Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile
Abstract
1. Introduction
2. Results
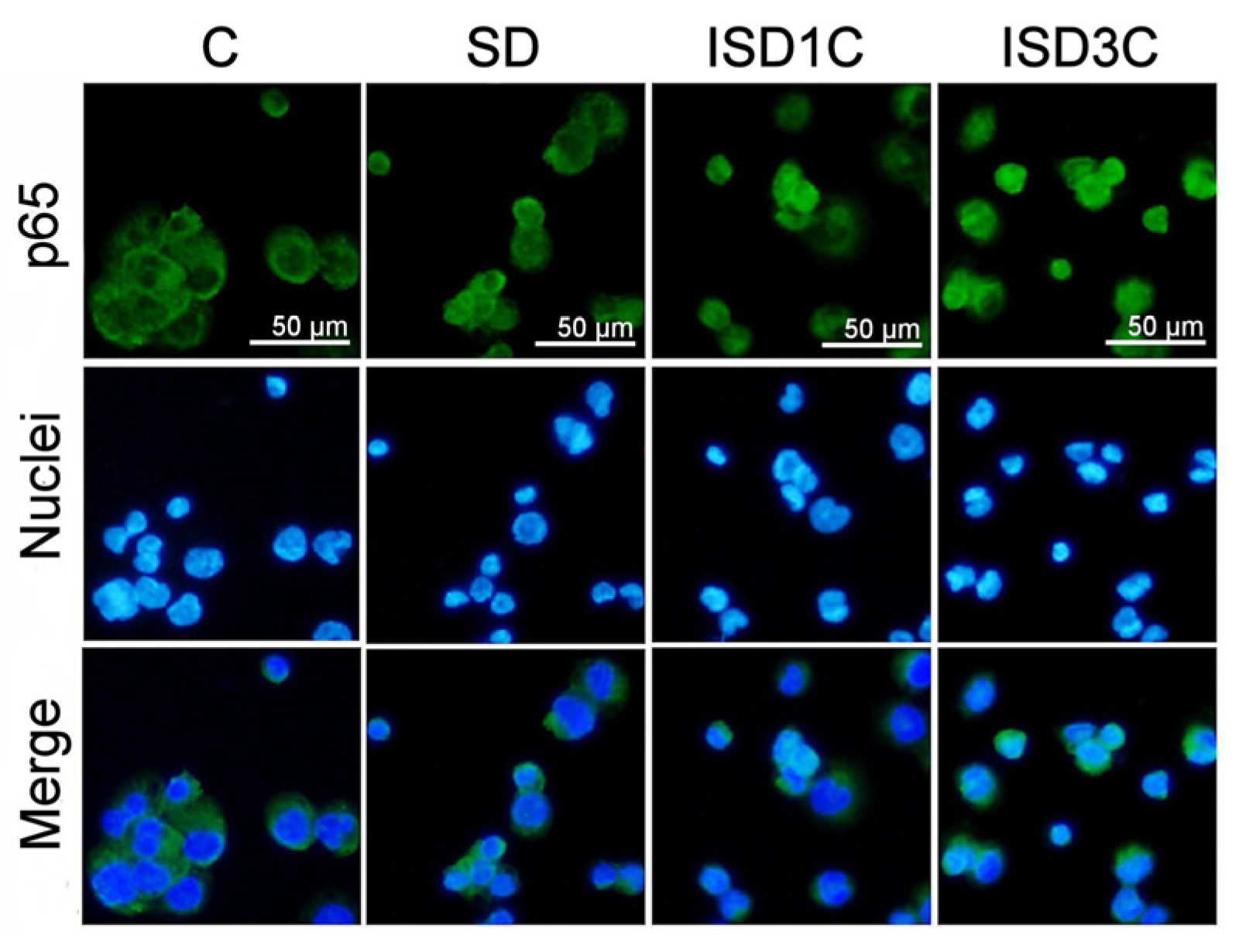
2.1. SD Modulates Macrophage Phenotype and Its Response to LPS Stimulation
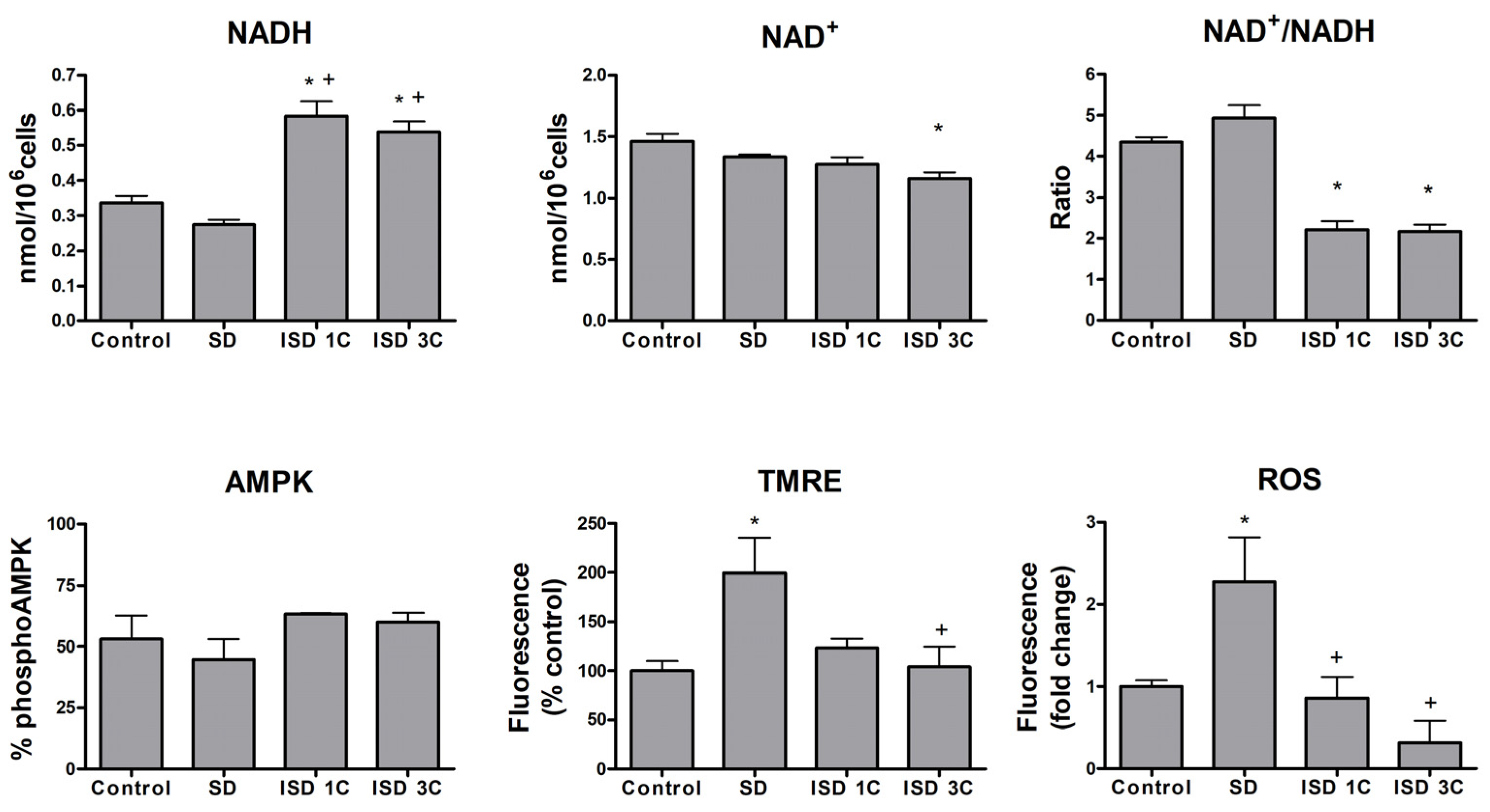
2.2. SD Affects Macrophage Metabolic Activity
2.3. MTS Reduction and Macrophage Profile Correlate to Metabolic Changes
3. Discussion
4. Materials and Methods
4.1. Cell Line
4.2. Cell Treatments
4.3. Cell Counting
4.4. MTS Assay
4.5. RT-PCR and qPCR
4.6. Immunofluorescence
4.7. NAD+ and NADH Quantitation
4.8. Phospho-AMPK Alpha 1 and Total AMPK Alpha 1
4.9. Reactive Oxygen Species
4.10. Mitochondrial Membrane Potential
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales-Ivorra, I.; Romera-Baures, M.; Roman-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients 2018, 10, 1030. [Google Scholar] [CrossRef]
- Cooper, I.; Brukner, P.; Devlin, B.L.; Reddy, A.J.; Fulton, M.; Kemp, J.L.; Culvenor, A.G. An Anti-Inflammatory Diet Intervention for Knee Osteoarthritis: A Feasibility Study. BMC Musculoskelet. Disord. 2022, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Shipton, E.A.; Stanwix, A.E.; Joffe, B.I.; Ramokgadi, J. Beneficial Effects of Weight Loss Associated with Moderate Calorie/Carbohydrate Restriction, and Increased Proportional Intake of Protein and Unsaturated Fat on Serum Urate and Lipoprotein Levels in Gout: A Pilot Study. Ann. Rheum. Dis. 2000, 59, 539–543. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Wieczorek, M.; Balanescu, A.; Bischoff-Ferrari, H.A.; Boonen, A.; Cavalli, G.; de Souza, S.; de Thurah, A.; Dorner, T.E.; Moe, R.H.; et al. 2021 EULAR Recommendations Regarding Lifestyle Behaviours and Work Participation to Prevent Progression of Rheumatic and Musculoskeletal Diseases. Ann. Rheum. Dis. 2023, 82, 48–56. [Google Scholar] [CrossRef]
- Cutolo, M.; Nikiphorou, E. Nutrition and Diet in Rheumatoid Arthritis. Nutrients 2022, 14, 888. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Shab-Bidar, S.; Rostamian, A.; Mohammadi, H.; Djafarian, K. The Effects of Intermittent Fasting Diet on Quality of Life, Clinical Symptoms, Inflammation, and Oxidative Stress in Overweight and Obese Postmenopausal Women with Rheumatoid Arthritis: Study Protocol of a Randomized Controlled Trial. Trials 2024, 25, 168. [Google Scholar] [CrossRef]
- Ben Nessib, D.; Maatallah, K.; Ferjani, H.; Triki, W.; Kaffel, D.; Hamdi, W. Sustainable Positive Effects of Ramadan Intermittent Fasting in Rheumatoid Arthritis. Clin. Rheumatol. 2022, 41, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Ben Nessib, D.; Maatallah, K.; Ferjani, H.; Kaffel, D.; Hamdi, W. Impact of Ramadan Diurnal Intermittent Fasting on Rheumatic Diseases. Clin. Rheumatol. 2020, 39, 2433–2440. [Google Scholar] [CrossRef]
