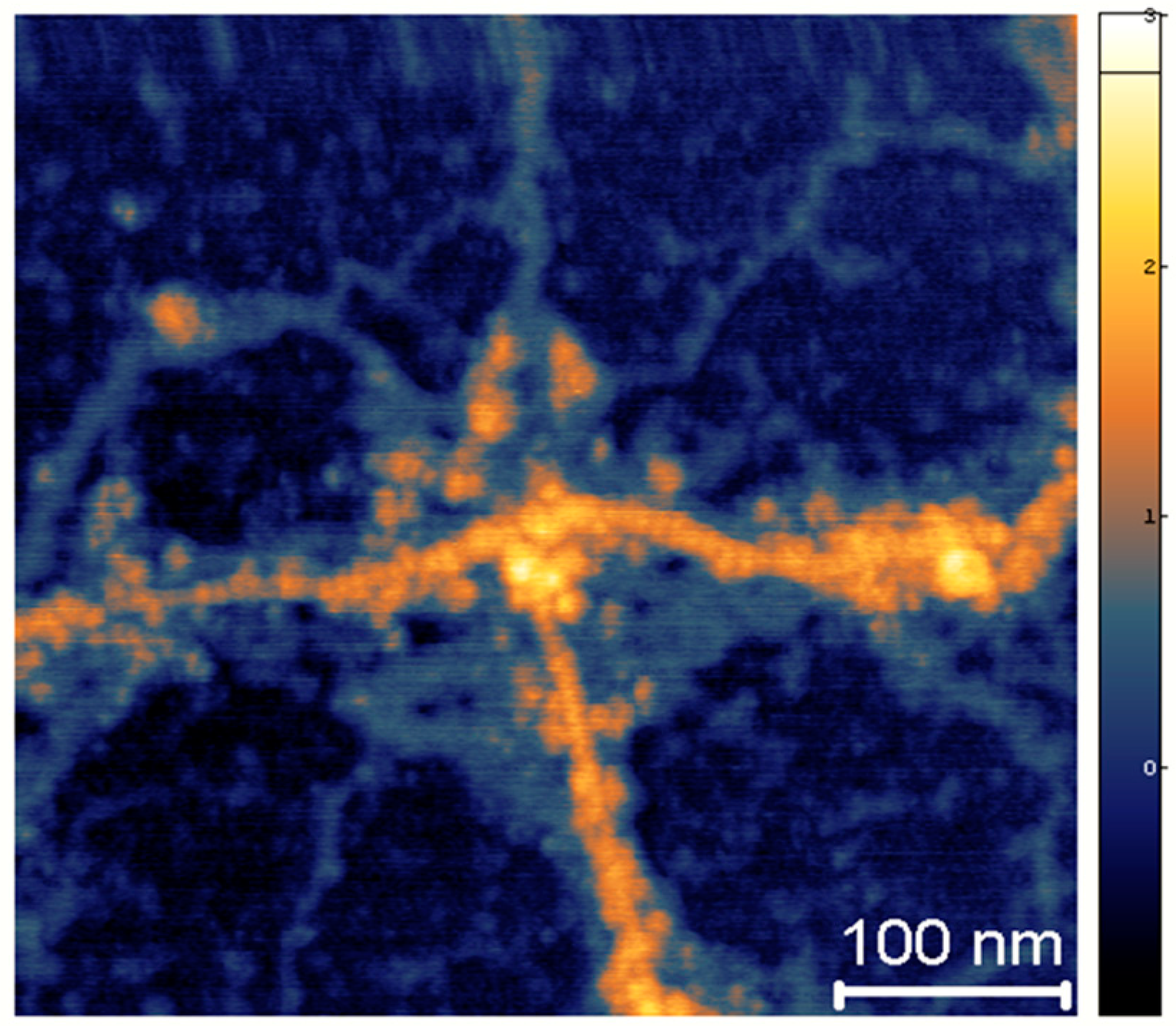
Appendix A. The Chemical Nature of Xerosydryle
Appendix A.1. Production Techniques of Xerosydryle
Appendix A.2. Chemical Analysis of the Solid Sample, Which Should Make It Possible to Derive a Chemical Formula for This Solid-State Water Polymer


| Element | Weight (%) |
|---|---|
| C | 61.69 |
| N | 17.6 |
| O | 18.47 |
| S | 2.24 |
Appendix A.3. Why the First Quantization (Hydrogen Bonding-Addressed by Reviewer #1) Is Inadequate to Justify Its Existence and Why the Second Quantization Is More Suitable
Appendix A.4. Is There a Possibility of Contamination? How Many Impurities (That May Result from the Processing of the Liquid Phase) Can Be Ruled Out?
