Lactoferrin Affects the Viability of Bacteria in a Biofilm and the Formation of a New Biofilm Cycle of Mannheimia haemolytica A2
Abstract
1. Introduction
2. Results
2.1. Effect of Sub-Inhibitory Concentrations of bLf on the Growth of M. haemolytica A2
2.2. Biofilm Formation of M. haemolytica A2 Is Negatively Affected When Bacteria Are Pre-Incubated with bLf but Increases When bLf Is Added during the Formation of Biofilm
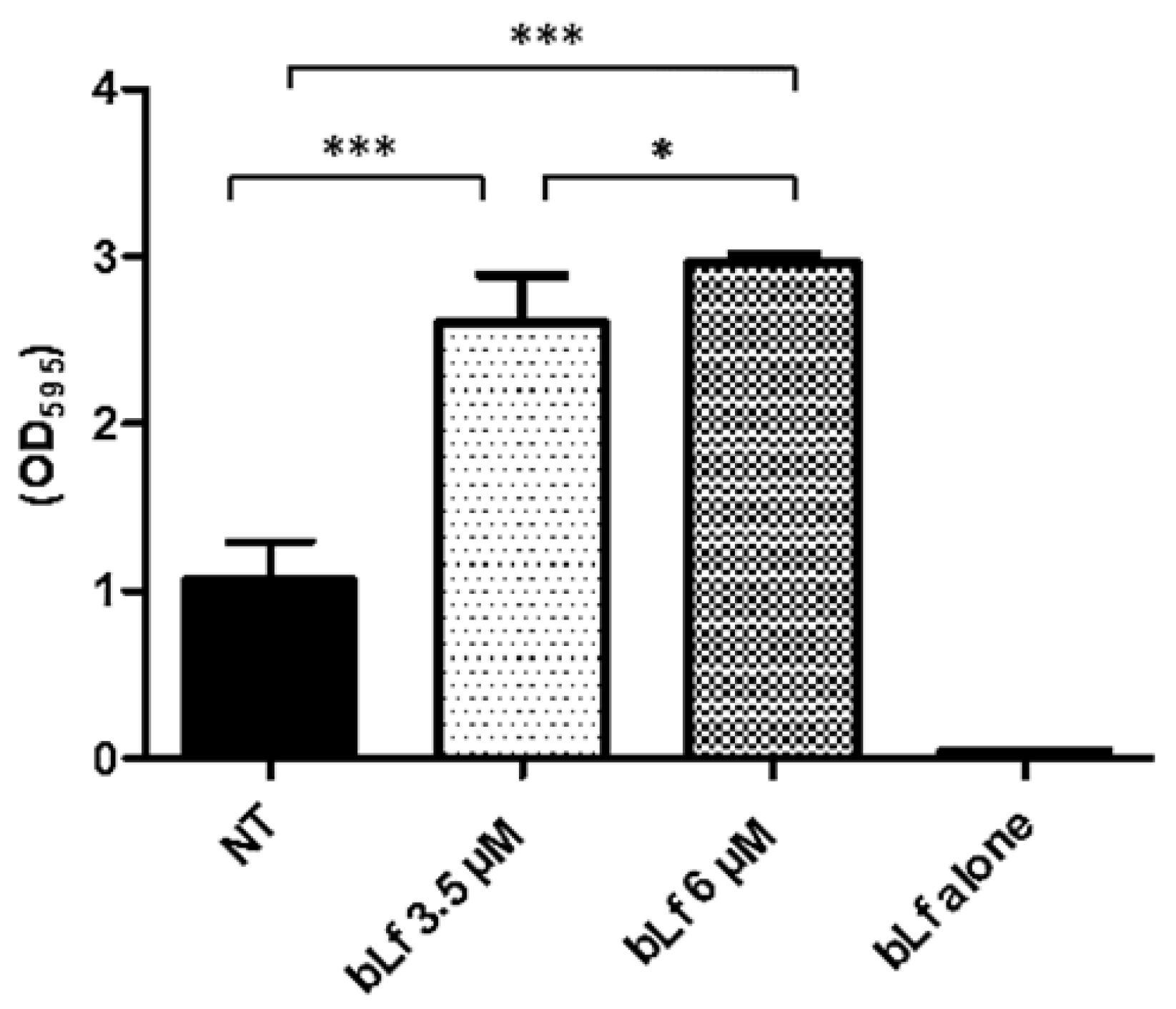
2.3. Bovine Lactoferrin Increases the Mature Biofilm of M. haemolytica A2
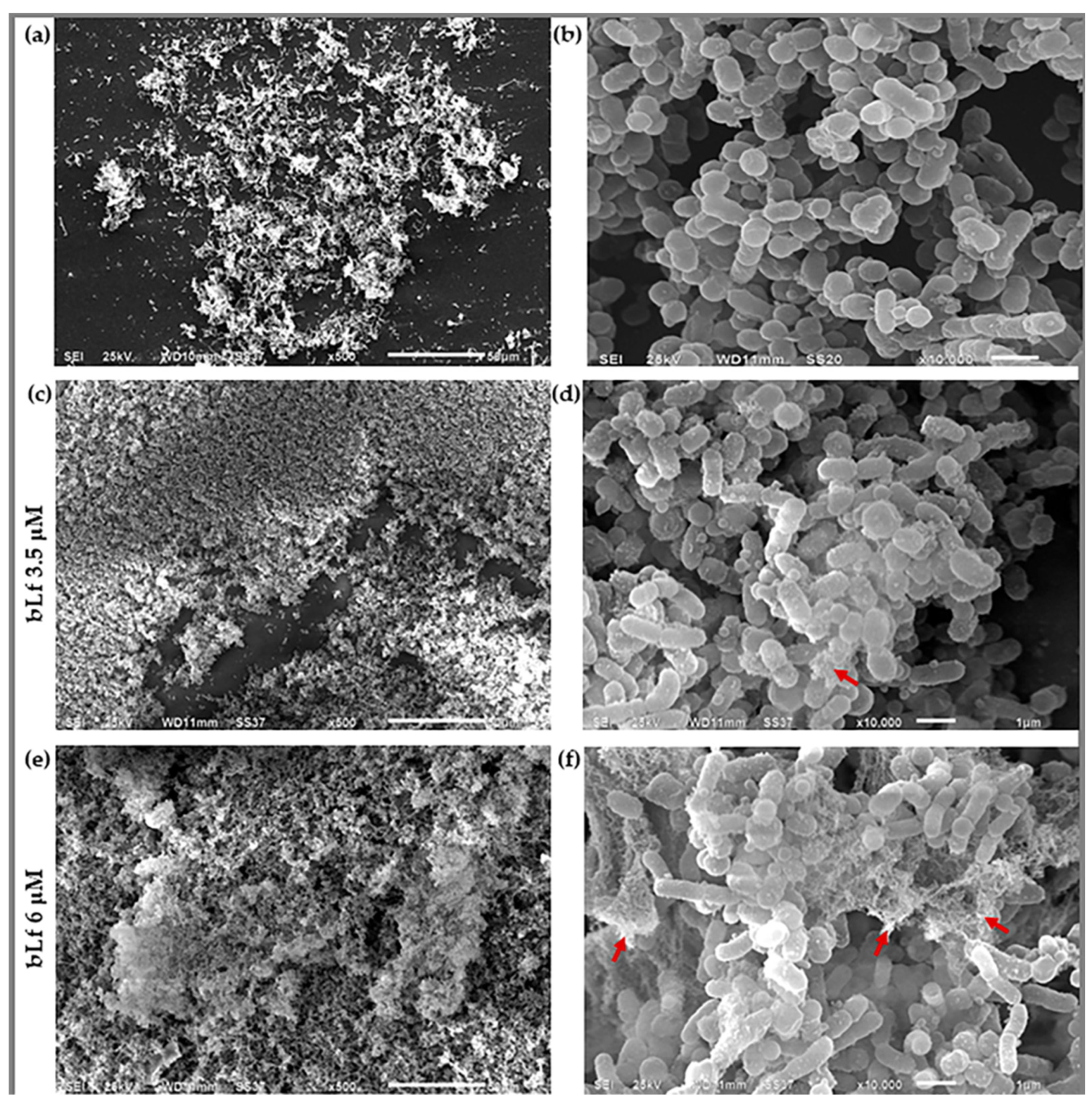
2.4. Scanning Electron Microscopy
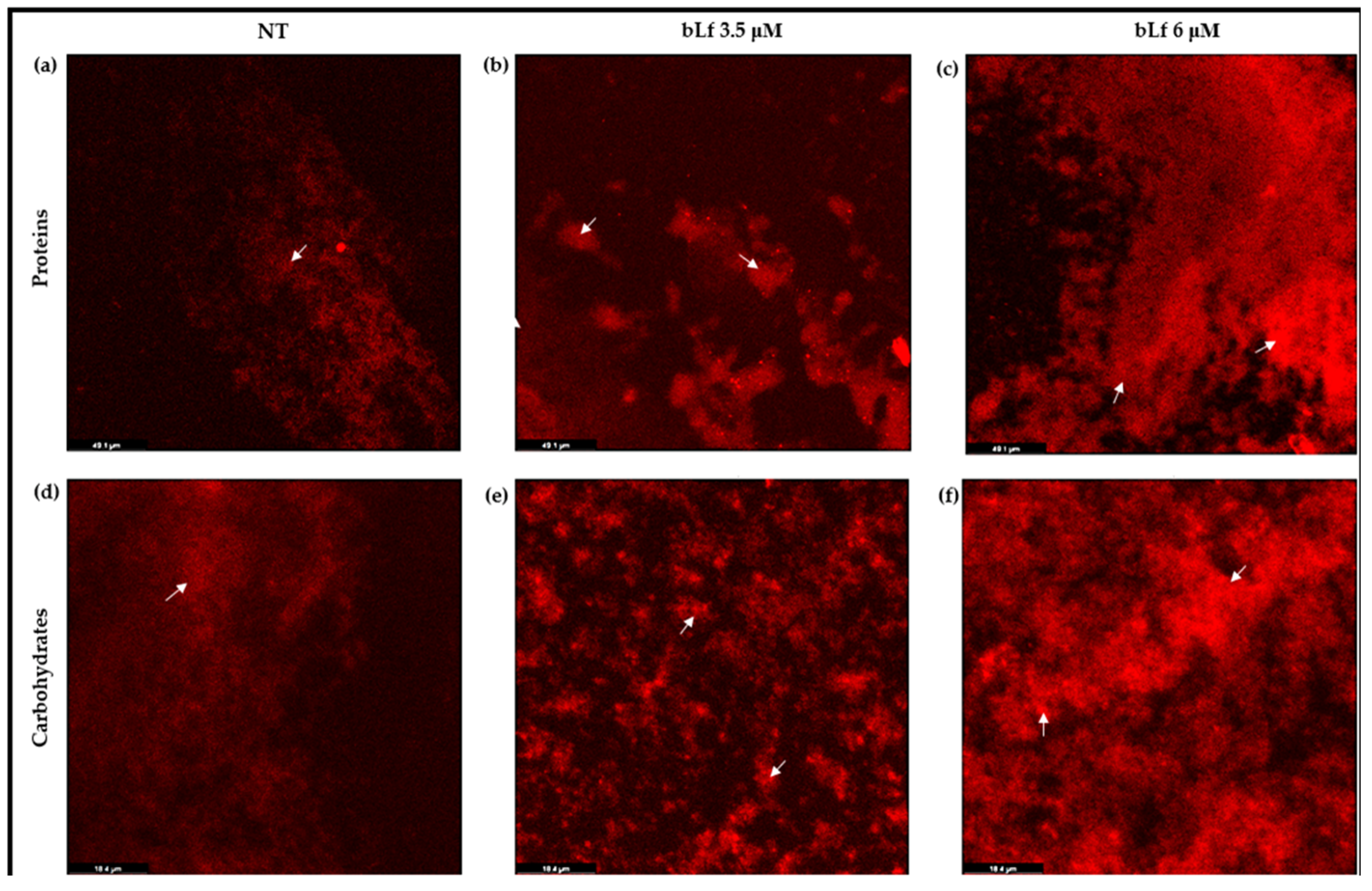
2.5. Proteins and Carbohydrates Are Increased in Biofilms When bLf Is Added
2.6. Protein Pattern of the Biofilm of M. haemolytica A2
2.7. Bovine Lactoferrin Is Present on the Biofilm of M. haemolytica A2
2.8. Viability of M. haemolytica A2 Cells in Biofilms with bLf
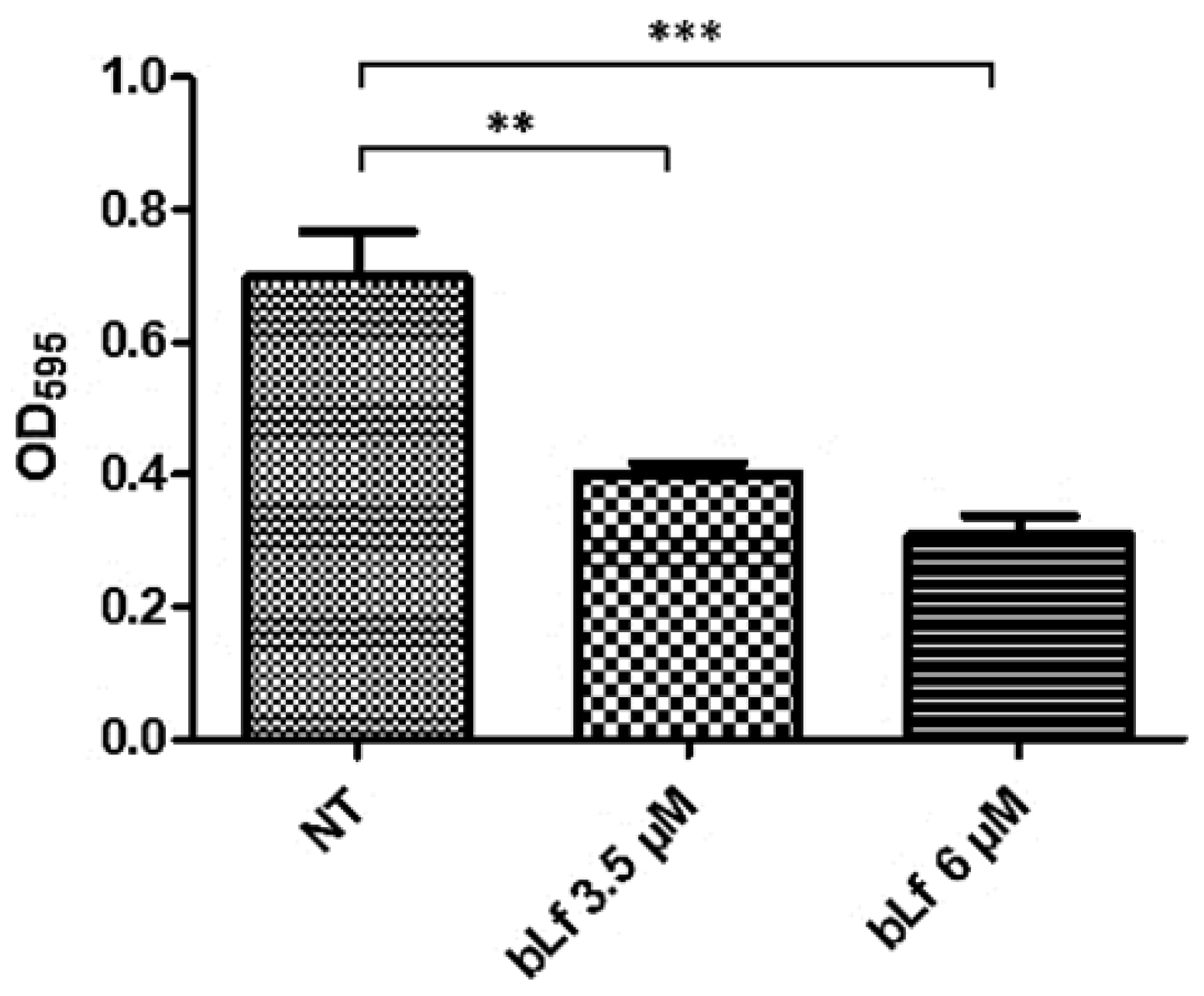
2.9. Bovine Lactoferrin Affects the Formation of a New Biofilm Cycle in M. haemolytica A2
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Culture
4.2. Viability of M. haemolytica A2 in the Presence of Bovine Lactoferrin
4.3. Effect of Bovine Lactoferrin on Biofilm Formation of M. haemolytica A2
4.4. Morphological Observation
4.5. Biofilm Macromolecular Analysis
4.6. SDS-PAGE
4.7. Western Blot
4.8. FITC-Labelled bLf and Laser Confocal Microscopy
4.9. Viability of Bacteria in a Biofilm with bLf
4.10. Formation of a New Biofilm Cycle
