Morphographic Changes in the Electrocardiogram of Colossoma macropomum Caused by Exposure to Manganese
Abstract
1. Introduction
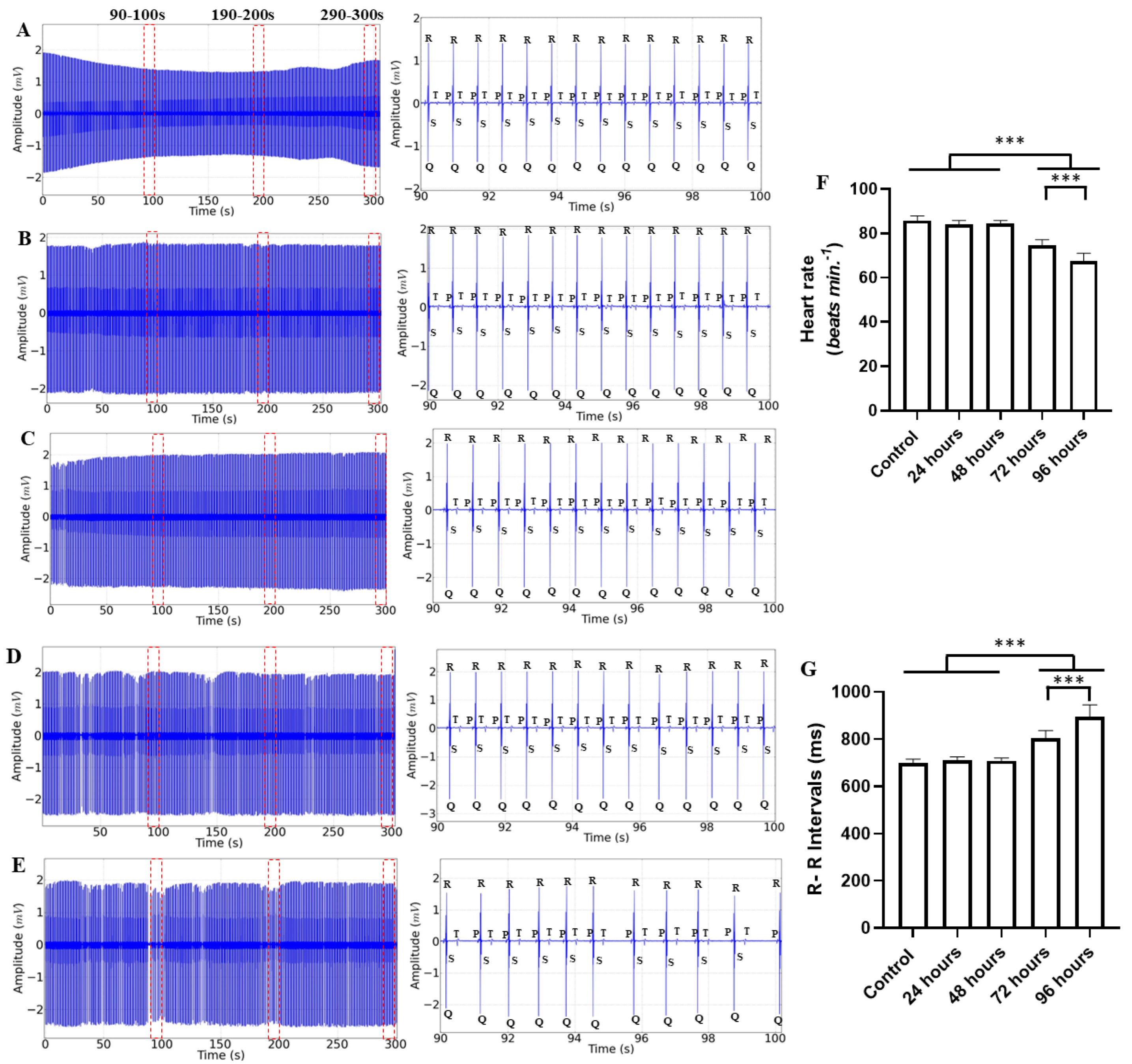
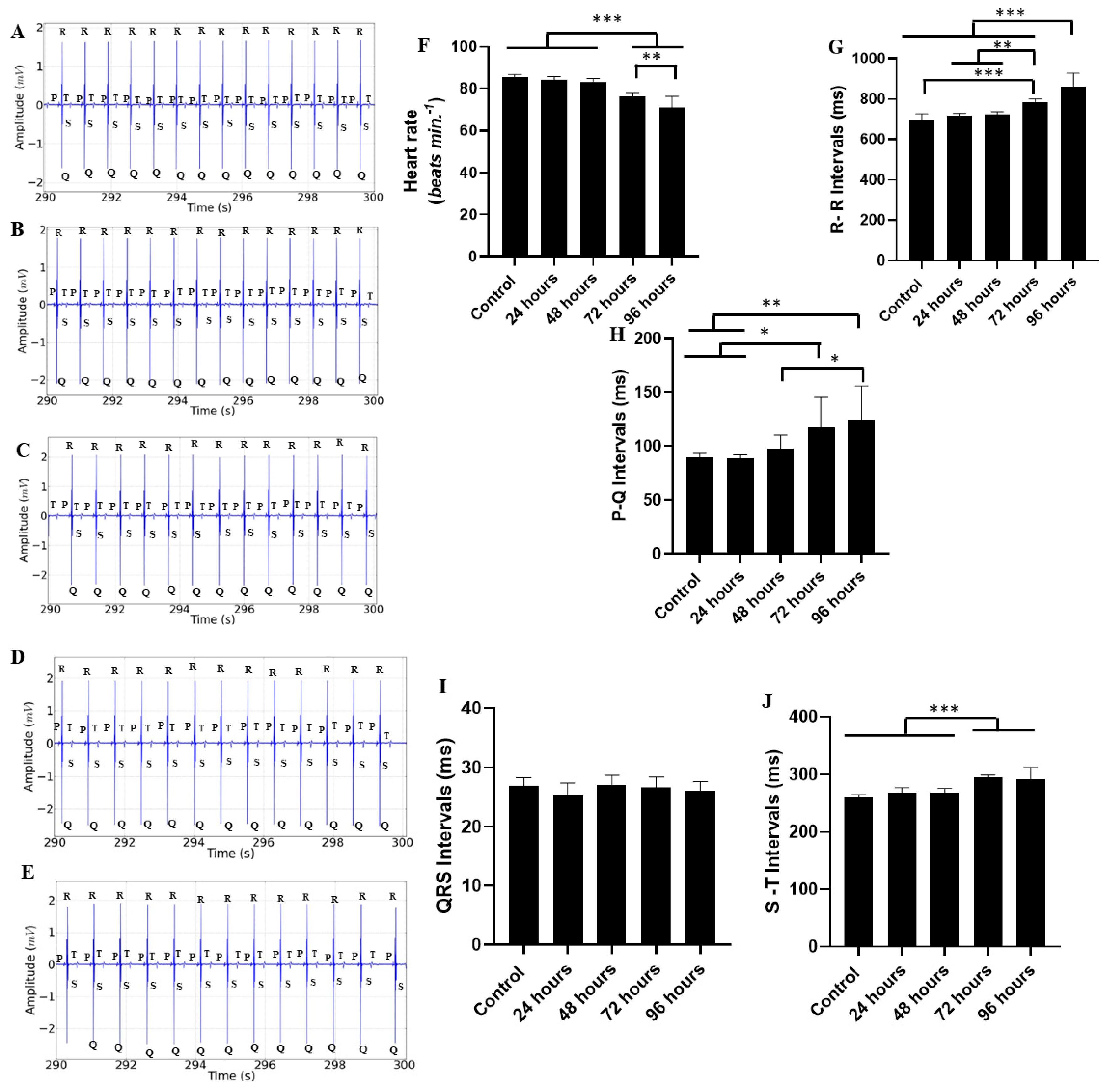
2. Results
3. Discussion
4. Material and Methods
4.1. Study Animals
4.2. Experimental Design
4.2.1. Manganese Experiment
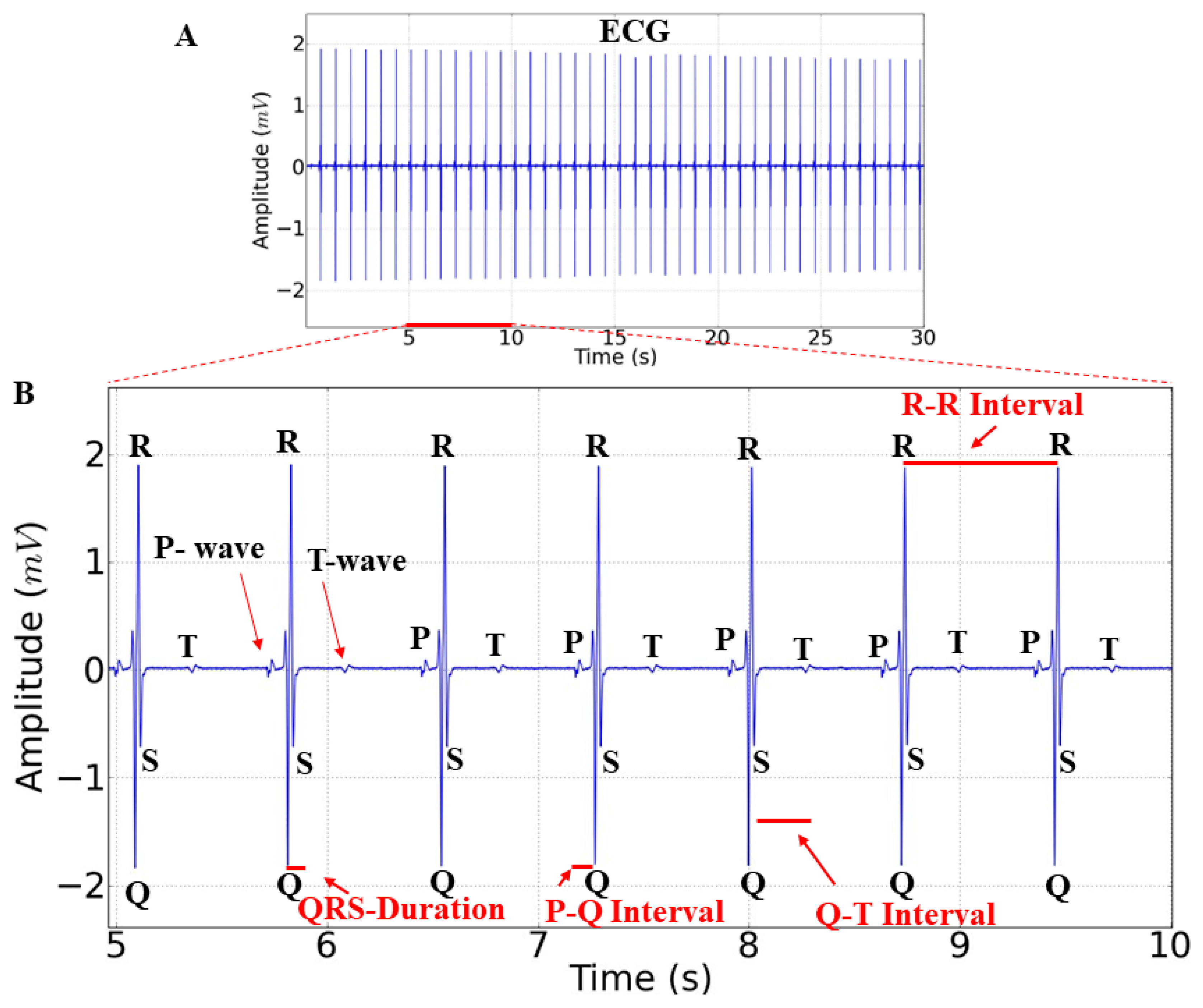
4.2.2. Electrocardiogram Analysis
4.2.3. Recording and Analysis
4.2.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; Paula, S.O.; Minim, V.P.R.; Bressan, J. Oxidative stress: Concept, implications and modulating factors. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Flores, M.V.B.; Eichwald, T.; Mantovani, A.; Glaser, V.; Schimitz, C.R.R.; Rossoni, C.; Steffani, J.A.; Perinetto, D.; Carvalho, D.; Remor, A.P. Manganese subacute intoxication in adult wistar rats: Evaluation of oxidative parameters in cns and metal deposition in different tissues. Estud. Interdiscip. Saúde 2018, 7, 193–210. Available online: https://periodicos.uniarp.edu.br/index.php/ries/article/view/1446 (accessed on 18 July 2023).