- -
- consistently reproducible results across various chemical and physical characteristics of water;
- -
- uniform behavior across different insoluble materials used to perturb water (e.g., an increase in electric conductivity with the number of procedure iterations).
Appendix A.5. Is There a Possibility That Xerosydryle and Biological Objects May Interact in One Way or Another?
References
- Capolupo, A.; Del Giudice, E.; Elia, V.; Germano, R.; Napoli, E.; Niccoli, M.; Tedeschi, A.; Vitiello, G. Self-Similarity Properties of Nafionized and Filtered Water and Deformed Coherent States. Int. J. Mod. Phys. B 2013, 28, 1450007. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R.; Oliva, R.; Roviello, V.; Niccoli, M.; Amoresano, A.; Naviglio, D.; Ciaravolo, M.; Trifuoggi, M.; et al. New Chemical-Physical Properties of Water after Iterative Procedure Using Hydrophilic Polymers: The Case of Paper Filter. J. Mol. Liq. 2019, 296, 111808. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R.; Roviello, V.; Oliva, R.; Niccoli, M.; Amoresano, A.; Toscanesi, M.; Trifuoggi, M.; Fabozzi, A.; et al. Water Perturbed by Cellophane: Comparison of Its Physicochemical Properties with Those of Water Perturbed with Cotton Wool or Nafion. J. Therm. Anal. Calorim. 2021, 146, 2073–2088. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R.; Naviglio, D.; Ciaravolo, M.; Dal Poggetto, G.; Caputo, D.; Oliva, R.; Yinnon, T.A. New Physicochemical Properties of Liquid Water Resulting from Recurrent Contact with Hydrophilic Polymers. Characteristics of the Resulting Supramolecular Aggregates: The xerosydryle. Water 2022, 12, 72–85. [Google Scholar] [CrossRef]
- Sharma, A.; Traynor-Kaplan, A.; Pollack, G.H. Solid Water at Room Temperature? Arab. J. Chem. 2023, 16, 104537. [Google Scholar] [CrossRef]
- Germano, R. Water’s quantum structures and life. Electromagn. Biol. Med. 2015, 34, 133–137. [Google Scholar] [CrossRef]
- Papendick, R.I.; Campbell, G.S. Theory and Measurement of Water Potential. In Water Potential Relations in Soil Microbiology; Wiley: Hoboken, NJ, USA, 2015; pp. 1–22. [Google Scholar] [CrossRef]
- Dürr, C.; Dickie, J.; Yang, X.-Y.; Pritchard, H. Ranges of Critical Temperature and Water Potential Values for the Germination of Species Worldwide: Contribution to a Seed Trait Database. Agric. For. Meteorol. 2015, 200, 222–232. [Google Scholar] [CrossRef]
- Bidgoly, R.O.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of Temperature and Water Potential on Carthamus tinctorius L. Seed Germination: Quantification of the Cardinal Temperatures and Modeling Using Hydrothermal Time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, D.; Liu, H.; Guo, C.; Tang, L.; Wang, H.; Chen, Y.; Luo, K. Effect of Temperature and Water Potential on the Germination of Seeds from Three Different Populations of Bidens pilosa as a Potential Cd Hyperaccumulator. BMC Plant Biol. 2022, 22, 487. [Google Scholar] [CrossRef]
- Onofri, A.; Benincasa, P.; Mesgaran, M.B.; Ritz, C. Hydrothermal-Time-to-Event Models for Seed Germination. Eur. J. Agron. 2018, 101, 129–139. [Google Scholar] [CrossRef]
