shRNA-Targeting Caspase-3 Inhibits Cell Detachment Induced by Pemphigus Vulgaris Autoantibodies in HaCaT Cells
Abstract
:1. Introduction
2. Results
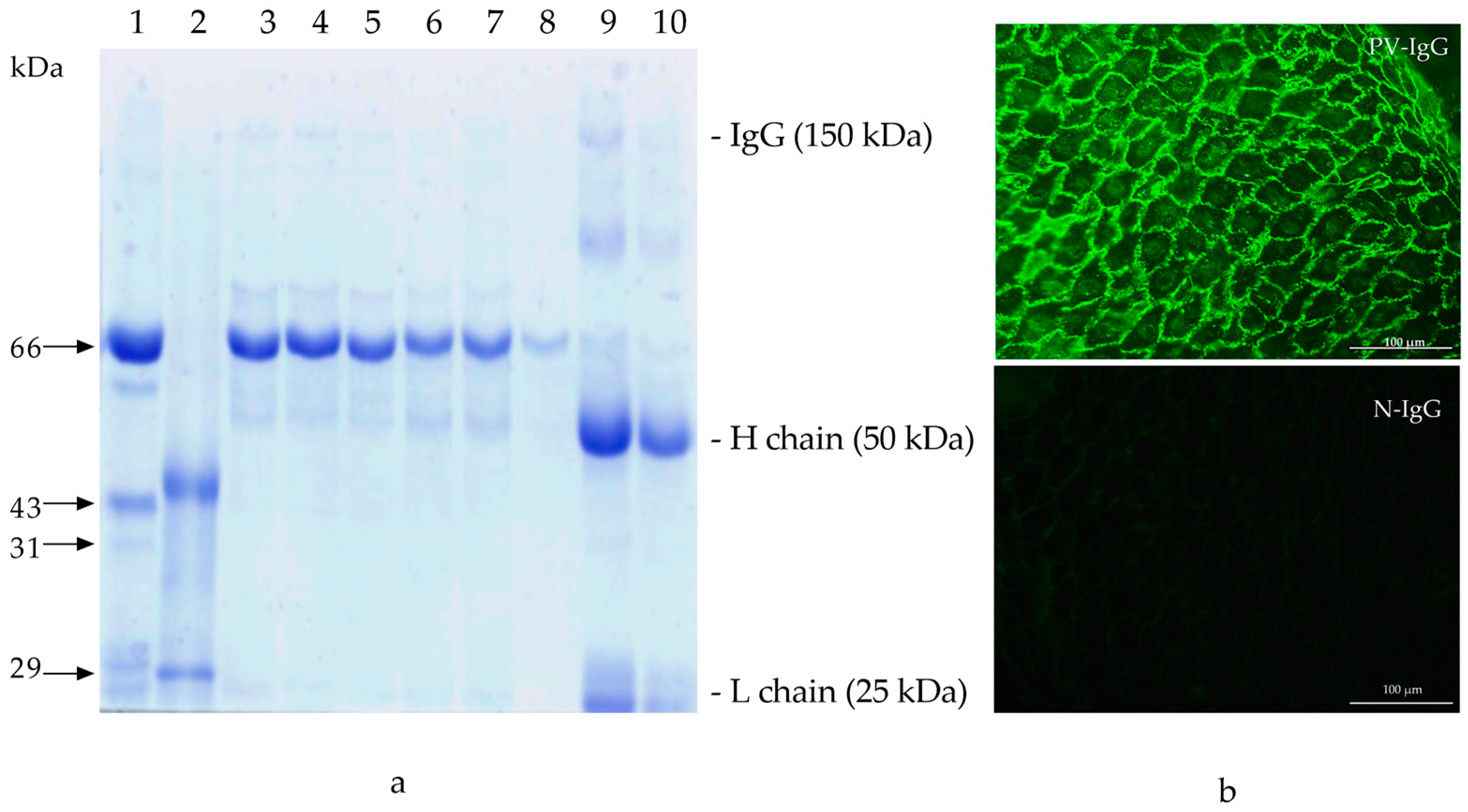
2.1. PV-IgG
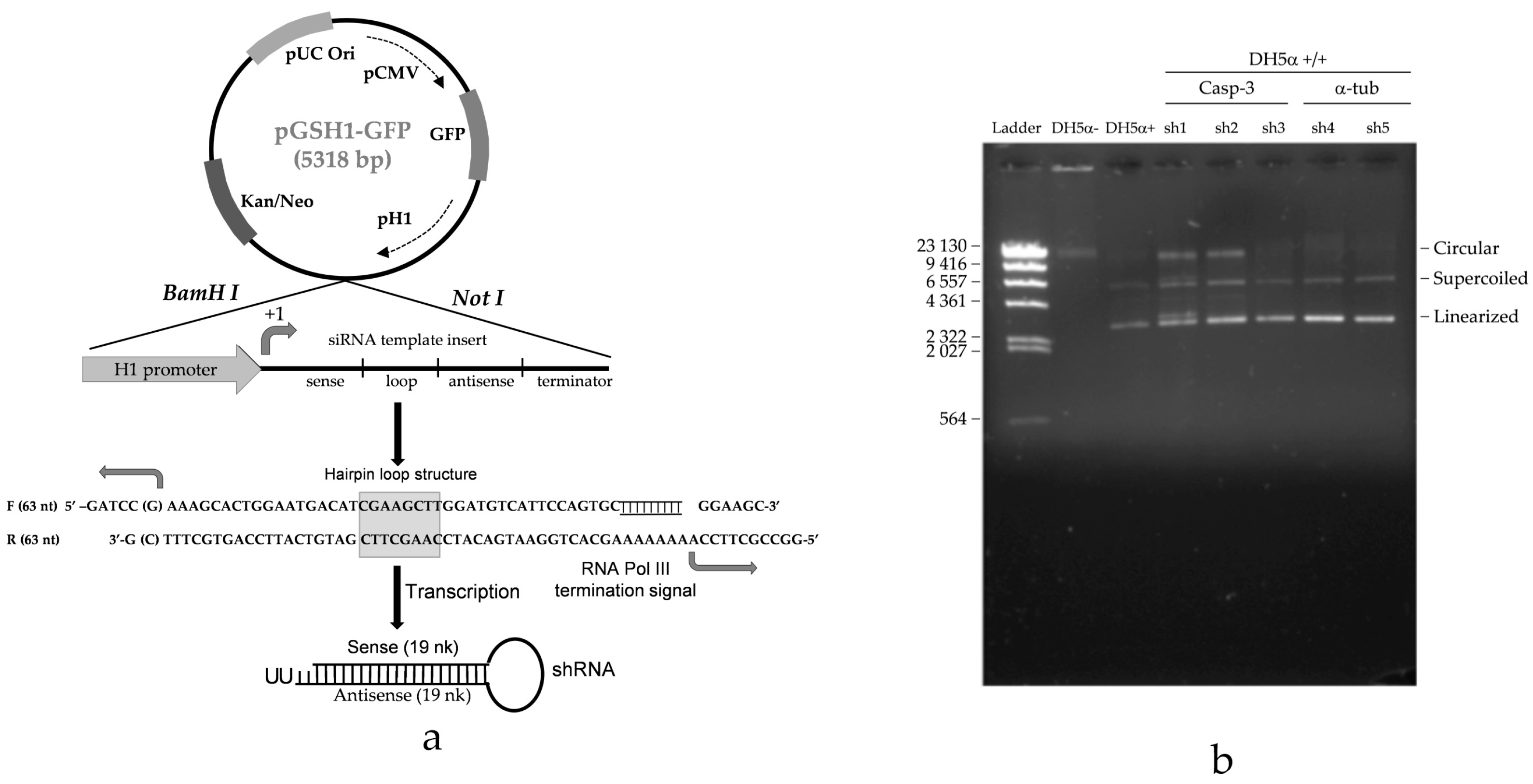
2.2. Casp-3-shRNA Expression in Transformants
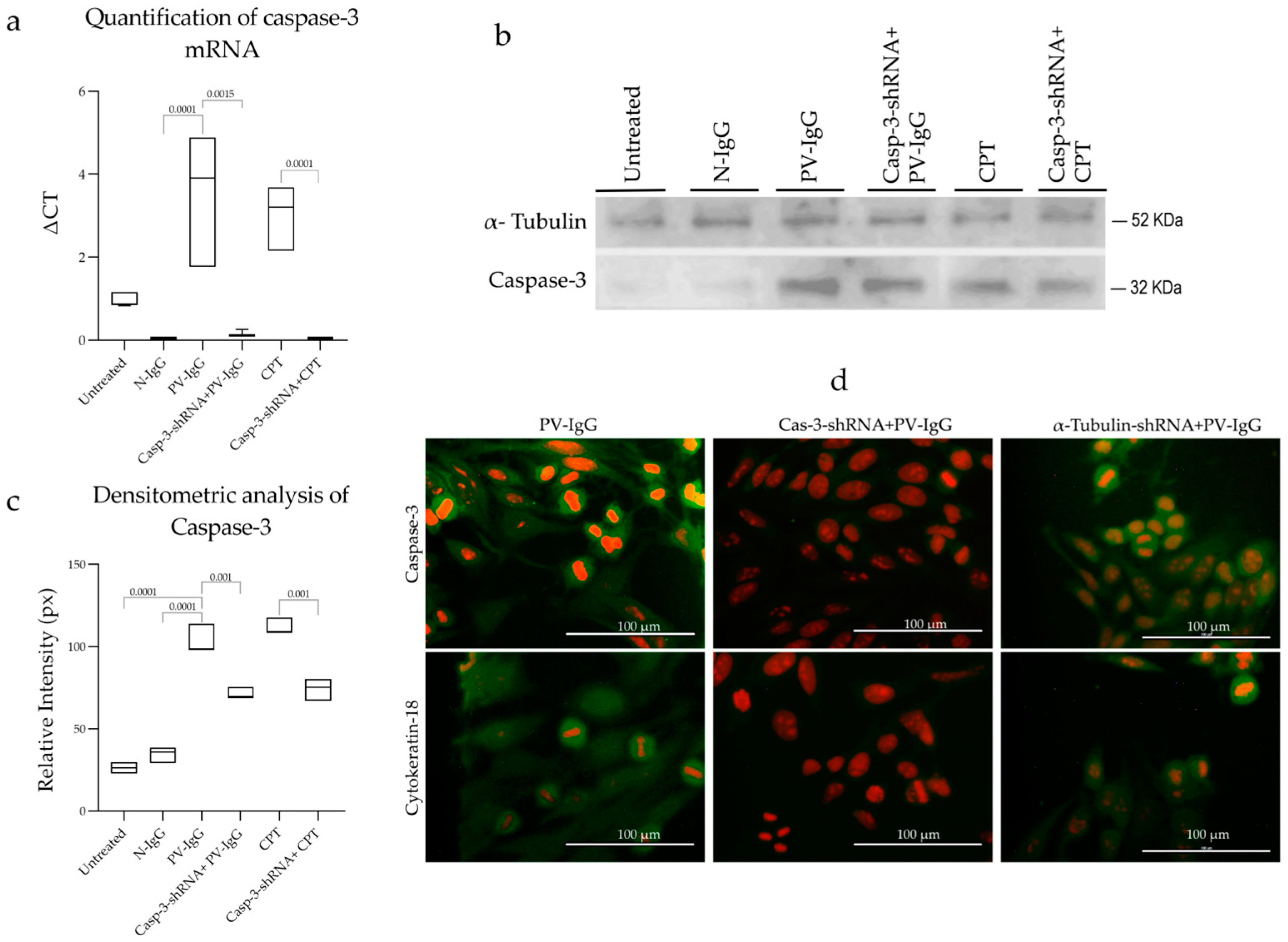
2.3. Effect of shRNA on Caspase-3 of Transfected Cells
2.4. Cell Viability
2.5. Cell Adhesion
2.6. Caspase-3-shRNA Reduces Cell Death Induced by PV-IgG
2.7. Phosphatidylserine (PS) Exposure on the Cell Surface
3. Discussion
4. Materials and Methods
4.1. PV-IgG Characterization
4.2. PV-IgG Purification
4.3. Cell Culture
4.4. Cell Adhesion Assays
4.5. Caspase-3-shRNA-Expressing Vector and Oligo Design for Simple Hairpin
4.6. Transformation of DH5α E. coli (F− Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rk−, mk+) phoA supE44 thi-1 gyrA96 relA1 λ−)
4.7. Isolation of Casp-3-shRNA Expression Vectors from E. coli Transformants
4.8. Transfection of Caspase-3-shRNA
4.9. Nonadherent Cell Viability Monitoring via Flow Cytometry
4.10. Labeled Annexin Affinity Assay
4.11. Caspase-3 Gene Expression Analysis via qRT-PCR
4.12. Caspase-3 Protein Expression
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPT | Camptothecin |
| DEVD-cmk | Caspase-3 inhibitor |
| DMEM-HG | Dulbecco’s Modified Eagle Medium high-glucose |
| Dsc | Desmocolin |
| Dsg1 | Desmoglein 1 |
| Dsg3 | Desmoglein 3 |
| EGFR | Epidermal Growth Factor Receptor |
| FITC | Fluorescein Isothiocyanate |
| H&E | Hematoxylin and Eosin |
| IFI | Indirect Immunofluorescence |
| KSFM | Keratinocyte Serum-Free Medium |
| MES | 4-Morpholineethanesulfonic acid hydrate |
| MWCO | Molecular Weight Cutoff Membrane |
| N-IgG | Normal IgG |
| PV-IgG | pemphigus vulgaris-IgG |
| PV | pemphigus vulgaris |
| qRT-PCR | quantitative Reverse Transcriptase Polymerase Chain Reaction |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| shRNA | short hairpin RNA |
References
