Galectin-2 Agglutinates Helicobacter pylori via Lipopolysaccharide Containing H Type I Under Weakly Acidic Conditions
Abstract
:1. Introduction
2. Results
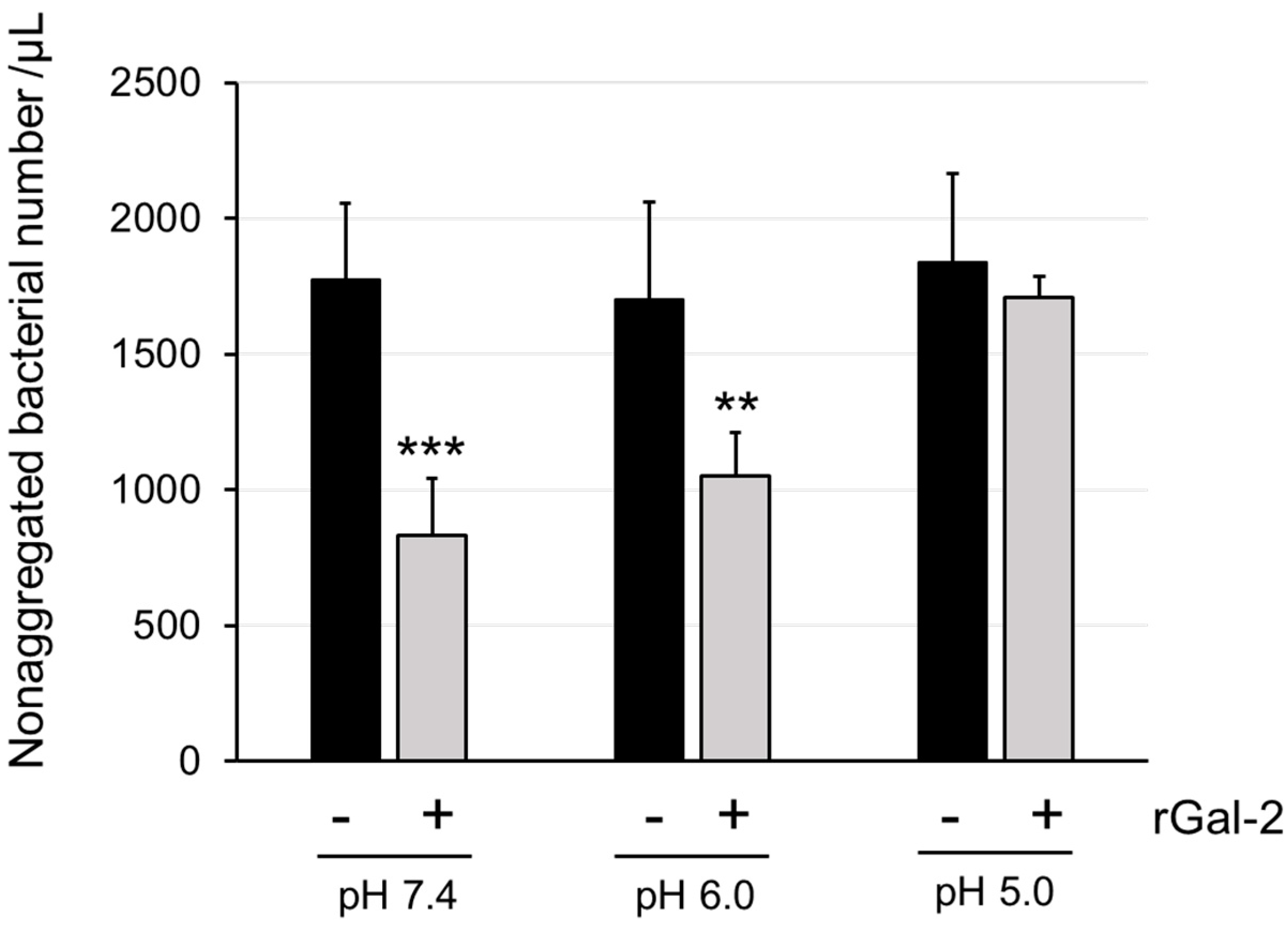
2.1. Gal-2 Agglutinates H. pylori at pH 6.0 but Not at pH 5.0
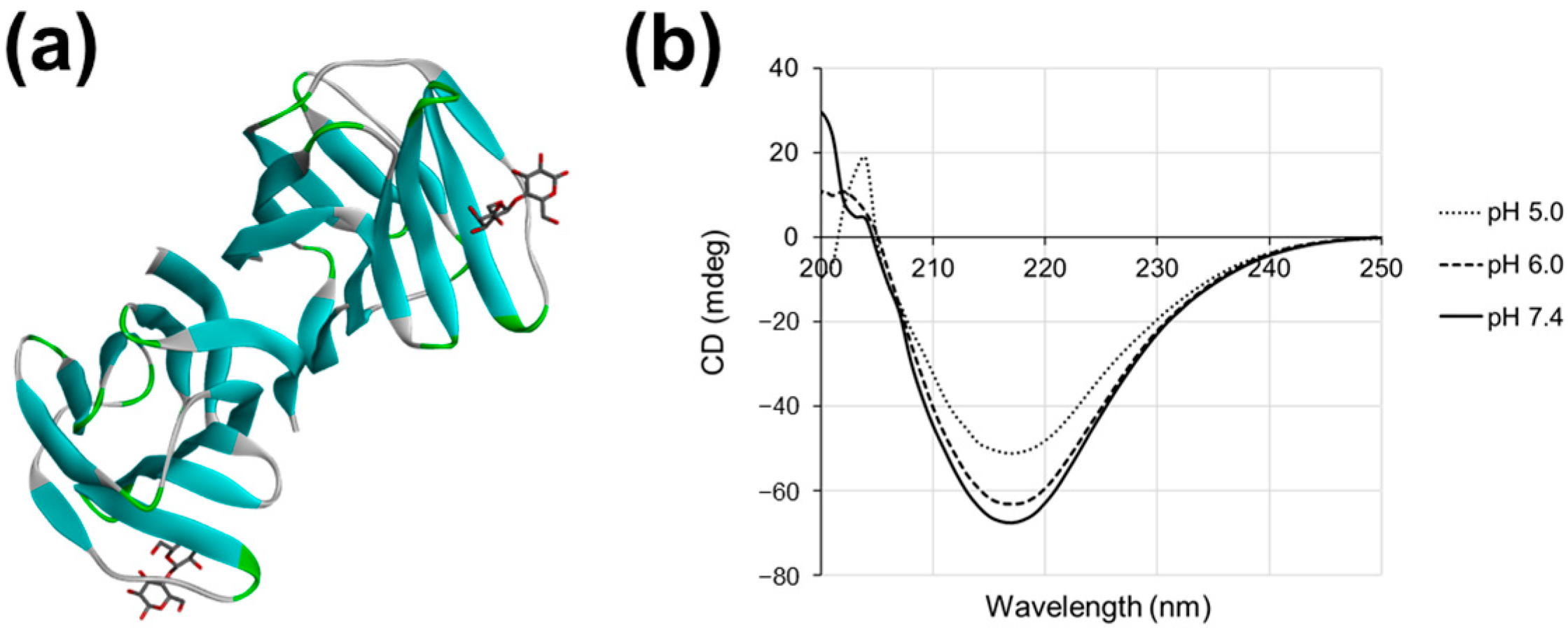
2.2. Gal-2 Structure Is Altered at pH 5.0
2.3. Helicobacter pylori Contains LPS with Lewis Antigen
2.4. The Structure of Gal-3 Is Altered at pH 4.3 and Binds to H. pylori LPS Containing H Type I and Lewis X Structures
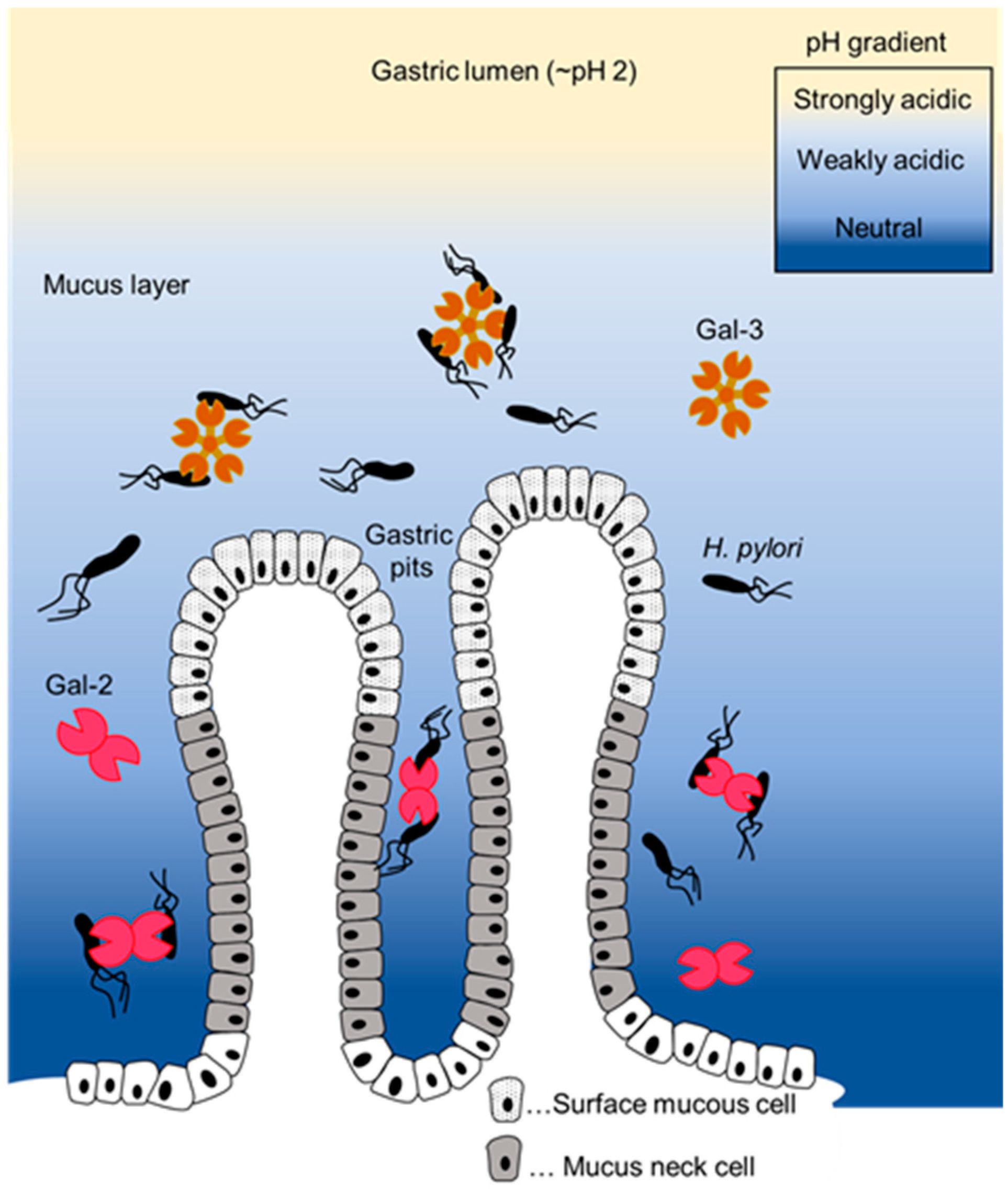
3. Discussion
4. Materials and Methods
4.1. Preparation of Recombinant Gal-2 and -3 Proteins
4.2. Helicobacter pylori Aggregation Assay
4.3. Circular Dichroism Spectroscopy
4.4. Preparation of H. pylori Lipopolysaccharides
4.5. Purification and Chemical Analysis of LPS
4.6. Affinity Chromatography
4.7. Silver Staining and Western Blotting
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nio-Kobayashi, J. Histological Mapping and Subtype-Specific Functions of Galectins in Health and Disease. Trends Glycosci. Glycotechnol. 2018, 30, SE89–SE96. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.-T.; Rabinovich, G.A.; Stowell, S.R.; Vasta, G.R. Galectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2022. [Google Scholar]
- Günther, J.; Galuska, S.P. A brief history of galectin evolution. Front. Immunol. 2023, 14, 1147356. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Elola, M.T.; Blidner, A.G.; Sarrias, L.; Espelt, M.V.; Rabinovich, G.A. The universe of galectin-binding partners and their functions in health and disease. J. Biol. Chem. 2023, 299, 105400. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Baum, L.G.; Tinari, N.; Paganelli, R.; Natoli, C.; Liu, F.T.; Iacobelli, S. Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002, 23, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.; Dey, C.; Bojarová, P.; Křen, V.; Elling, L. Methods of in vitro study of galectin-glycomaterial interaction. Biotechnol. Adv. 2022, 58, 107928. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Negedu, M.N.; Duckworth, C.A.; Yu, L.G. Galectin-2 in Health and Diseases. Int. J. Mol. Sci. 2022, 24, 341. [Google Scholar] [CrossRef] [PubMed]