- Munz, E.; Rolletschek, H.; Oeltze-Jafra, S.; Fuchs, J.; Guendel, A.; Neuberger, T.; Ortleb, S.; Jakob, P.M.; Borisjuk, L. A functional Imaging Study of Germinating Oilseed Rape Seed. New Phytol. 2017, 216, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Merieux, N.; Cordier, P.; Wagner, M.-H.; Ducournau, S.; Aligon, S.; Job, D.; Grappin, P.; Grappin, E. ScreenSeed as a Novel High Throughput Seed Germination Phenotyping Method. Sci. Rep. 2021, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, H.W.M. Metabolomic Analysis of Tomato Seed Germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An Integrative Approach to Analyze Seed Germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Narsai, R.; Gouil, Q.; Secco, D.; Srivastava, A.; Karpievitch, Y.V.; Liew, L.C.; Lister, R.; Lewsey, M.G.; Whelan, J. Extensive Transcriptomic and Epigenomic Remodelling Occurs during Arabidopsis thaliana Germination. Genome Biol. 2017, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Cherrate, M.; Radouane, N.; Ezrari, S.; Echchgadda, G.; Maissour, A.; Makroum, K.; Plavan, G.; Abd-Elkader, O.H.; Bourioug, M. Effects of Temperature, pH, and Salinity on Seed Germination of Acinos alpinus subsp. Meridionalis and FTIR Analysis of Molecular Composition Changes. Sustainability 2023, 15, 4793. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Mami, Y.; Ashournezhad, M.; Abri, F.; Amani, M. Effect of Salinity and Drought Stress on Germination of Fenugreek. Afr. J. Agric. Res. 2011, 6, 5529–5532. [Google Scholar] [CrossRef]
- Zandi, P.; Basu, S.K.; Khatibani, L.B.; Balogun, M.O.; Aremu, M.O.; Sharma, M.; Kumar, A.; Sengupta, R.; Li, X.; Li, Y.; et al. Fenugreek (Trigonella foenum-graecum L.) Seed: A Review of Physiological and Biochemical Properties and Their Genetic Improvement. Acta Physiol. Plant. 2015, 37, 1714. [Google Scholar] [CrossRef]
- Arshad, K.; Ullah, A.; Ullah, S.; Bogari, H.A.; Ashour, M.L.; Noor, J.; Amin, F.; Shah, S. Quantifying Osmotic Stress and Temperature Effects on Germination and Seedlings Growth of Fenugreek (Trigonella foenum-graecum L.) via Hydrothermal Time Model. Sustainability 2022, 14, 12049. [Google Scholar] [CrossRef]
- Ali, R.R.S.; Nassar, I.N.; Ghallab, A.; Ali, E.F.; Alqubaie, A.I.; Rady, M.M.; Awad, A.A.M. Alleviation of Water-Deficit Stress on Seed Germination of Barley and Fenugreek in a Sandy Soil Using Superabsorbent Polymer. Agronomy 2023, 13, 2324. [Google Scholar] [CrossRef]
- Bhandal, I.S.; Malik, C.P. Potassium Estimation, Uptake, and Its Role in the Physiology and Metabolism of Flowering Plants. Int. Rev. Cytol. 1988, 110, 205–254. [Google Scholar] [CrossRef]
- Cocucci, S.; Cocucci, M. Effect of ABA, GA3 and FC on the Development of Potassium Uptake in Germinating Radish Seeds. Plant Sci. Lett. 1977, 10, 85–95. [Google Scholar] [CrossRef]
- Jia, Q.; Kong, D.; Li, Q.; Sun, S.; Song, J.; Zhu, Y.; Liang, K.; Ke, Q.; Lin, W.; Huang, J. The Function of Inositol Phosphatases in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2019, 20, 3999. [Google Scholar] [CrossRef] [PubMed]
- Khavari-Nejad, S. A review on plant peroxidases. Nova Biol. Reperta 2019, 5, 428–437. [Google Scholar] [CrossRef][Green Version]
- Baumberger, N.; Doesseger, B.; Guyot, R.; Diet, A.; Parsons, R.L.; Clark, M.A.; Simmons, M.; Bedinger, P.; Goff, S.A.; Ringli, C.; et al. Whole-Genome Comparison of Leucine-Rich Repeat Extensins in Arabidopsis and Rice. A Conserved Family of Cell Wall Proteins Form a Vegetative and a Reproductive Clade. Plant Physiol. 2003, 131, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Herger, A.; Dünser, K.; Kleine-Vehn, J.; Ringli, C. Leucine-Rich Repeat Extensin Proteins and Their Role in Cell Wall Sensing. Curr. Biol. 2019, 29, R851–R858. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2023, 25, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Estévez, I.H.; Hernández, M.R. Plant Glutathione S-Transferases: An Overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in Integrating Plant Responses to Drought and Salt Stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Liu, Y.; Sod, B.; Li, M.; Yang, T.; Gao, T.; Yang, Q.; Long, R. Overexpression of the Elongation factor MtEF1A1 Promotes Salt Stress Tolerance in Arabidopsis thaliana and Medicago truncatula. BMC Plant Biol. 2023, 23, 138. [Google Scholar] [CrossRef]
