Pathogenesis of Cerebral Small Vessel Disease: Role of the Glymphatic System Dysfunction
Abstract
1. Introduction
2. Ischemic Cognitive Impairment on CSVD
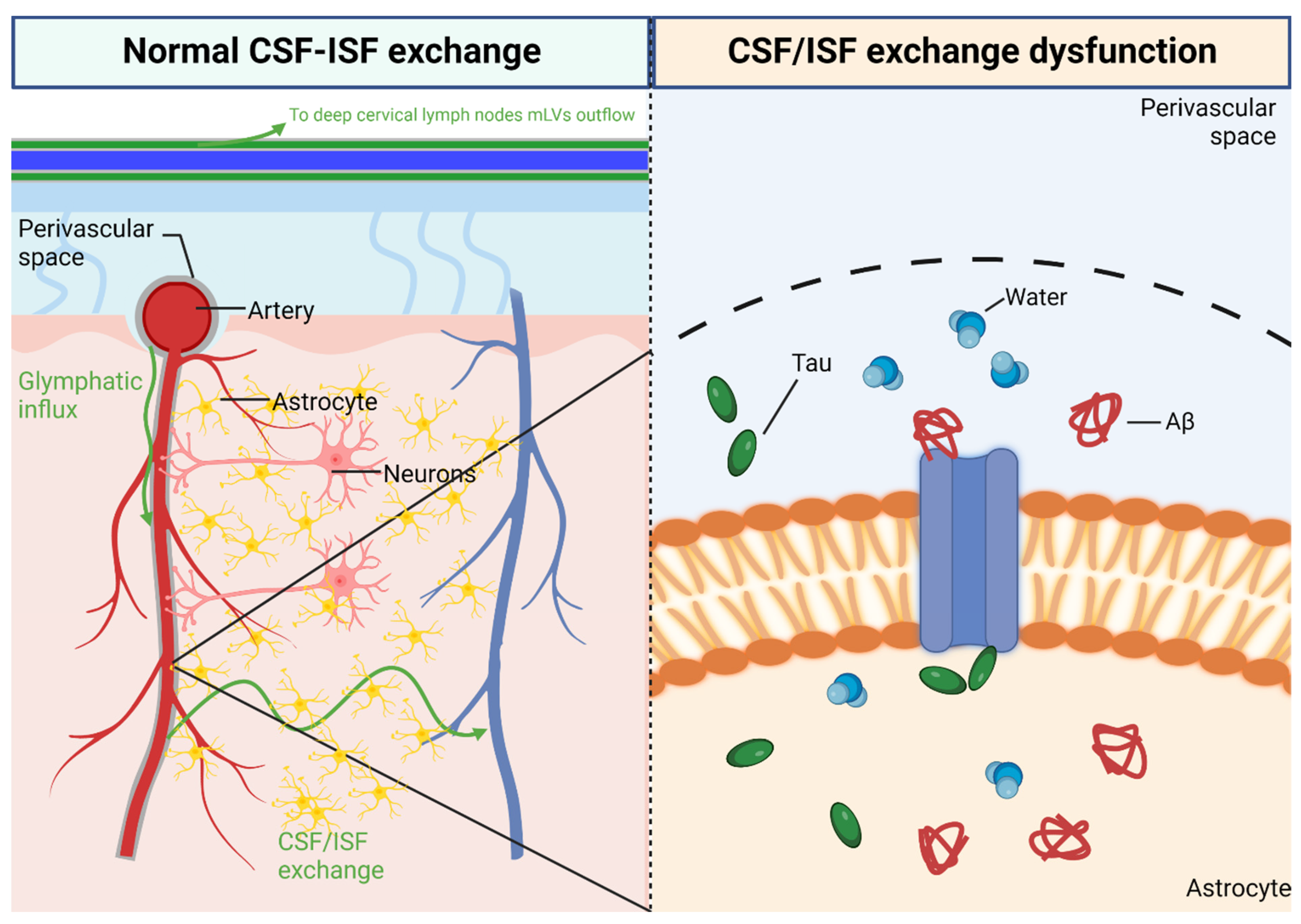
3. Glymphatic System in CNS
3.1. Perivascular Space
3.2. Aquaporin Channel
4. Glymphatic System Dysfunction on CSVD
5. Risk Factors of Glymphatic System Dysfunction on CSVD
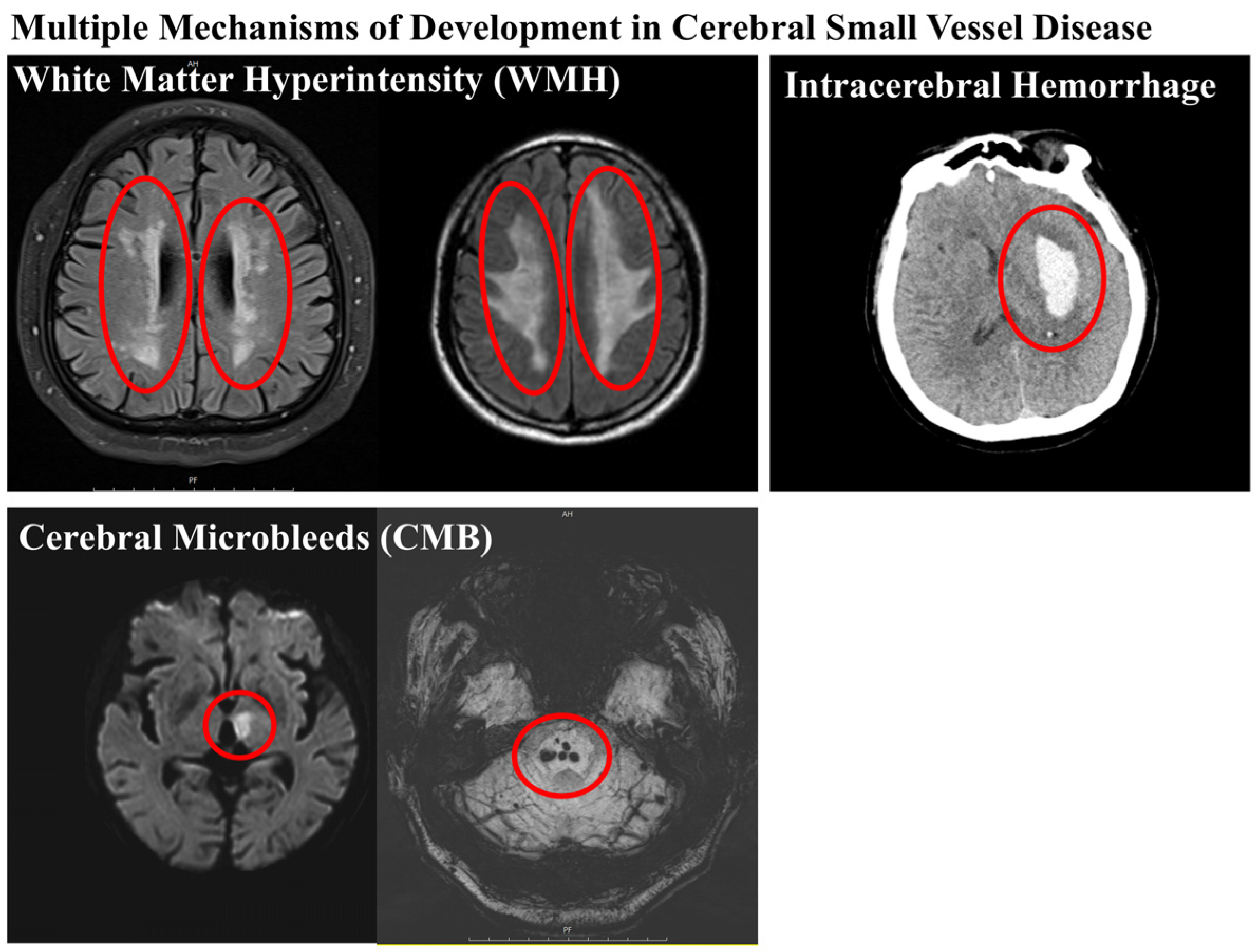
5.1. Cerebral Microbleeds
5.2. White Matter Hyperintensities
5.3. Lacunar Infarct
5.4. Long-Term Outcome of CSVD Patients
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chojdak-Lukasiewicz, J.; Dziadkowiak, E.; Zimny, A.; Paradowski, B. Cerebral small vessel disease: A review. Adv. Clin. Exp. Med. 2021, 30, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Choi, K.S.; Yi, H.J.; Ko, Y.; Kim, Y.S.; Bak, K.H.; Chun, H.J.; Lee, Y.J.; Lee, J.Y. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Sci. Rep. 2018, 8, 4819. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kitamura, A.; Beverley, J.; Koudelka, J.; Duncombe, J.; Lennen, R.; Jansen, M.A.; Marshall, I.; Platt, B.; Wiegand, U.K.; et al. Impaired Glymphatic Function and Pulsation Alterations in a Mouse Model of Vascular Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 788519. [Google Scholar] [CrossRef] [PubMed]
- Bacyinski, A.; Xu, M.; Wang, W.; Hu, J. The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front. Neuroanat. 2017, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yao, D.; Li, R.; Guo, X.; Hao, J.; Xie, M.; Li, J.; Pan, D.; Luo, X.; Yu, Z.; et al. Digoxin Ameliorates Glymphatic Transport and Cognitive Impairment in a Mouse Model of Chronic Cerebral Hypoperfusion. Neurosci. Bull. 2022, 38, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Y.; Reis, C.; Tao, T.; Li, W.; Li, X.; Zhang, J.H. Cerebral Small Vessel Disease. Cell Transplant. 2018, 27, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Schuhmann, M.K.; Garz, C.; Jandke, S.; Urlaub, D.; Mencl, S.; Zernecke, A.; Heinze, H.J.; Carare, R.O.; Kleinschnitz, C.; et al. Hypercholesterolemia induced cerebral small vessel disease. PLoS ONE 2017, 12, e0182822. [Google Scholar] [CrossRef] [PubMed]