- Nio-Kobayashi, J. Tissue- and cell-specific localization of galectins, beta-galactose-binding animal lectins, and their potential functions in health and disease. Anat. Sci. Int. 2017, 92, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Nio, J.; Kon, Y.; Iwanaga, T. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J. Histochem. Cytochem. 2005, 53, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Sato, D.; Nakajima, M.; Saito, M.; Sasaki, T.; Tanaka, T.; Hatanaka, T.; Takeuchi, T.; Arata, Y. Identification of Galectin-2-Mucin Interaction and Possible Formation of a High Molecular Weight Lattice. Biol. Pharm. Bull. 2017, 40, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, H.J.; Yeom, J.; Yoo, C.; Shin, J.; Yoo, J.; Kang, C.S.; Lee, C. Lowered expression of galectin-2 is associated with lymph node metastasis in gastric cancer. J. Gastroenterol. 2012, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Wang, T.C. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007, 98, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Saito, R.; Oyama, M.; Takeuchi, T.; Tanaka, T.; Natsume, H.; Tamura, M.; Arata, Y.; Hatanaka, T. Galectin-2 Has Bactericidal Effects against Helicobacter pylori in a β-galactoside-Dependent Manner. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.M.; Ross, I.N.; Turnberg, L.A. Demonstration of a pH gradient across the mucus layer on the surface of human gastric mucosa in vitro. Gut 1982, 23, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Scheid, P. Gastric mucus of the guinea pig: Proton carrier and diffusion barrier. Am. J. Physiol. 1997, 272, G63–G70. [Google Scholar] [CrossRef] [PubMed]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Konradt, M.; Groll, C.; Scheid, P.; Hanauer, G.; Werling, H.O.; Josenhans, C.; Suerbaum, S. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl. Acad. Sci. USA 2004, 101, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers. 