- Athar, H.-U.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt Stress Proteins in Plants: An Overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of Saline Water on Seed Germination and Early Seedling Growth of the Halophyte quinoa. AoB Plants 2014, 6, plu047. [Google Scholar] [CrossRef] [PubMed]
- Uçarlı, C. Effects of Salinity on Seed Germination and Early Seedling Stage. In Abiotic Stress Plants; Intech Open: London, UK, 2021. [Google Scholar] [CrossRef]
- Abley, K.; Formosa-Jordan, P.; Tavares, H.; Chan, E.Y.T.; Afsharinafar, M.; Leyser, O.; Locke, J.C.W. An ABA-GA Bistable Switch Can Account for Natural Variation in the Variability of Arabidopsis Seed Germination Time. eLife 2021, 10, e59485. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Follmer, C.M.; Hummes, A.P.; Lângaro, N.C.; Petry, C.; Moterle, D.F.; Bortoluzzi, E.C. Nutrient Availability and pH Level Affect Germination Traits and Seedling Development of Conyza canadensis. Sci. Rep. 2021, 11, 15607. [Google Scholar] [CrossRef] [PubMed]
- Vats, S. Biotic and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–367. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Liang, Y.; Urano, D.; Liao, K.-L.; Hedrick, T.L.; Gao, Y.; Jones, A.M. A Nondestructive Method to Estimate the Chlorophyll Content of Arabidopsis Seedlings. Plant Methods 2017, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.N. Chlorophyll Fluorescence: A Practical Approach to Study Ecophysiology of Green Plants. In Advances in Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 77–97. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental Stress—What Can We Learn from Chlorophyll a Fluorescence Analysis in Woody Plants? A review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; D’andria, R.; Jacobsen, S.-E. Non-Destructive evaluation of Chlorophyll Content in Quinoa and Amaranth Leaves by Simple and Multiple Regression Analysis of RGB Image Components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Gaiduk, A.P.; Pham, T.A.; Govoni, M.; Paesani, F.; Galli, G. Electron Affinity of Liquid Water. Nat. Commun. 2018, 9, 247. [Google Scholar] [CrossRef]
- Pollack, G. The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor; Ebner & Sons: Seattle, WA, USA, 2013. [Google Scholar]
- Kowacz, M.; Pollack, G.H. Moving Water Droplets: The Role of Atmospheric CO2 and Incident Radiant Energy in Charge Separation at the Air–Water Interface. J. Phys. Chem. B 2019, 123, 11003–11013. [Google Scholar] [CrossRef]
- Greil, F.; Punampalam, R.; Walther, T.H.; Heißler, S.; Ulrich, A.S. “Iteratively Nafionated Water” in Its Solid Phase at Room Temperature Is in Fact a Mixture of Lyophilized Biological and Non-Biological Contaminants. J. Mol. Liq. 2023, 385, 122351. [Google Scholar] [CrossRef]
- Elia, V.; Yinnon, T.A.; Oliva, R.; Napoli, E.; Germano, R.; Bobba, F.; Amoresano, A. DNA and the Chiral Water Superstructure. J. Mol. Liq. 2017, 248, 1028–1029. [Google Scholar] [CrossRef]
- Cha, S.-H. Comprehensive Survey on Distance/Similarity Measures between Probability Density Functions. Int. J. Math. Model. Meth. Appl. Sci. 2007, 1, 1. [Google Scholar]
- Nuzzo, R. Scientific method: Statistical errors. Nature 2014, 506, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Giorgino, T. Computing and Visualizing Dynamic Time Warping Alignments in R: ThedtwPackage. J. Stat. Softw. 2009, 31, 1–24. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5439. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2018, 47, D309–D314. [Google Scholar] [CrossRef]