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, R.; Mahmood, F.; Ali, H.M.; Siddique, A.B. Bacterial, PCR and Clinico-Pathological Diagnosis of Naturally Occurring Pneumonic Pasturellosis (Mannheimiosis) during Subtropical Climate in Sheep. Microb. Pathog. 2017, 112, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, M.; Nigussie, T.; Kebede, I.A. Isolation and Identification of Pasteurella multocida and Mannheimia haemolytica from Pneumonic Small Ruminants and Their Antibiotic Susceptibility in Haramaya District, Eastern Ethiopia. Sci. World J. 2024, 2024, 5605552. [Google Scholar] [CrossRef]
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial-Host Interactions in Bovine Pneumonia. Vet. Pathol. 2011, 48, 338–348. [Google Scholar] [CrossRef]
- Highlander, S.K. Molecular Genetic Analysis of Virulence in Mannheimia (pasteurella) haemolytica. Front. Biosci. 2001, 6, 1128–1150. [Google Scholar] [CrossRef]
- het Lam, J.; Derkman, T.H.J.; van Garderen, E.; Dijkman, R.; van Engelen, E. Distinct Mannheimia haemolytica Serotypes Isolated from Fatal Infections in Veal Calves and Dairy Cows. Vet. J. 2023, 292, 105940. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.C.; Jolley, W.L.; Purdy, C.W. Characterization of Neuraminidases Produced by Various Serotypes of Pasteurella haemolytica. Infect. Immun. 1993, 61, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Zecchinon, L.; Fett, T.; Desmecht, D. How Mannheimia haemolytica Defeats Host Defence through a Kiss of Death Mechanism. Vet. Res. 2005, 36, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Rico, G.; Martínez-Castillo, M.; González-Ruíz, C.; Luna-Castro, S.; de la Garza, M. Mannheimia haemolytica A2 Secretes Different Proteases into the Culture Medium and in Outer Membrane Vesicles. Microb. Pathog. 