- Kasperkiewicz, M.; Ellebrecht, C.T.; Takahashi, H.; Yamagami, J.; Zillikens, D.; Payne, A.S.; Amagai, M. Pemphigus. Nat. Rev. Dis. Primers. 2017, 3, 17026. [Google Scholar] [CrossRef]
- Hammers, C.M.; Stanley, J.R. Recent Advances in Understanding Pemphigus and Bullous Pemphigoid. J. Investg. Dermatol. 2020, 140, 733–741. [Google Scholar] [CrossRef]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Caldelari, R.; de Bruin, A.; Baumann, D.; Suter, M.M.; Balmer, V.; Müller, E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgarir. J. Cell Biol. 2001, 153, 823–834. [Google Scholar] [CrossRef]
- Waschke, J.; Spindler, V. Desmosomes and extradesmosomal adhesive signaling contacts in pemphigus. Med. Res. Rev. 2014, 34, 1127–1145. [Google Scholar] [CrossRef]
- Vielmuth, F.; Walter, E.; Fuchs, M.; Radeva, M.; Buechau, F.; Magin, T.M.; Spinder, V.; Waschke, K.J. Keratins regulate p38MAP-dependent desmoglein binding properties in pemphigus. Front. Immunol. 2018, 9, 528. [Google Scholar] [CrossRef]
- Pacheco-Tovar, M.G.; Avalos-Díaz, E.; Vega-Memije, E.; Bollain-y-Goytia, J.J.; López-Robles, E.; Hojyo-Tomoka, M.T.; Domínguez-Soto, L.; Herrera-Esparza, R. The final destiny of acantholytic cells in pemphigus is Fas mediated. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 697–701. [Google Scholar] [CrossRef]
- Lotti, R.; Shu, E.; Petrachi, T.; Marconi, A.; Palazzo, E.; Quadri, M.; Lin, A.; O’Reilly, L.A.; Pincelli, C. Soluble Fas ligand is essential for blister formation in Pemphigus. Front. Immunol. 2018, 9, 370. [Google Scholar] [CrossRef]
- Frusić-Zlotkin, M.; Raichenberg, D.; Wang, X.; David, M.; Michel, B.; Milner, Y. Apoptotic mechanism in pemphigus autoimmunoglobulins-induced acantholysis—Possible involvement of the EGF receptor. Autoimmunity 2006, 39, 563–575. [Google Scholar] [CrossRef]
- Puviani, M.; Marconi, A.; Cozzani, E.; Pincelli, C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J. Investg. Dermatol. 2003, 120, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Bumiller-Bini Hoch, V.; Schneider, L.; Pumpe, A.E.; Lüders, E.; Hundt, J.E.; Boldt, A.B.W. Marked to die-cell death mechanisms for keratinocyte acantholysis in pemphigus diseases. Life 2022, 12, 329. [Google Scholar] [CrossRef]
