High-Frequency Imaging Reveals Synchronised Delta- and Theta-Band Ca2+ Oscillations in the Astrocytic Soma In Vivo
Abstract
:1. Introduction
2. Results
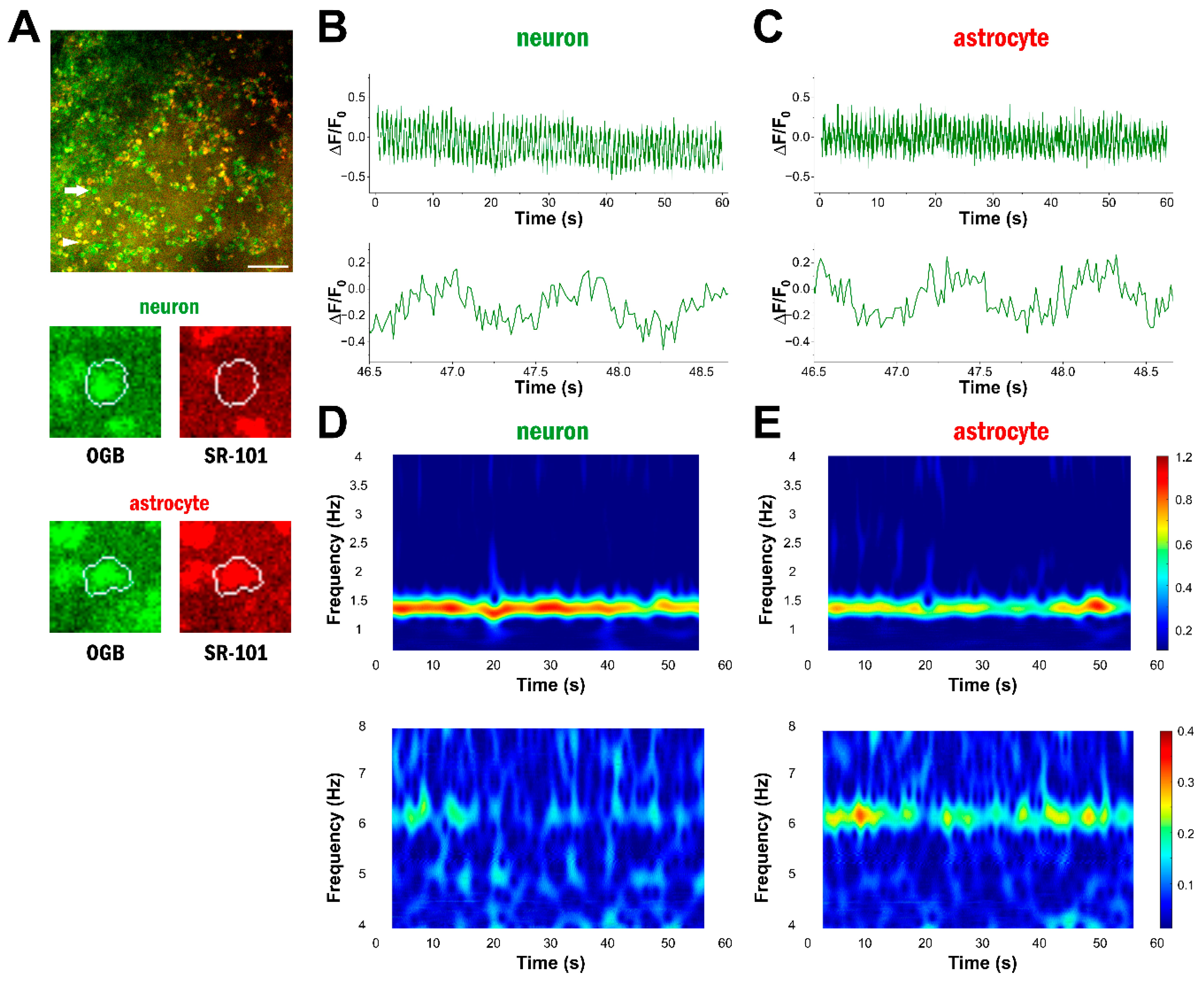
2.1. Astrocytes Display High-Frequency Ca2+ Oscillations
2.2. High-Frequency Ca2+ Signals Do Not Emerge in Fentanyl Anaesthesia
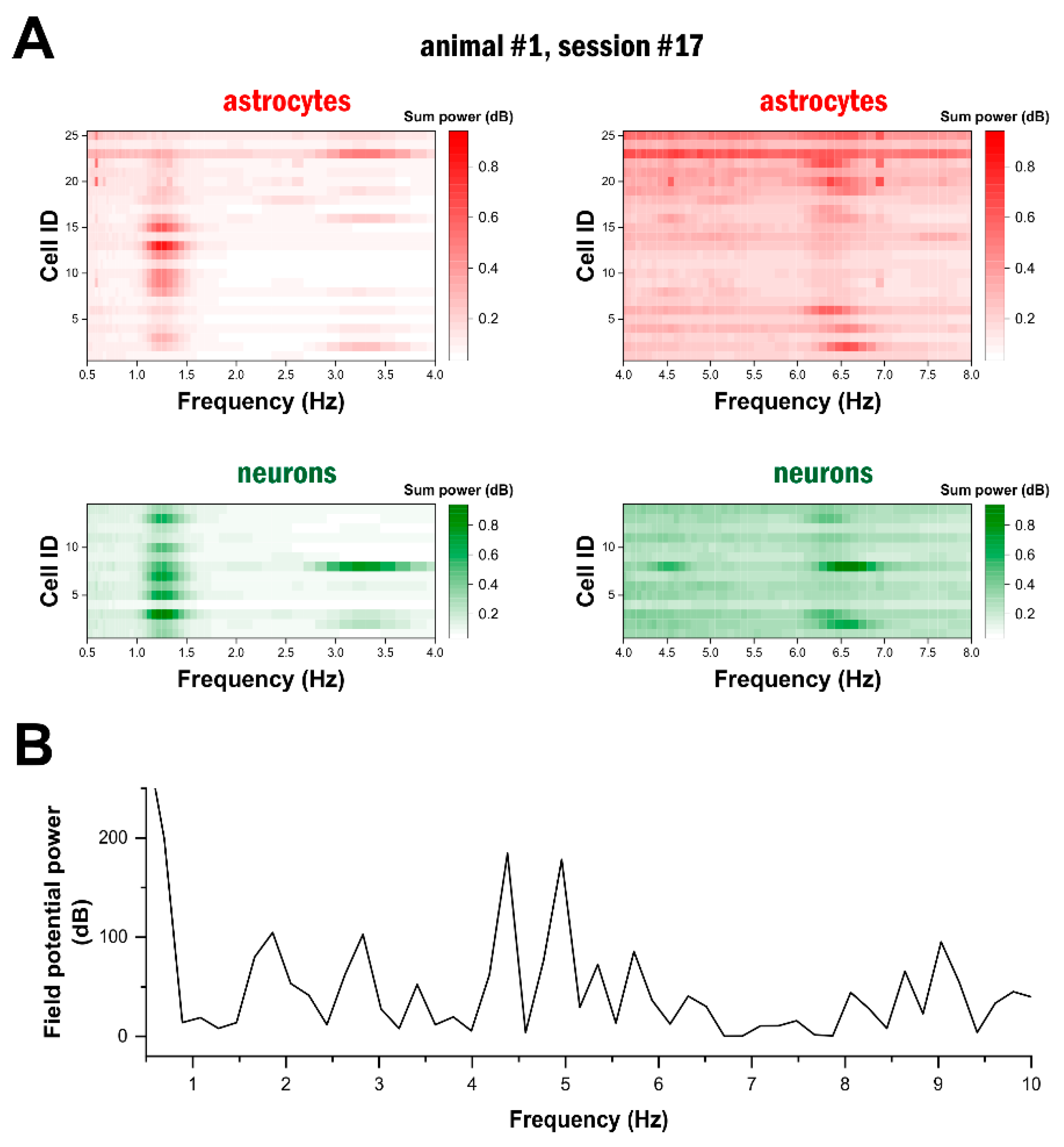
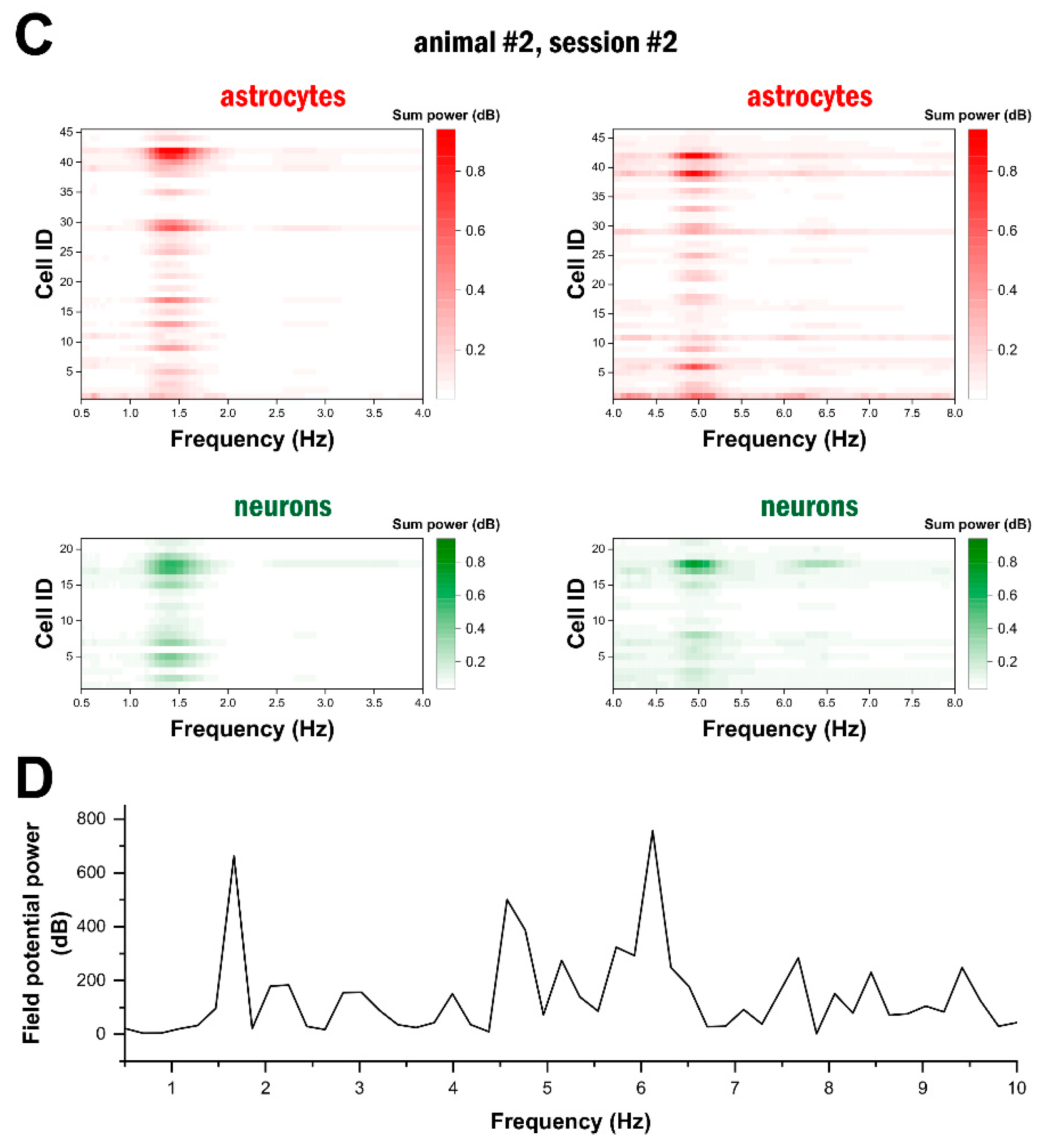
2.3. High-Frequency Astrocytic Ca2+ Signals Are Present at the Network Level
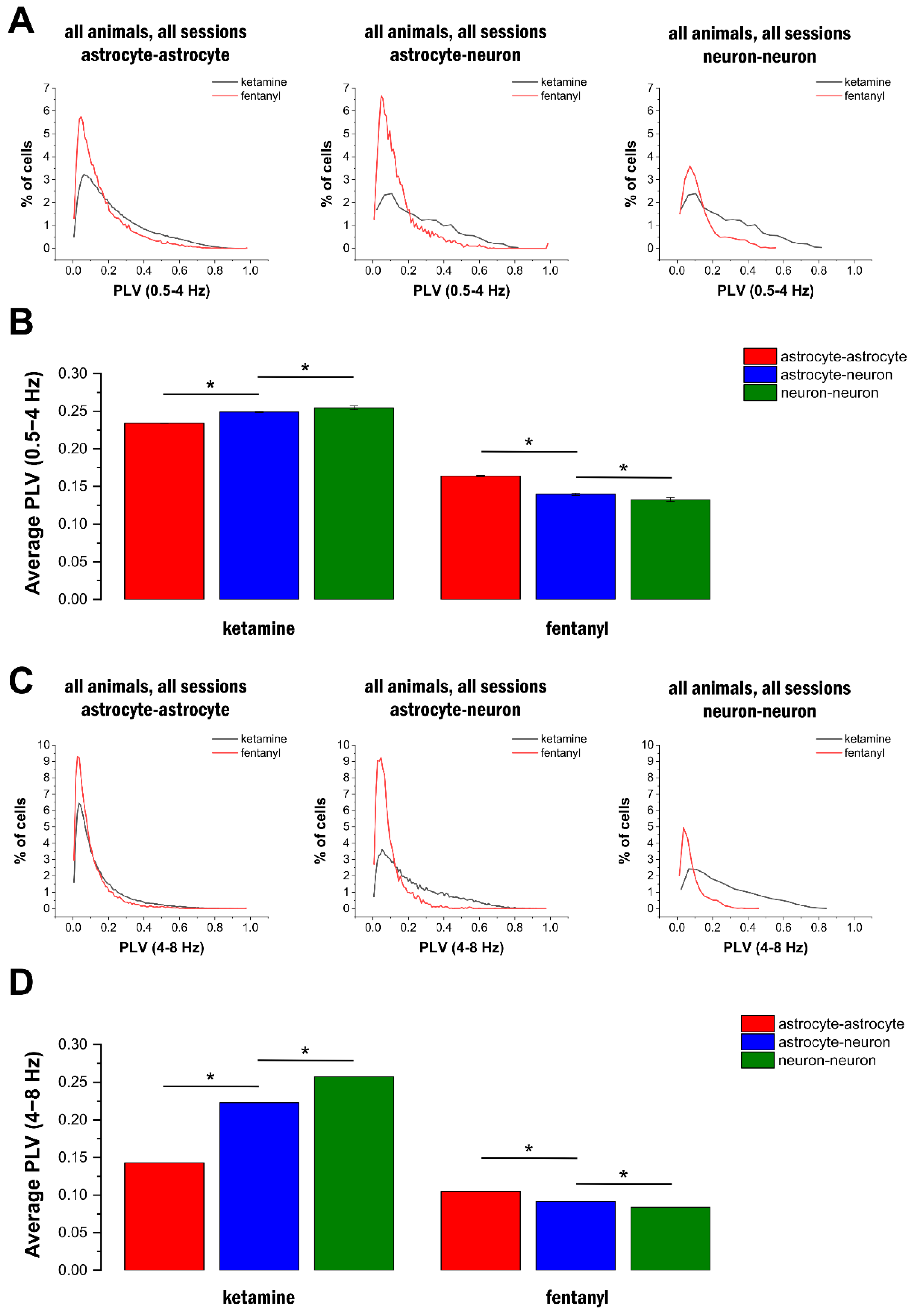
2.4. Delta- and Theta-Band Oscillations in Astrocytes Are Synchronised in the Network
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Preparation
4.3. Two-Photon Microscopy Coupled with Field Potential Recording
4.4. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130595. [Google Scholar] [CrossRef]
- Kuga, N.; Sasaki, T.; Takahara, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef]
- Szabó, Z.; Héja, L.; Szalay, G.; Kékesi, O.; Füredi, A.; Szebényi, K.; Dobolyi, Á.; Orbán, T.I.; Kolacsek, O.; Tompa, T.; et al. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci. Rep. 2017, 7, 6018. [Google Scholar] [CrossRef]
- Poskanzer, K.E.; Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E2675–E2684. [Google Scholar] [CrossRef]
- Kozachkov, L.; Michmizos, K.P. The Causal Role of Astrocytes in Slow-Wave Rhythmogenesis: A Computational Modelling Study. arXiv 2017, arXiv:1702.03993. [Google Scholar]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 2020, 11, 3240. [Google Scholar] [CrossRef]