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ge, Z.; Rasko, D.A.; Taylor, D.E. Lewis antigens in Helicobacter pylori: Biosynthesis and phase variation. Mol. Microbiol. 2000, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.; Thomas, R.J.; Atherton, J.; Roberts, I.S.; High, N.J. Galectin-3 binds to Helicobacter pylori O-antigen: It is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006, 8, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Hagiwara, S.; Hsu, D.K.; Liu, F.T.; Yoshie, O. Galectin-3 Plays an Important Role in Innate Immunity to Gastric Infection by Helicobacter pylori. Infect. Immun. 2016, 84, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, G.O.; Monteiro, M.A.; Pang, H.; Walsh, E.J.; Moran, A.P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): Structure of the O antigen chain and core oligosaccharide regions. Biochemistry 1996, 35, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Altman, E.; Chandan, V.; Li, J.; Vinogradov, E. A reinvestigation of the lipopolysaccharide structure of Helicobacter pylori strain Sydney (SS1). FEBS. J. 2011, 278, 3484–3493. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Takeuchi, K.; Takeuchi, H. Involvement of histidine residues in the pH-dependent β-galactoside binding activity of human galectin-1. Biochemistry 2013, 52, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, M.; Tamura, M.; Fujii, N.; Takeuchi, T.; Hatanaka, T.; Kishimoto, S.; Arata, Y.; Takahashi, H. Structural mechanisms for the S-nitrosylation-derived protection of mouse galectin-2 from oxidation-induced inactivation revealed by NMR. FEBS. J. 2018, 285, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
- von Mach, T.; Carlsson, M.C.; Straube, T.; Nilsson, U.; Leffler, H.; Jacob, R. Ligand binding and complex formation of galectin-3 is modulated by pH variations. Biochem. J. 2014, 457, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Liao, T.; Debowski, A.W.; Nilsson, H.O.; Fulurija, A.; Haslam, S.M.; Mulloy, B.; Dell, A.; Stubbs, K.A.; et al. The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 2017, 13, e1006280. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Correia, V.G.; Moreira, A.S.P.; Domingues, M.R.M.; Ferreira, R.M.; Figueiredo, C.; Azevedo, N.F.; Marcos-Pinto, R.; Carneiro, F.; Magalhães, A.; et al. Helicobacter pylori lipopolysaccharide structural domains and their recognition by immune proteins revealed with carbohydrate microarrays. Carbohydr. Polym. 2021, 253, 117350. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tsujii, M.; Tanimura, H.; Tsutsui, S.; Tsuji, S.; Takeda, A.; Inoue, A.; Fukui, H.; Yoshio, T.; Kishida, O.; et al. Comparative study of esomeprazole and lansoprazole in triple therapy for eradication of Helicobacter pylori in Japan. World J. Gastroenterol. 2014, 20, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, M.; Miura, M.; Hanawa, M.; Hirose, Y.; Kitahashi, A.; Imamura, N.; Watanabe, I.; Takaso, K.; Shimura, N.; Yoda, Y. Efficacy and tolerability of second-line metronidazole triple therapy using vonoprazan for helicobacter pylori eradication in japan—Comparative study: Vonoprazan vs. proton pump inhibitors. Open J. Gastroenterol. 2018, 8, 27–38. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Timmer, B.J.J.; Kooijman, A.; Schaapkens, X.; Mooibroek, T.J. A Synthetic Galectin Mimic. Angew. Chem. Int. Ed. Engl. 2021, 60, 16178–16183. [Google Scholar] [CrossRef] [PubMed]
- Bukholm, G.; Tannaes, T.; Nedenskov, P.; Esbensen, Y.; Grav, H.J.; Hovig, T.; Ariansen, S.; Guldvog, I. Colony variation of Helicobacter pylori: Pathogenic potential is correlated to cell wall lipid composition. Scand. J. Gastroenterol. 1997, 32, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Amirmozaffari, N.; Tabarraei, B.; Jeddi-Tehrani, M.; Zarei, O.; Alizadeh, R.; Masjedian, F.; Zarnani, A.H. Extraction, Purification and Characterization of Lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna. J. Med. Biotechnol. 2011, 3, 3–9. [Google Scholar] [PubMed]
- Davis, M.R., Jr.; Goldberg, J.B. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J. Vis. Exp. 2012, 63, 3916. [Google Scholar] [CrossRef]