- Hariton, W.V.J.; Galichet, A.; Berghe, T.V.; Overmiller, A.M.; Mahoney, M.G.; Declercq, W.; Müller, E.J. Feasibility study for clinical application of caspase-3 inhibitors in Pemphigus vulgaris. Exp. Dermatol. 2017, 26, 1274–1277. [Google Scholar] [CrossRef]
- Ning, L.; Wang, M.; Zhao, J.; Liu, Z.; Diaz, L.A. Involvement of apoptotic mechanism in pemphigus foliaceous autoimmune injury of the skin. J. Immunol. 2009, 182, 711–717. [Google Scholar] [CrossRef]
- Pacheco-Tovar, D.; López-Luna, A.; Herrera-Esparza, R.; Avalos-Díaz, E. The caspase pathway as a possible therapeutic target in experimental pemphigus. Autoimmune Dis. 2011, 2011, 563091. [Google Scholar] [CrossRef]
- Fichou, Y.; Férec, C. The potential of oligonucleotides for therapeutic applications. Trends. Biotechnol. 2006, 24, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Yamaguchi, K.; Liu, Q.; Niimi, T.; Maturana, A.D.; Iijima, M.; Yoshimoto, N.; Kuroda, S. One-step scalable preparation method for non-cationic liposomes with high siRNA content. Int. J. Pharm. 2015, 490, 316–323. [Google Scholar] [CrossRef]
- Mannan, T.; Jing, S.; Foroushania, S.H.; Fortune, F.; Wan, H. RNAi-mediated inhibition of the desmosomal cadherin (desmoglein 3) impairs epithelial cell proliferation. Cell Prolif. 2011, 44, 301–310. [Google Scholar] [CrossRef]
- Wan, H.; South, A.P.; Hart, I.R. Increased keratinocyte proliferation initiated through downregulation of desmoplakin by RNA interference. Exp. Cell Res. 2007, 313, 2336–2344. [Google Scholar] [CrossRef]
- Anhalt, G.J.; Labib, R.S.; Voorhees, J.J.; Beals, T.F.; Diaz, L.A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N. Engl. J. Med. 1982, 306, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, P.; Hu, P.; Warren, S.; Liu, Z.; Diaz, L.A.; Rubenstein, D.S. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc. Natl. Acad. Sci. USA. 2006, 103, 12855–12860. [Google Scholar] [CrossRef] [PubMed]