2017, 113, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kragh, K.N.; Tolker-Nielsen, T.; Lichtenberg, M. The Non-Attached Biofilm Aggregate. Commun. Biol. 2023, 6, 898. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial Resistance in Biofilm-Associated Bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Boukahil, I.; Czuprynski, C.J. Characterization of Mannheimia haemolytica Biofilm Formation in Vitro. Vet. Microbiol. 2015, 175, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, F.; Rezaee, M.A.; Ebrahimzadeh, S.; Yousefi, L.; Nouri, R.; Kafil, H.S.; Gholizadeh, P. Novel Strategies to Combat Bacterial Biofilms. Mol. Biotechnol. 2021, 63, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Grooters, K.E.; Ku, J.C.; Richter, D.M.; Krinock, M.J.; Minor, A.; Li, P.; Kim, A.; Sawyer, R.; Li, Y. Strategies for Combating Antibiotic Resistance in Bacterial Biofilms. Front. Cell. Infect. Microbiol. 2024, 14, 1352273. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Gómez, C.; Ramírez-Rico, G.; Ruiz-Mazón, L.; Sicairos, N.L.; Serrano-Luna, J.; de la Garza, M. Lactoferrin: An Effective Weapon in the Battle Against Bacterial Infections. Curr. Pharm. Des. 2022, 28, 3243–3260. [Google Scholar] [CrossRef]
- Dashper, S.G.; Pan, Y.; Veith, P.D.; Chen, Y.Y.; Toh, E.C.Y.; Liu, S.W.; Cross, K.J.; Reynolds, E.C. Lactoferrin Inhibits Porphyromonas gingivalis Proteinases and Has Sustained Biofilm Inhibitory Activity. Antimicrob. Agents Chemother. 2012, 56, 1548–1556. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Kobayashi, T.; Yaeshima, T.; Iwatsuki, K.; Yoshie, H. Inhibitory Effects of Lactoferrin on Growth and Biofilm Formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents Chemother. 2009, 53, 3308–3316. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Brown, E.L.; Guion, C.E.; Chen, J.Z.; McMahon, R.J.; Cleary, T.G. Effect of Lactoferrin on Enteroaggregative E. coli (EAEC). Biochem. Cell. Biol. 2006, 84, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Ehara, T.; Matsumoto, T. Inhibitory Effects of Lactoferrin on Biofilm Formation in Clinical Isolates of Pseudomonas aeruginosa. J. Infect. Chemother. 2012, 18, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Francis, J.D.; Guevara, M.A.; Moore, R.E.; Chambers, S.A.; Doster, R.S.; Eastman, A.J.; Rogers, L.M.; Noble, K.N.; Manning, S.D.; et al. Antibacterial and Anti-Biofilm Activity of the Human Breast Milk Glycoprotein Lactoferrin against Group B Streptococcus. ChemBioChem 2021, 22, 2124–2133. [Google Scholar] [CrossRef]
- Francesca, B.; Ajello, M.; Bosso, P.; Morea, C.; Andrea, P.; Giovanni, A.-O.; Piera, V. Both Lactoferrin and Iron Influence Aggregation and Biofilm Formation in Streptococcus mutans. Biometals 2004, 17, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Luna-Castro, S.; Aguilar-Romero, F.; Samaniego-Barrón, L.; Godínez-Vargas, D.; De La Garza, M. Effect of Bovine Apo-Lactoferrin on the Growth and Virulence of Actinobacillus pleuropneumoniae. BioMetals 2014, 27, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Nunogami, S.; Taya, M. Antibiofilm Effect of Warfarin on Biofilm Formation of Escherichia coli Promoted by Antimicrobial Treatment. J. Glob. Antimicrob. Resist. 