- Isshiki, Y.; Kondo, S. Characterization of the carbohydrate backbone of Vibrio parahaemolyticus O6 lipopolysaccharides. Microbiol. Immunol. 2011, 55, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Roberts, N.R.; Leiner, K.Y.; Wu, M.L.; Farr, A.L. The quantitative histochemistry of brain. I. Chemical methods. J. Biol. Chem. 1954, 207, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Nakamura, R.; Hamasaki, M.; Oyama, M.; Hamano, S.; Hatanaka, T. In vitro evaluation of the effect of galectins on Schistosoma mansoni motility. BMC Res. Notes 2023, 16, 266. [Google Scholar] [CrossRef] [PubMed]


| Components | % (w/w) | nmol/mg | Molar Ratio |
|---|---|---|---|
| Sugars | |||
| Fucose | 3.0 | 181 | 3.2 |
| Ribose | 8.6 | 572 | 10.1 |
| Galactose | 6.4 | 355 | 6.3 |
| Glucose | 4.2 | 234 | 4.1 |
| D-glycero-D-manno-Heptose | 3.8 | 179 | 3.2 |
| L-glycero-D-manno-Heptose | 2.4 | 113 | 2.0 |
| N-Acetylglucosamine | 7.3 | 332 | 5.9 |
| Phosphorous | |||
| Total | 3.3 | 333 | 5.9 |
| Fatty acids | |||
| Palmitic acid | 0.5 | 20 | 0.4 |
| Octadecanoic acid | 3.2 | 113 | 2.0 |
| 3-Hydroxy-palmitic acid | 0.9 | 35 | 0.6 |
| 3-Hydroxy-octadecanoic acid | 4.5 | 149 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T.; Oyama, M.; Kubota, M.; Isshiki, Y.; Takeuchi, T.; Tanaka, T.; Tanikawa, T.; Tamura, M.; Arata, Y.; Hatanaka, T. Galectin-2 Agglutinates Helicobacter pylori via Lipopolysaccharide Containing H Type I Under Weakly Acidic Conditions. Int. J. Mol. Sci. 2024, 25, 8725. https://doi.org/10.3390/ijms25168725
Sasaki T, Oyama M, Kubota M, Isshiki Y, Takeuchi T, Tanaka T, Tanikawa T, Tamura M, Arata Y, Hatanaka T. Galectin-2 Agglutinates Helicobacter pylori via Lipopolysaccharide Containing H Type I Under Weakly Acidic Conditions. International Journal of Molecular Sciences. 2024; 25(16):8725. https://doi.org/10.3390/ijms25168725
Chicago/Turabian StyleSasaki, Takaharu, Midori Oyama, Mao Kubota, Yasunori Isshiki, Tomoharu Takeuchi, Toru Tanaka, Takashi Tanikawa, Mayumi Tamura, Yoichiro Arata, and Tomomi Hatanaka. 2024. "Galectin-2 Agglutinates Helicobacter pylori via Lipopolysaccharide Containing H Type I Under Weakly Acidic Conditions" International Journal of Molecular Sciences 25, no. 16: 8725. https://doi.org/10.3390/ijms25168725