- Chernyavsky, A.I.; Arredondo, J.; Kitajima, Y.; Sato-Nagai, M.; Grando, S.A. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: Characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J. Biol. Chem. 2007, 282, 13804–13812. [Google Scholar] [CrossRef]
- Ding, X.; Diaz, L.A.; Fairley, J.A.; Giudice, G.J.; Liu, Z. The anti-desmoglein1 autoantibodies in pemphigus vulgaris sera are pathogenic. J. Investg. Dermatol. 1999, 112, 739–743. [Google Scholar] [CrossRef]
- Spindler, V.; Waschke, J. Desmosomal cadherins and signaling: Lessons from autoimmune disease. Cell Comun. Adhes. 2014, 21, 77–84. [Google Scholar] [CrossRef]
- Meza-Lamas, E.; Bollain-y-Goytia, J.J.; Ramírez-Sandoval, R.; Sánchez-Rodríguez, S.H.; López-Robles, E.; Avalos-Díaz, E.; Herrera-Esparza, R. Camptothecin induces the transit of FasL trimers to the cell surface in apoptotic HEp-2 cells. Cell Mol. Biol. Lett. 2006, 11, 299–311. [Google Scholar] [CrossRef]
- Pelacho, B.; Natal, C.; España, A.; Sánchez-Carpintero, I.; Iraburu, M.J.; López-Zabalza, M.J. Pemphigus vulgaris autoantibodies induce apoptosis in HaCaT keratinocytes. FEBS Lett. 2004, 566, 6–10. [Google Scholar] [CrossRef]
- Arredondo, J.; Chernyavsky, A.I.; Karaouni, A.; Grando, S.A. Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in Pemphigus. Am. J. Pathol. 2005, 167, 1531–1544. [Google Scholar] [CrossRef]
- Lee, H.E.; Berkowitz, P.; Jolly, P.S.; Diaz, L.A.; Chua, M.P.; Rubenstein, D.S. Biphasic activation of P38MAPK suggests that apoptosis is a downstream event in pemphigus acantholysis. J. Biol. Chem. 2009, 284, 12524–12532. [Google Scholar] [CrossRef]
- Janse, I.C.; van-der-Wier, G.; Jonkman, M.F.; Pas, H.H.; Diercks, G.F.H. No evidence of apoptotic cells in pemphigus acantholysis. J. Investg. Dermatol. 2014, 134, 2039–2041. [Google Scholar] [CrossRef]