- Ter Telgte, A.; van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; de Leeuw, F.E. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.N.; Valdes-Hernandez, M.; Wang, X.; Chappell, F.M.; Shuler, K.; Armitage, P.A.; Carpenter, T.C.; Dennis, M.S. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013, 44, 525–527. [Google Scholar] [CrossRef]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef]
- Salvadori, E.; Brambilla, M.; Maestri, G.; Nicotra, A.; Cova, I.; Pomati, S.; Pantoni, L. The clinical profile of cerebral small vessel disease: Toward an evidence-based identification of cognitive markers. Alzheimer’s Dement. 2023, 19, 244–260. [Google Scholar] [CrossRef]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Rangroo Thrane, V.; Thrane, A.S.; Plog, B.A.; Thiyagarajan, M.; Iliff, J.J.; Deane, R.; Nagelhus, E.A.; Nedergaard, M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013, 3, 2582. [Google Scholar] [CrossRef]
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Gross, P.M.; Weindl, A. Peering through the windows of the brain. J. Cereb. Blood Flow Metab. 1987, 7, 663–672. [Google Scholar] [CrossRef]
- Fayeye, O.; Pettorini, B.L.; Foster, K.; Rodrigues, D. Mesencephalic enlarged Virchow-Robin spaces in a 6-year-old boy: A case-based update. Child’s Nerv. Syst. 2010, 26, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, I.; Galea, I.; Perry, V.H. What is the blood-brain barrier (not)? Trends Immunol. 2007, 28, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Masseguin, C.; Corcoran, M.; Carcenac, C.; Daunton, N.G.; Guell, A.; Verkman, A.S.; Gabrion, J. Altered gravity downregulates aquaporin-1 protein expression in choroid plexus. J. Appl. Physiol. 2000, 88, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Speake, T.; Freeman, L.J.; Brown, P.D. Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim. Biophys. Acta 2003, 1609, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Bhat, R.V.; Preston, G.M.; Guggino, W.B.; Baraban, J.M.; Agre, P. Molecular characterization of an aquaporin cDNA from brain: Candidate osmoreceptor and regulator of water balance. Proc. Natl. Acad. Sci. USA 1994, 91, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Halsey, A.M.; Conner, A.C.; Bill, R.M.; Logan, A.; Ahmed, Z. Aquaporins and Their Regulation after Spinal Cord Injury. Cells 2018, 7, 174. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C. Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010, 168, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.A.; Hsu, M.S.; Seldin, M.M.; Binder, D.K. Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain. ASN Neuro 2015, 7, 1759091415605486. [Google Scholar] [CrossRef]