- Robinson Mark, D.; McCarthy Davis, J.; Smyth Gordon, K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Elia, V.; Ausanio, G.; De Ninno, A.; Gentile, F.; Germano, R. Experimental Evidence of Stable Aggregates of Water at Room Temperature and Normal Pressure After Iterative Contact with a Nafion® Polymer Membrane. Water J. 2013, 5, 16–26. [Google Scholar]
- Elia, V.; Ausanio, G.; De Ninno, A.; Germano, R.; Napoli, E.; Niccoli, M. Experimental Evidences of Stable Water Nanostructures at Standard Pressure and Temperature Obtained by Iterative Filtration. Water 2014, 5, 121–130. [Google Scholar] [CrossRef]
- Elia, V.; Germano, R.; Napoli, E. Permanent Dissipative Structures in Water: The Matrix of Life? Experimental Evidences and Their Quantum Origin. Curr. Top. Med. Chem. 2015, 15, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Yinnon, T.; Elia, V.; Napoli, E.; Germano, R.; Liu, Z.-Q. Water Ordering Induced by Interfaces: An Experimental and Theoretical Study. Water 2016, 7, 96. [Google Scholar] [CrossRef]
- Elia, V.; Yinnon, T.; Oliva, R.; Napoli, E.; Germano, R.; Bobba, F.; Amoresano, A. Chiral Micron-Sized H2O Aggregates in Water: Circular Dichroism of Supramolecular H2O Architectures Created by Perturbing Pure Water. Water 2017, 8, 1–29. [Google Scholar] [CrossRef]
- Elia, V.; Oliva, R.; Napoli, E.; Germano, R.; Pinto, G.; Lista, L.; Niccoli, M.; Toso, D.; Vitiello, G.; Trifuoggi, M.; et al. Experimental Study of Physicochemical Changes in Water by Iterative Contact with Hydrophilic Polymers: A Comparison between Cellulose and Nafion. J. Mol. Liq. 2018, 268, 598–609. [Google Scholar] [CrossRef]
- Signanini, P.; Vessia, G.; Elia, V.; Napoli, E.; Germano, R. A Study on the Changes in Physical Properties of Demineralized Water Put in Contact with Porous Hydrophilic Materials: Experimental Evidences on Metabrick Material. J. Porous Media 2019, 22, 1609–1625. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R.; Naviglio, D.; Ciaravolo, M.; Dal Poggetto, G.; Caputo, D.; Oliva, R.; Yinnon, T.A. A Study on the Changes in Physical Properties of Distilled Water Put In Contact with Porous Hydrophilic Materials: Experimental Evidence on Neapolitan Yellow Tuff. Water 2022, 12, 119–129. [Google Scholar] [CrossRef]
- Elia, V.; Marchettini, N.; Napoli, E.; Niccoli, M. The Role of Ethanol in Extremely Diluted Solutions. J. Therm. Anal. Calorim. 2014, 116, 477–483. [Google Scholar] [CrossRef]
- Yinnon, T.A.; Elia, V. Dynamics in perturbed very dilute aqueous solutions: Theory and experimental evidence. Int. J. Mod. Phys. B 2013, 27, 1350005. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. On the Stability of Extremely Diluted Solutions to Temperature. J. Therm. Anal. Calorim. 2013, 113, 963–970. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. Physical-Chemical Study of Water in Contact with a Hydrophilic Polymer: Nafion. J. Therm. Anal. Calorim. 2013, 112, 937–944. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. Calorimetric and Conductometric Titrations of Nanostructures of Water Molecules in Iteratively Filtered Water. J. Therm. Anal. Calorim. 2013, 111, 815–821. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E. Nanostructures of Water Molecules in Iteratively Filtered Water. Key Eng. Mater. 2011, 495, 37–40. [Google Scholar] [CrossRef]
- Betti, L.; Elia, V.; Napoli, E.; Trebbi, G.; Zurla, M.; Nani, D.; Peruzzi, M.; Brizzi, M. Biological Effects and Physico-Chemical Properties of Extremely Diluted Aqueous Solutions as a Function of Aging-Time. Front. Life Sci. 2011, 5, 117–126. [Google Scholar] [CrossRef][Green Version]