- Schmidt, E.; Gutberlet, J.; Siegmund, D.; Berg, D.; Wajant, H.; Waschke, J. Apoptosis is not Required for acantholysis in pemphigus vulgaris. Am. J. Physiol.-Cell Physiol. 2009, 296, C162–C172. [Google Scholar] [CrossRef]
- Luyet, C.; Schulze, K.; Sayar, B.S.; Howald, D.; Müller, J.E.; Galichet, A. Preclinical studies identify Non-Apoptotic low-level Caspase-3 as therapeutic target in Pemphigus vulgaris. PLoS ONE 2015, 10, e0119809. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Svandova, E.; Lesot, H.; Sharpe, P.; Matalova, E. Making the head: Caspases in life and death. Front. Cell Dev. Biol. 2023, 10, 1075751. [Google Scholar] [CrossRef]
- Hyuk-Kwon, K.; Jae-Hyeok, L.; Hyeon-Jun, S.; Jae-Ho, K.; Sangdun, C. Structural and functional analysis of cell adhesion and nuclear envelope nano-topography in cell death. Sci. Rep. 2015, 5, 15623. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta. 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Brancolini, C.; Lazarevic, D.; Rodriguez, J.; Schneider, C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J. Cell Biol. 1997, 139, 759–771. [Google Scholar] [CrossRef]
- Brentnall, M.; Weir, D.B.; Rongvaux, A.; Marcus, A.I.; Boise, L.H. Procaspase-3 regulates fibronectin secretion and influences adhesion, migration and survival independently of catalytic function. J. Cell Sci. 2014, 127, 2217–2226. [Google Scholar] [CrossRef]
- Sabolinski, M.L.; Beutner, E.H.; Krasny, S.; Kumar, V.; Huang, J.; Chorzelski, T.P.; Sampaio, S.; Bystryn, J.C. Substrate specificity of anti-epithelial antibodies of pemphigus vulgaris and pemphigus foliaceous sera in immunofluorescence tests on monkey and guinea pig esophagous sections. J. Investg. Dermatol. 1987, 88, 545–549. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yamada, K.M.; Kennedy, D.W. Dualistic nature of adhesive protein function: Fibronectin and its biologically active peptide fragments can autoinhibit fibronectin function. J. Cell Biol. 1984, 99, 29–36. [Google Scholar] [CrossRef]