- Hubbard, J.A.; Szu, J.I.; Binder, D.K. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res. Bull. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Mathiisen, T.M.; Ottersen, O.P. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Zacharek, A.; Cui, C.; Zhang, L.; Li, Q.; Lu, M.; Zhang, T.; Liu, A.; Chen, J. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol. Aging 2017, 50, 96–106. [Google Scholar] [CrossRef]
- Chu, H.; Huang, C.; Ding, H.; Dong, J.; Gao, Z.; Yang, X.; Tang, Y.; Dong, Q. Aquaporin-4 and Cerebrovascular Diseases. Int. J. Mol. Sci. 2016, 17, 1249. [Google Scholar] [CrossRef]
- Ramiro, L.; Simats, A.; Penalba, A.; Garcia-Tornel, A.; Rovira, A.; Mancha, F.; Bustamante, A.; Montaner, J. Circulating Aquaporin-4 as A biomarker of early neurological improvement in stroke patients: A pilot study. Neurosci. Lett. 2020, 714, 134580. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, X.; Zhang, Y.; Alzheimer’s Disease Neuroimaging Initiative. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. Am. J. Neuroradiol. 2011, 32, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Pollock, H.; Hutchings, M.; Weller, R.O.; Zhang, E.T. Perivascular spaces in the basal ganglia of the human brain: Their relationship to lacunes. J. Anat. 1997, 191 Pt 3, 337–346. [Google Scholar] [CrossRef]
- Etemadifar, M.; Hekmatnia, A.; Tayari, N.; Kazemi, M.; Ghazavi, A.; Akbari, M.; Maghzi, A.H. Features of Virchow-Robin spaces in newly diagnosed multiple sclerosis patients. Eur. J. Radiol. 2011, 80, e104–e108. [Google Scholar] [CrossRef] [PubMed]
- Charisis, S.; Rashid, T.; Liu, H.; Ware, J.B.; Jensen, P.N.; Austin, T.R.; Li, K.; Fadaee, E.; Hilal, S.; Chen, C.; et al. Assessment of Risk Factors and Clinical Importance of Enlarged Perivascular Spaces by Whole-Brain Investigation in the Multi-Ethnic Study of Atherosclerosis. JAMA Netw. Open 2023, 6, e239196. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Ois Santiago, A.; Roquer Gonzalez, J.