- Gyorgy, B.; Andreas, D. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar]
- Buzsáki, G. Rhythms of the Brain; Oxford Academic: New York, NY, USA, 2006; pp. 1–464. [Google Scholar] [CrossRef]
- Prevedel, R.; Verhoef, A.J.; Pernía-Andrade, A.J.; Weisenburger, S.; Huang, B.S.; Nöbauer, T.; Fernández, A.; Delcour, J.E.; Golshani, P.; Baltuska, A.; et al. Fast volumetric calcium imaging across multiple cortical layers using sculpted light. Nat. Methods 2016, 13, 1021–1028. [Google Scholar] [CrossRef]
- Geiller, T.; Vancura, B.; Terada, S.; Troullinou, E.; Chavlis, S.; Tsagkatakis, G.; Tsakalides, P.; Ócsai, K.; Poirazi, P.; Rózsa, B.J.; et al. Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice. Neuron 2020, 108, 968–983.e9. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishikawa, T.; Abe, R.; Nakayama, R.; Asada, A.; Matsuki, N.; Ikegaya, Y. Astrocyte calcium signalling orchestrates neuronal synchronization in organotypic hippocampal slices. J. Physiol. 2014, 592, 2771–2783. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Tang, X.; Arizono, M.; Bannai, H.; Shih, P.-Y.; Dembitskaya, Y.; Kazantsev, V.; Tanaka, M.; Itohara, S.; Mikoshiba, K.; et al. Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium 2014, 55, 119–129. [Google Scholar] [CrossRef]
- Ding, F.; O’Donnell, J.; Thrane, A.S.; Zeppenfeld, D.; Kang, H.; Xie, L.; Wang, F.; Nedergaard, M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013, 54, 387–394. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Mukamel, E.A.; Schnitzer, M.J. Motor Behavior Activates Bergmann Glial Networks. Neuron 2009, 62, 400–412. [Google Scholar] [CrossRef]