- Elia, V.; Marchettini, N.; Napoli, E.; Niccoli, M. Calorimetric, Conductometric and Density Measurements of Iteratively Filtered Water Using 450, 200, 100 and 25 Nm Millipore Filters. J. Therm. Anal. Calorim. 2013, 114, 927–936. [Google Scholar] [CrossRef]
- Cattaneo, T.M.P.; Stefania, V.; Elena, N.; Vittorio, E. Influence of Filtration Processes on Aqueous Nanostructures by NIR Spectroscopy. J. Chem. Chem. Eng. 2011, 5, 1046. [Google Scholar]
- Elia, V.; Marrari, L.; Napoli, E. Aqueous Nanostructures in Water Induced by Electromagnetic Fields Emitted by EDS: A Conductometric Study of Fullerene and Carbon Nanotube EDS. J. Therm. Anal. Calorim. 2012, 107, 843–851. [Google Scholar] [CrossRef]
- Brizzi, M.; Elia, V.; Trebbi, G.; Nani, D.; Peruzzi, M.; Betti, L. The Efficacy of Ultramolecular Aqueous Dilutions on a Wheat Germination Model as a Function of Heat and Aging-Time. Evid. Based Complement. Altern. Med. 2011, 2011, 696298. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. Thermodynamic Parameters for the Binding Process of the OH− Ion with the Dissipative Structures. Calorimetric and Conductometric Titrations. J. Therm. Anal. Calorim. 2010, 102, 1111–1118. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. A Molecular Model of Interaction between Extremely Diluted Solutions and NaOH Solutions Used as Titrant.: Conductometric and PHmetric Titrations. J. Mol. Liq. 2009, 148, 45–50. [Google Scholar] [CrossRef]
- Cacace, C.M.; Elia, L.; Elia, V.; Napoli, E.; Niccoli, M. Conductometric and PHmetric Titrations of Extremely Diluted Solutions Using HCl Solutions as Titrant. J. Mol. Liq. 2009, 146, 122–126. [Google Scholar] [CrossRef]
- Del Giudice, E.; Elia, V.; Napoli, E.; Tedeschi, A. The Role of Water in Living Organisms. Neural Netw. World 2009, 19, 355–360. [Google Scholar]
- Ciavatta, L.; Elia, V.; Napoli, E.; Niccoli, M. New Physico-Chemical Properties of Extremely Diluted Solutions. Electromotive Force Measurements of Galvanic Cells Sensible to the Activity of NaCl at 25 °C. J. Solut. Chem. 2008, 37, 1037–1049. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Marchettini, N.; Napoli, E.; Niccoli, M.; Tiezzi, E. Physicochemical Properties of Aqueous Extremely Diluted Solutions in Relation to Ageing. J. Therm. Anal. Calorim. 2008, 93, 1003–1011. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M. On the Stability of Extremely Diluted Aqueous Solutions at High Ionic Strength: A Calorimetric Study at 298 K. J. Therm. Anal. Calorim. 2008, 92, 643–648. [Google Scholar] [CrossRef]
- Belon, P.; Elia, V.; Elia, L.; Montanino, M.; Napoli, E.; Niccoli, M. Conductometric and Calorimetric Studies of the Serially Diluted and Agitated Solutions-On the Combined Anomalous Effect of Time and Volume Parameters. J. Therm. Anal. Calorim. 2008, 93, 459–469. [Google Scholar] [CrossRef]
- Corti, H. Comments on “New Physico-Chemical Properties of Extremely Dilute Solutions. A Conductivity Study at 25 °C in Relation to Ageing". J. Solut. Chem. 2008, 37, 1819–1824. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M.; Marchettini, N.; Tiezzi, E. New PhysicoChemical Properties of Extremely Dilute Solutions. A Conductivity Study at 25 °C in Relation to Ageing. J. Solut. Chem. 2008, 37, 85–96. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R. The “Memory of Water”: An Almost Deciphered Enigma. Dissipative Structures in Extremely Diluted Aqueous Solutions of the Homeopathic Medicine. Homeopathy 2007, 96, 163–169. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Marchese, M.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L.; Savarese, F. Interaction of “Extremely Diluted Solutions” with Aqueous Solutions of Hydrochloric Acid and Sodium Hydroxide-A Calorimetric Study at 298 K. J. Mol. Liq. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L. Conductometric Studies of the Serially Diluted and Agitated Solutions on an Anomalous Effect That Depends on the Dilution Process. J. Mol. Liq. 2007, 135, 158–165. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Napoli, E.; Niccoli, M. Conductometric and Calorimetric Studies of Serially Diluted and Agitated Solutions: The Dependence of Intensive Parameters on Volume. Int. J. Ecodynam. 2007, 1, 361–372. [Google Scholar] [CrossRef]