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, A.; Fan, Y.H.; Mok, V.C.T.; Shi, L. Magnetic resonance imaging manifestations of cerebral small vessel disease: Automated quantification and clinical application. Chin. Med. J. 2020, 134, 151–160. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Puy, L.; Pasi, M.; Rodrigues, M.; van Veluw, S.J.; Tsivgoulis, G.; Shoamanesh, A.; Cordonnier, C. Cerebral microbleeds: From depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 598–607. [Google Scholar] [CrossRef]
- Charidimou, A.; Shams, S.; Romero, J.R.; Ding, J.; Veltkamp, R.; Horstmann, S.; Eiriksdottir, G.; van Buchem, M.A.; Gudnason, V.; Himali, J.J.; et al. Clinical significance of cerebral microbleeds on MRI: A comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int. J. Stroke 2018, 13, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Akoudad, S.; Portegies, M.L.; Koudstaal, P.J.; Hofman, A.; van der Lugt, A.; Ikram, M.A.; Vernooij, M.W. Cerebral Microbleeds Are Associated with an Increased Risk of Stroke: The Rotterdam Study. Circulation 2015, 132, 509–516. [Google Scholar] [CrossRef]
- Charidimou, A.; Imaizumi, T.; Moulin, S.; Biffi, A.; Samarasekera, N.; Yakushiji, Y.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.C.; et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology 2017, 89, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.D.; Tivarus, M.E.; Schifitto, G.; Uddin, M.N.; Zhong, J. Brain iron imaging markers in the presence of white matter hyperintensities. Magn. Reson. Imaging 2023, 98, 115–123. [Google Scholar] [CrossRef]
- Young, V.G.; Halliday, G.M.; Kril, J.J. Neuropathologic correlates of white matter hyperintensities. Neurology 2008, 71, 804–811. [Google Scholar] [CrossRef]
- Moran, C.; Phan, T.G.; Srikanth, V.K. Cerebral small vessel disease: A review of clinical, radiological, and histopathological phenotypes. Int. J. Stroke 2012, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Steinke, W.; Ley, S.C. Lacunar stroke is the major cause of progressive motor deficits. Stroke 2002, 33, 1510–1516. [Google Scholar] [CrossRef]
- Schonewille, W.J.; Tuhrim, S.; Singer, M.B.; Atlas, S.W. Diffusion-weighted MRI in acute lacunar syndromes. A clinical-radiological correlation study. Stroke 1999, 30, 2066–2069. [Google Scholar] [CrossRef]