- Thrane, A.S.; Thrane, V.R.; Zeppenfeld, D.; Lou, N.; Xu, Q.; Nagelhus, E.A.; Nedergaard, M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 18974–18979. [Google Scholar] [CrossRef]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.J.P.; Glück, C.; Stobart, M.J.; Zuend, M.; Weber, B. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 2018, 98, 726–735.e4. [Google Scholar] [CrossRef]
- Arizono, M.; Inavalli, V.V.G.K.; Panatier, A.; Pfeiffer, T.; Angibaud, J.; Levet, F.; Ter Veer, M.J.T.; Stobart, J.; Bellocchio, L.; Mikoshiba, K.; et al. Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat. Commun. 2020, 11, 1906. [Google Scholar] [CrossRef]
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making sense of astrocytic calcium signals—From acquisition to interpretation. Nat. Rev. Neurosci. 2020, 21, 551–564. [Google Scholar] [CrossRef]
- Semyanov, A. Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium 2019, 78, 15–25. [Google Scholar] [CrossRef]
- Héja, L.; Szabó, Z.; Péter, M.; Kardos, J. Spontaneous Ca2+ Fluctuations Arise in Thin Astrocytic Processes with Real 3D Geometry. Front. Cell. Neurosci. 2021, 15, 617989. [Google Scholar] [CrossRef]
- Héja, L.; Kardos, J. NCX activity generates spontaneous Ca2+ oscillations in the astrocytic leaflet microdomain. Cell Calcium 2020, 86, 102137. [Google Scholar] [CrossRef]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Neuroscience: Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Kerr, J.N.; Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 