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. BioMetals 2003, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, O.V.D.; Cavalcante, K.S.; da Silva, W.K.B.; Nascimento, W.; Filho, S.A. miRNAs activate mitochondrial energy metabolism: A narrative view. Concilium 2023, 23, 118–129. [Google Scholar] [CrossRef]
- Ward, E.J.; Edmondson, D.A.; Nour, M.M.; Snyder, S.; Rosenthal, F.S.; Dydak, U. Toenail Manganese: A Sensitive and Specific Biomarker of Exposure to Manganese in Career Welders. Ann. Work. Expo. Health 2017, 62, 101–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ntihabose, R.; Surette, C.; Foucher, D.; Clarisse, O.; Bouchard, M.F. Assessment of saliva, hair and toenails as biomarkers of low level exposure to manganese from drinking water in children. Neurotoxicology 2018, 64, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shilnikova, N.; Karyakina, N.; Farhat, N.; Ramoju, S.; Cline, B.; Momoli, F.; Mattison, D.; Jensen, N.; Terrell, R.; Krewski, D. Biomarkers of environmental manganese exposure. Crit. Rev. Toxicol. 2022, 52, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Kile, M.; Dobson, C.; Amarasiriwardena, C.; Quamruzzaman, Q.; Rahman, M.; Golam, M.; Christiani, D.C. Maternal–infant biomarkers of prenatal exposure to arsenic and manganese. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 639–648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbas, L.A.L.; Torres, M.F.; da Costa, B.M.P.; Feitosa, M.J.M.; Maltez, L.C.; Amado, L.L.; Toda, Y.P.S.; Batista, P.d.S.; Cabral, D.A.C.; Hamoy, M. Eugenol induces body immobilization yet evoking an increased neuronal excitability in fish during short-term baths. Aquat. Toxicol. 2021, 231, 105734. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, C.S.; da Silva, R.A.; da Costa, B.M.P.A.; Torres, M.F.; de Mello, V.J.; Noronha, R.C.R.; da Silva, J.K.D.R.; Hamoy, M.; Barbas, L.A.L.; Nascimento, L.A.S.D. Cardiac response in tambaqui Colossoma macropomum anaesthetised with Piper divaricatum essential oil. Fish Physiol. Biochem. 2022, 48, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.M.A.; Torres, M.F.; da Silva, R.A.; Aydın, B.; Amado, L.L.; Hamoy, M.; Barbas, L.A.L. Integrated behavioural, neurological, muscular and cardiorespiratory response in tambaqui, Colossoma macropomum anaesthetized with menthol. Aquaculture 2022, 560, 738553. [Google Scholar] [CrossRef]
- Hamoy, A.O.; da Fonseca, S.M.; Cei, G.L.; Júnior, F.L.D.A.; Hamoy, M.K.O.; Ribeiro, R.M.; Barbas, L.A.L.; Muto, N.; Hamoy, M. Behavioral, electrocorticographic and electrocardiologic changes in Colossoma macropomum (Tambaqui) in the effect of cunaniol. PLoS ONE 2023, 18, e0287681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Araújo, E.R.L.; Torres, M.F.; Hamoy, M.; Barbas, L.A.L.; Sampaio, L.A. Cardiac response of tambaqui Colossoma macropomum anaesthetised with geraniol and citronellol. Aquaculture 2023, 565, 739101. [Google Scholar] [CrossRef]
- da Paz, C.A.; da Costa, B.M.P.A.; Hamoy, M.K.O.; dos Santos, M.F.; da Rocha, L.L.; Deiga, Y.d.S.; Barbosa, A.d.S.; Amaral, A.L.G.D.; Câmara, T.M.; Barbosa, G.B.; et al. Establishing a safe anesthesia concentration window for Nile tilapia (Oreochromis niloticus) (Linnaeus 1758) by monitoring cardiac activity in eugenol immersion baths. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 278, 109839. [Google Scholar] [CrossRef] [PubMed]
- Gavin, C.E.; Gunter, K.K.; Gunter, T.E. Manganese and calcium transport in mitochondria: Implications for manganese toxicity. Neurotoxicology 1999, 20, 445–453. [Google Scholar] [PubMed]