- Kueng, W.; Silber, E.; Eppenberger, U. Quantification of cells cultured on 96-well plates. Anal. Biochem. 1989, 182, 16–19. [Google Scholar] [CrossRef]
- Sheng, P.; Flood, K.A.; Xie, M. Short hairpin RNAs for strand-specific small interfering RNA production. Front. Bioeng. Biotechnol. 2020, 7–8, 940. [Google Scholar] [CrossRef]
- Zamora-Avila, D.E.; Franco-Molina, M.A.; Trejo-Avila, L.M.; Rodríguez-Padilla, C.; Reséndez-Pérez, D.; Zapata-Benavides, P. RNAi silencing of the WT1 gene inhibits cell proliferation and induces apoptosis in the B16F10 murine melanoma cell line. Melanoma Res. 2007, 17, 341–348. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Caulín, C.; Salvesen, G.S.; Oshima, R.G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 1997, 138, 1379–1394. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Tovar, D.; Pacheco-Tovar, M.-G.; Saavedra-Alonso, S.; Zapata-Benavides, P.; Torres-del-Muro, F.-d.-J.; Bollain-y-Goytia, J.-J.; Herrera-Esparza, R.; Rodríguez-Padilla, C.; Avalos-Díaz, E. shRNA-Targeting Caspase-3 Inhibits Cell Detachment Induced by Pemphigus Vulgaris Autoantibodies in HaCaT Cells. Int. J. Mol. Sci. 2024, 25, 8864. https://doi.org/10.3390/ijms25168864
Pacheco-Tovar D, Pacheco-Tovar M-G, Saavedra-Alonso S, Zapata-Benavides P, Torres-del-Muro F-d-J, Bollain-y-Goytia J-J, Herrera-Esparza R, Rodríguez-Padilla C, Avalos-Díaz E. shRNA-Targeting Caspase-3 Inhibits Cell Detachment Induced by Pemphigus Vulgaris Autoantibodies in HaCaT Cells. International Journal of Molecular Sciences. 2024; 25(16):8864. https://doi.org/10.3390/ijms25168864
Chicago/Turabian StylePacheco-Tovar, Deyanira, María-Guadalupe Pacheco-Tovar, Santiago Saavedra-Alonso, Pablo Zapata-Benavides, Felipe-de-Jesús Torres-del-Muro, Juan-José Bollain-y-Goytia, Rafael Herrera-Esparza, Cristina Rodríguez-Padilla, and Esperanza Avalos-Díaz. 2024. "shRNA-Targeting Caspase-3 Inhibits Cell Detachment Induced by Pemphigus Vulgaris Autoantibodies in HaCaT Cells" International Journal of Molecular Sciences 25, no. 16: 8864. https://doi.org/10.3390/ijms25168864