2004, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.; Pullon, R.M.; Vlisides, P.E.; Warnaby, C.E. Electroencephalographic slow wave dynamics and loss of behavioural responsiveness induced by ketamine in human volunteers. Br. J. Anaesth. 2019, 123, 592–600. [Google Scholar] [CrossRef]
- Fontanini, A.; Spano, P.F.; Bower, J.M. Ketamine-xylazine-induced slow (<1.5 Hz) oscillations in the rat piriform (olfactory) cortex are functionally correlated with respiration. J. Neurosci. 2003, 23, 7993–8001. [Google Scholar] [CrossRef] [PubMed]
- Munro Krull, E.; Sakata, S.; Toyoizumi, T. Theta Oscillations Alternate With High Amplitude Neocortical Population Within Synchronized States. Front. Neurosci. 2019, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.U.; Morton, A.J. Characteristic patterns of EEG oscillations in sheep (Ovis aries) induced by ketamine may explain the psychotropic effects seen in humans. Sci. Rep. 2020, 10, 9440. [Google Scholar] [CrossRef]
- Minelli, A.; DeBiasi, S.; Brecha, N.C.; Zuccarello, L.V.; Conti, F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 1996, 16, 6255–6264. [Google Scholar] [CrossRef]
- Danbolt, N.C.; Storm-Mathisen, J.; Kanner, B.I. An [Na+ + K+]coupled l-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 1992, 51, 295–310. [Google Scholar] [CrossRef]
- Boddum, K.; Jensen, T.P.; Magloire, V.; Kristiansen, U.; Rusakov, D.A.; Pavlov, I.; Walker, M.C. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 2016, 7, 13572. [Google Scholar] [CrossRef]