2016, 7, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Avalos-Gómez, C.; Reyes-López, M.; Ramírez-Rico, G.; Díaz-Aparicio, E.; Zenteno, E.; González-Ruiz, C.; De La Garza, M. Effect of Apo-Lactoferrin on Leukotoxin and Outer Membrane Vesicles of Mannheimia haemolytica A2. Vet. Res. 2020, 51, 36. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Martinez-Castillo, M.; Avalos-Gómez, C.; de la Garza, M. Bovine Apo-Lactoferrin Affects the Secretion of Proteases in Mannheimia haemolytica A2. Access Microbiol. 2021, 3, 000269. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.R. Treatment and Control of Respiratory Disease in Sheep. Vet. Clin. North Am.—Food Anim. Pract. 2011, 27, 175–186. [Google Scholar] [CrossRef]
- Boukahil, I.; Czuprynski, C.J. Mannheimia haemolytica Biofilm Formation on Bovine Respiratory Epithelial Cells. Vet. Microbiol. 2016, 197, 129–136. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Zamudio, U.A.; Vidal, J.E.; Nazmi, K.; Bolscher, J.G.M.; Leon-Sicairos, C.; Antezana, B.S.; Canizalez-Roman, A.; León-Sicairos, N. Lactoferrin Disaggregates Pneumococcal Biofilms and Inhibits Acquisition of Resistance Through Its DNase Activity. Front. Microbiol. 2019, 10, 2386. [Google Scholar] [CrossRef]
- Ammons, M.C.; Copié, V. Mini-Review: Lactoferrin: A Bioinspired, Anti-Biofilm Therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Hagiwara, K.; Matsuyama, J.; Kiyosawa, I. Binding of Lactoferrin to Bacterial Cells of the Clostridium Species and Their Agglutination. Biosci. Biotechnol. Biochem. 1998, 62, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Erdei, J.; Forsgren, A.; Naidu, A.S. Lactoferrin Binds to Porins OmpF and OmpC in Escherichia coli. Infect. Immun. 1994, 62, 1236–1240. [Google Scholar] [CrossRef]