- Cai, M.; Jacob, M.A.; van Loenen, M.R.; Bergkamp, M.; Marques, J.; Norris, D.G.; Duering, M.; Tuladhar, A.M.; de Leeuw, F.E. Determinants and Temporal Dynamics of Cerebral Small Vessel Disease: 14-Year Follow-Up. Stroke 2022, 53, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Kim, J.; Song, D.; Yoo, J.; Lee, H.S.; Kim, Y.J.; Nam, H.S.; Heo, J.H.; Kim, Y.D. Total Cerebral Small-Vessel Disease Score is Associated with Mortality during Follow-Up after Acute Ischemic Stroke. J. Clin. Neurol. 2017, 13, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Hong, D.Y.; Lee, D.H.; Park, S.W.; Lee, J.Y.; Jeong, J.H.; Kim, E.Y.; Chung, H.M.; Hong, K.S.; Park, S.P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Berezuk, C.; Ramirez, J.; Gao, F.; Scott, C.J.; Huroy, M.; Swartz, R.H.; Murray, B.J.; Black, S.E.; Boulos, M.I. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep 2015, 38, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Kerr, S.; McVey, C.; Godwin, J. The effectiveness of secondary prevention lifestyle interventions designed to change lifestyle behavior following stroke: Summary of a systematic review. Int. J. Stroke 2012, 7, 243–247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Lee, E.C.; Park, S.-W.; Lee, J.Y.; Lee, M.R.; Oh, J.S. Pathogenesis of Cerebral Small Vessel Disease: Role of the Glymphatic System Dysfunction. Int. J. Mol. Sci. 2024, 25, 8752. https://doi.org/10.3390/ijms25168752
Lee D-H, Lee EC, Park S-W, Lee JY, Lee MR, Oh JS. Pathogenesis of Cerebral Small Vessel Disease: Role of the Glymphatic System Dysfunction. International Journal of Molecular Sciences. 2024; 25(16):8752. https://doi.org/10.3390/ijms25168752
Chicago/Turabian StyleLee, Dong-Hun, Eun Chae Lee, Sang-Won Park, Ji Young Lee, Man Ryul Lee, and Jae Sang Oh. 2024. "Pathogenesis of Cerebral Small Vessel Disease: Role of the Glymphatic System Dysfunction" International Journal of Molecular Sciences 25, no. 16: 8752. https://doi.org/10.3390/ijms25168752
APA StyleLee, D.-H., Lee, E. C., Park, S.-W., Lee, J. Y., Lee, M. R., & Oh, J. S. (2024). Pathogenesis of Cerebral Small Vessel Disease: Role of the Glymphatic System Dysfunction. International Journal of Molecular Sciences, 25(16), 8752. https://doi.org/10.3390/ijms25168752