- Lia, A.; Henriques, V.J.; Zonta, M.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M.; Losi, G. Calcium Signals in Astrocyte Microdomains, a Decade of Great Advances. Front. Cell. Neurosci. 2021, 15, 673433. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.B.; Hori, K.; Novakovic, M.M.; Bernstein, N.G.; Lambot, L.; Prakriya, M. CRAC channels regulate astrocyte Ca2+ signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci. Signal. 2019, 12, eaaw5450. [Google Scholar] [CrossRef]
- Oschmann, F.; Mergenthaler, K.; Jungnickel, E.; Obermayer, K. Spatial separation of two different pathways accounting for the generation of calcium signals in astrocytes. PLoS Comput. Biol. 2017, 13, e1005377. [Google Scholar] [CrossRef]
- Kékesi, O.; Ioja, E.E.E.; Szabó, Z.; Kardos, J.; Héja, L. Recurrent seizure-like events are associated with coupled astroglial synchronization. Front. Cell. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Brain oscillations in perception and memory. Int. J. Psychophysiol. 2000, 35, 95–124. [Google Scholar] [CrossRef] [PubMed]
- Headley, D.B.; Paré, D. Common oscillatory mechanisms across multiple memory systems. npj Sci. Learn. 2017, 2, 1. [Google Scholar] [CrossRef]
- Vaidyanathan, T.V.; Collard, M.; Yokoyama, S.; Reitman, M.E.; Poskanzer, K.E. Cortical astrocytes independently regulate sleep depth and duration via separate gpcr pathways. Elife 2021, 10, e63329. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–62.e14. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Gerashchenko, D.; Timofeev, I.; Bacskai, B.J.; Kastanenka, K.V. Slow Wave Sleep Is a Promising Intervention Target for Alzheimer’s Disease. Front. Neurosci. 