- Samaniego-Barrón, L.; Luna-Castro, S.; Piña-Vázquez, C.; Suárez-Güemes, F.; De La Garza, M. Two Outer Membrane Proteins Are Bovine Lactoferrin-Binding Proteins in Mannheimia haemolytica A1. Vet. Res. 2016, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.B.; Ward, L.S.; Fisher, S.T.; Wolcott, R.D.; James, G.A. In Vitro Susceptibility of Established Biofilms Composed of a Clinical Wound Isolate of Pseudomonas aeruginosa Treated with Lactoferrin and Xylitol. Int. J. Antimicrob. Agents 2009, 33, 230–236. [Google Scholar] [CrossRef]
- Ruangcharoen, S.; Suwannarong, W.; Lachica, M.R.C.T.; Bolscher, J.G.M.; Nazmi, K.; Khunkitti, W.; Taweechaisupapong, S. Killing Activity of LFchimera on Periodontopathic Bacteria and Multispecies Oral Biofilm Formation in Vitro. World J. Microbiol. Biotechnol. 2017, 33, 1–10. [Google Scholar] [CrossRef]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm Dispersal: Multiple Elaborate Strategies for Dissemination of Bacteria with Unique Properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rico, G.; Martinez-Castillo, M.; Ruiz-Mazón, L.; Meneses-Romero, E.P.; Palacios, J.A.F.; Díaz-Aparicio, E.; Abascal, E.N.; de la Garza, M. Identification, Biochemical Characterization, and In Vivo Detection of a Zn-Metalloprotease with Collagenase Activity from Mannheimia haemolytica A2. Int. J. Mol. Sci. 2024, 25, 1289. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2010, 47, e2437. [Google Scholar] [CrossRef]