- Bachmann, E.; Weber, E.; Zbinden, G. Effects of seven anthracyclin antibiotics on electrocardiogram and mitochondrial function of rat hearts. Inflamm. Res. 1975, 5, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Berkaya, S.K.; Uysal, A.K.; Gunal, E.S.; Ergin, S.; Gunal, S.; Gulmezoglu, M.B. A survey on ECG analysis. Biomed. Signal Process. Control. 2018, 43, 216–235. [Google Scholar] [CrossRef]
- Saint-Paul, U. Potential for aquaculture of South American freshwater fishes: A review. Aquaculture 1986, 54, 205–240. [Google Scholar] [CrossRef]
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American fish for continental aquaculture. Rev. Aquac. 2016, 10, 351–369. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.-Q.; Wang, X.-Y.; Fang, Y.-Y.; Xu, L.; Zhao, K.-F.; Huang, B.; Liu, B.-L. Bioaccumulation of manganese and its effects on oxidative stress and immune response in juvenile groupers (Epinephelus moara ♀ × E. lanceolatus ♂). Chemosphere 2022, 297, 134235. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Yousafzai, A.M.; Sher, N.; Muhammad, I.; Nayab, G.E.; Aqeel, S.A.M.; Shah, S.T.; Aschner, M.; Khan, I.; Khan, H. Toxicity and bioaccumulation of manganese and chromium in different organs of common carp (Cyprinus carpio) fish. Toxicol. Rep. 2021, 8, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Brurok, H.; Schjø/Snm, J.; Berg, K.; Karlsson, J.O.G.; Jynge, P. Manganese and the heart: Acute cardiodepression and myocardial accumulation of manganese. Acta Physiol. Scand. 1997, 159, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Brurok, H.; Schjøtt, J.; Berg, K.; Karlsson, J.O.; Jynge, P. Effects of MnDPDP, DPDP--, and MnCl2 on Cardiac Energy Metabolism and Manganese Accumulation. Investig. Radiol. 1997, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Browning, C.L.; Green, A.; Gray, E.P.; Hurt, R.; Kane, A.B. Manganese dioxide nanosheets induce mitochondrial toxicity in fish gill epithelial cells. Nanotoxicology 2021, 15, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.; Dias, V.; Roversi, K.; Pase, C.; Segat, H.; Teixeira, A.; Benvegnú, D.; Trevizol, F.; Barcelos, R.; Riffel, A.; et al. Moderate hypoxia is able to minimize the manganese-induced toxicity in tissues of silver catfish (Rhamdia quelen). Ecotoxicol. Environ. Saf. 2013, 91, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.C.; Torronteras, R.; Córdoba, F.; Canalejo, A. Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol. Environ. Saf. 2012, 78, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Davis, K.; Saddler, C.; Joseph, J.; Catapane, E.J.; Carroll, M.A. The Ability of PAS, Acetylsalicylic Acid and Calcium Disodium EDTA to Protect Against the Toxic Effects of Manganese on Mitochondrial Respiration in Gill of Crassostrea virginica. In Vivo 2011, 33, 7–14. [Google Scholar] [PubMed]
- Gabriel, D.; Riffel, A.P.K.; Finamor, I.A.; Saccol, E.M.H.; Ourique, G.M.; Goulart, L.O.; Kochhann, D.; Cunha, M.A.; Garcia, L.O.; Pavanato, M.A.; et al. Effects of Subchronic Manganese Chloride Exposure on Tambaqui (Colossoma macropomum) Tissues: Oxidative Stress and Antioxidant Defenses. Arch. Environ. Contam. Toxicol. 2013, 64, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, K.G.; Boldrini-França, J.; Passos, L.S.; Gomes, A.S.; Coppo, G.C.; Pereira, T.M.; Chippari-Gomes, A.R. Multiple biomarkers response of Astyanax lacustris (Teleostei: Characidae) exposed to manganese and temperature increase. Environ. Toxicol. Pharmacol. 2023, 100, 104124. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, J.G.S.; Liu, M.-C.; Sakakibara, Y.; Suiko, M. Manganese administration induces the increased production of dopamine sulfate and depletion of dopamine in Sprague-Dawley rats. J. Biochem. 2000, 128, 477–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baj, J.; Flieger, W.; Barbachowska, A.; Kowalska, B.; Flieger, M.; Forma, A.; Teresiński, G.; Portincasa, P.; Buszewicz, G.; Radzikowska-Büchner, E.; et al. Consequences of Disturbing Manganese Homeostasis. Int. J. Mol. Sci. 2023, 24, 14959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanc, P.D. The early history of manganese and the recognition of its neurotoxicity, 1837–1936. Neurotoxicology 2018, 64, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Lamonzie, E.; Vaillant, F.; Abell, E.; Charron, S.; El Hamrani, D.; Quesson, B.; Brette, F. Assessment of Cardiac Toxicity of Manganese Chloride for Cardiovascular Magnetic Resonance. Front. Physiol. 2022, 13, 952043. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Brurok, H.; Berg, K.; Sneen, L.; Grant, D.; Karlsson, J.O.; Jynge, P. Cardiac metal contents after infusions of manganese: An experimental evaluation in the isolated rat heart. Investig. Radiol. 1999, 34, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.A.; Kour, J.; Nashiruddullah, N.; Chakraborty, D. Electrocardiographic interpretation of the nasopharyngealresponse in conscious rabbits. Indian J. Anim. Res. 2016, 50, 900–904. [Google Scholar] [CrossRef]
- da Fonseca, S.M.; da Paz, C.A.; Hamoy, M.K.O.; de Freitas, L.G.D.R.; de Araújo, D.B.; Farias, R.A.F.; Lopes, D.C.F.; Muto, N.A.; Barbas, L.A.L.; Hamoy, M. Cryoanesthesia in tambaqui Colossoma macropomum: Behavioral and electrocardiographic responses. Aquaculture 2024, 582, 740551. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, L.M.d.; dos Santos, M.F.; Paz, C.A.d.; de Araújo, D.B.; Ferreira, R.d.C.; Deiga, Y.d.S.; de Souza, L.V.; Câmara, T.M.; dos Santos, R.G.; Barbosa, A.d.S.; et al. Morphographic Changes in the Electrocardiogram of Colossoma macropomum Caused by Exposure to Manganese. Int. J. Mol. Sci. 2024, 25, 8910. https://doi.org/10.3390/ijms25168910
Nascimento LMd, dos Santos MF, Paz CAd, de Araújo DB, Ferreira RdC, Deiga YdS, de Souza LV, Câmara TM, dos Santos RG, Barbosa AdS, et al. Morphographic Changes in the Electrocardiogram of Colossoma macropomum Caused by Exposure to Manganese. International Journal of Molecular Sciences. 2024; 25(16):8910. https://doi.org/10.3390/ijms25168910
Chicago/Turabian StyleNascimento, Lorena Meirelis do, Murilo Farias dos Santos, Clarissa Araújo da Paz, Daniella Bastos de Araújo, Rayllan da Cunha Ferreira, Yris da Silva Deiga, Luana Vasconcelos de Souza, Tays Mata Câmara, Rodrigo Gonçalves dos Santos, Anara de Sousa Barbosa, and et al. 2024. "Morphographic Changes in the Electrocardiogram of Colossoma macropomum Caused by Exposure to Manganese" International Journal of Molecular Sciences 25, no. 16: 8910. https://doi.org/10.3390/ijms25168910
APA StyleNascimento, L. M. d., dos Santos, M. F., Paz, C. A. d., de Araújo, D. B., Ferreira, R. d. C., Deiga, Y. d. S., de Souza, L. V., Câmara, T. M., dos Santos, R. G., Barbosa, A. d. S., Hamoy, M. K. O., do Amaral, A. L. G., Eiró-Quirino, L., Cabral, T. d. S., da Silva, M. A. P. d. S., Muto, N. A., & Hamoy, M. (2024). Morphographic Changes in the Electrocardiogram of Colossoma macropomum Caused by Exposure to Manganese. International Journal of Molecular Sciences, 25(16), 8910. https://doi.org/10.3390/ijms25168910