2020, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Russ, A.N.; Zhao, Q.; Perle, S.J.; Maci, M.; Miller, M.R.; Hou, S.S.; Algamal, M.; Zhao, Z.; Li, H.; et al. Optogenetic targeting of astrocytes restores slow brain rhythm function and slows Alzheimer’s disease pathology. Sci. Rep. 2023, 13, 13075. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Semyanov, A. Decline of astrocyte Ca2+ signalling in Alzheimer’s disease: STIM1 to the rescue! Cell Calcium 2023, 113, 102756. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péter, M.; Héja, L. High-Frequency Imaging Reveals Synchronised Delta- and Theta-Band Ca2+ Oscillations in the Astrocytic Soma In Vivo. Int. J. Mol. Sci. 2024, 25, 8911. https://doi.org/10.3390/ijms25168911
Péter M, Héja L. High-Frequency Imaging Reveals Synchronised Delta- and Theta-Band Ca2+ Oscillations in the Astrocytic Soma In Vivo. International Journal of Molecular Sciences. 2024; 25(16):8911. https://doi.org/10.3390/ijms25168911
Chicago/Turabian StylePéter, Márton, and László Héja. 2024. "High-Frequency Imaging Reveals Synchronised Delta- and Theta-Band Ca2+ Oscillations in the Astrocytic Soma In Vivo" International Journal of Molecular Sciences 25, no. 16: 8911. https://doi.org/10.3390/ijms25168911
APA StylePéter, M., & Héja, L. (2024). High-Frequency Imaging Reveals Synchronised Delta- and Theta-Band Ca2+ Oscillations in the Astrocytic Soma In Vivo. International Journal of Molecular Sciences, 25(16), 8911. https://doi.org/10.3390/ijms25168911