- Shatila, F.; Yaşa, İ.; Yalçın, H.T. Biofilm Formation by Salmonella enterica Strains. Curr. Microbiol. 2021, 78, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J.S.C. Assays for Determination of Protein Concentration. Curr. Protoc. Pharmacol. 2016, 2016, A.3A.1–A.3A.32. [Google Scholar] [CrossRef]
- Chaganti, L.K.; Venkatakrishnan, N.; Bose, K. An Efficient Method for FITC Labelling of Proteins Using Tandem Affinity Purification. Biosci. Rep. 2018, 38, BSR20181764. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Mazón, L.; Ramírez-Rico, G.; de la Garza, M. Lactoferrin Affects the Viability of Bacteria in a Biofilm and the Formation of a New Biofilm Cycle of Mannheimia haemolytica A2. Int. J. Mol. Sci. 2024, 25, 8718. https://doi.org/10.3390/ijms25168718
Ruiz-Mazón L, Ramírez-Rico G, de la Garza M. Lactoferrin Affects the Viability of Bacteria in a Biofilm and the Formation of a New Biofilm Cycle of Mannheimia haemolytica A2. International Journal of Molecular Sciences. 2024; 25(16):8718. https://doi.org/10.3390/ijms25168718
Chicago/Turabian StyleRuiz-Mazón, Lucero, Gerardo Ramírez-Rico, and Mireya de la Garza. 2024. "Lactoferrin Affects the Viability of Bacteria in a Biofilm and the Formation of a New Biofilm Cycle of Mannheimia haemolytica A2" International Journal of Molecular Sciences 25, no. 16: 8718. https://doi.org/10.3390/ijms25168718
APA StyleRuiz-Mazón, L., Ramírez-Rico, G., & de la Garza, M. (2024). Lactoferrin Affects the Viability of Bacteria in a Biofilm and the Formation of a New Biofilm Cycle of Mannheimia haemolytica A2. International Journal of Molecular Sciences, 25(16), 8718. https://doi.org/10.3390/ijms25168718