- Elia, V.; Elia, L.; Cacace, P.; Napoli, E.; Niccoli, M.; Savarese, F. ‘Extremely Diluted Solutions’ as Multi-Variable Systems. J. Therm. Anal. Calorim. 2006, 84, 317–323. [Google Scholar] [CrossRef]
- Elia, V.; Marchese, M.; Montanino, M.; Napoli, E.; Niccoli, M.; Nonatelli, L.; Ramaglia, A. Hydrohysteretic Phenomena of “Extremely Diluted Solutions” Induced by Mechanical Treatments: A Calorimetric and Conductometric Study at 25 °C. J. Solut. Chem. 2005, 34, 947–960. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Niccoli, M.; Nonatelli, L.; Ramaglia, A.; Ventimiglia, E. New Physico-Chemical Properties of Extremely Diluted Aqueous Solutions: A Calorimetric and Conductivity Study at 25 °C. J. Therm. Anal. Calorim. 2004, 78, 331–342. [Google Scholar] [CrossRef]
- Elia, V.; Baiano, S.; Duro, I.; Napoli, E.; Niccoli, M.; Nonatelli, L. Permanent Physico-Chemical Properties of Extremely Diluted Aqueous Solutions of Homeopathic Medicines. Homeopathy 2004, 93, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Elia, V.; Niccoli, M. New Physico-Chemical Properties of Extremely Diluted Aqueous Solutions. J. Therm. Anal. Calorim. 2004, 75, 815–836. [Google Scholar] [CrossRef]
- Elia, V.; Niccoli, M. New Physicochemical Properties of Water Induced by Mechanical Treatments. A Calorimetric Study at 25 °C. J. Therm. Anal. Calorim. 2000, 61, 527–537. [Google Scholar] [CrossRef]
- Elia, V.; Niccoli, M. Thermodynamics of Extremely Diluted Aqueous Solutions. Ann. N. Y. Acad. Sci. 1999, 879, 241–248. [Google Scholar] [CrossRef]
- Renati, P.; Madl, P. What Is the “Hydrogen Bond”? A QFT-QED Perspective. Int. J. Mol. Sci. 2024, 25, 3846. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G. Fractals, Coherent States and Self-Similarity Induced Noncommutative Geometry. Phys. Lett. A 2012, 376, 2527–2532. [Google Scholar] [CrossRef]
- McDermott, M.; Vanselous, H.; Corcelli, S.; Petersen, P. DNA’s Chiral Spine of Hydration. ACS Cent. Sci. 2017, 3, 708–714. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geuna, F.; Pensotti, A.; Vecchione, R.; Germano, R. Hints of Biological Activity of Xerosydryle: Preliminary Evidence on the Early Stages of Seedling Development. Int. J. Mol. Sci. 2024, 25, 8717. https://doi.org/10.3390/ijms25168717
Geuna F, Pensotti A, Vecchione R, Germano R. Hints of Biological Activity of Xerosydryle: Preliminary Evidence on the Early Stages of Seedling Development. International Journal of Molecular Sciences. 2024; 25(16):8717. https://doi.org/10.3390/ijms25168717
Chicago/Turabian StyleGeuna, Filippo, Andrea Pensotti, Raffaele Vecchione, and Roberto Germano. 2024. "Hints of Biological Activity of Xerosydryle: Preliminary Evidence on the Early Stages of Seedling Development" International Journal of Molecular Sciences 25, no. 16: 8717. https://doi.org/10.3390/ijms25168717
APA StyleGeuna, F., Pensotti, A., Vecchione, R., & Germano, R. (2024). Hints of Biological Activity of Xerosydryle: Preliminary Evidence on the Early Stages of Seedling Development. International Journal of Molecular Sciences, 25(16), 8717. https://doi.org/10.3390/ijms25168717