- Iwashige, K.; Kouda, K.; Kouda, M.; Horiuchi, K.; Takahashi, M.; Nagano, A.; Tanaka, T.; Takeuchi, H. Calorie Restricted Diet and Urinary Pentosidine in Patients with Rheumatoid Arthritis. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Adawi, M.; Damiani, G.; Bragazzi, N.L.; Bridgewood, C.; Pacifico, A.; Conic, R.R.Z.; Morrone, A.; Malagoli, P.; Pigatto, P.D.M.; Amital, H.; et al. The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study. Nutrients 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the Maintenance of Homeostasis. Cell. Mol. Immunol. 2020, 18, 579–587. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Kolliniati, O.; Ieronymaki, E.; Vergadi, E.; Tsatsanis, C. Metabolic Regulation of Macrophage Activation. J. Innate Immun. 2022, 14, 51–68. [Google Scholar] [CrossRef]
- Williams, M.R.; Cauvi, D.M.; Rivera, I.; Hawisher, D.; De Maio, A. Changes in Macrophage Function Modulated by the Lipid Environment. Innate Immun. 2016, 22, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, Z.; Chen, Q.; Gu, J. Serum Deprivation Induced Apoptosis in Macrophage Is Mediated by Autocrine Secretion of Type I IFNs. Apoptosis 2006, 11, 545–554. [Google Scholar] [CrossRef]
- Castela, I.; Rodrigues, C.; Ismael, S.; Barreiros-Mota, I.; Morais, J.; Araújo, J.R.; Marques, C.; Silvestre, M.P.; Ângelo-Dias, M.; Martins, C.; et al. Intermittent Energy Restriction Ameliorates Adipose Tissue-Associated Inflammation in Adults with Obesity: A Randomised Controlled Trial. Clin. Nutr. 2022, 41, 1660–1666. [Google Scholar] [CrossRef]
- Gray, K.L.; Clifton, P.M.; Keogh, J.B.; Keogh, J.B. The Effect of Intermittent Energy Restriction on Weight Loss and Diabetes Risk Markers in Women with a History of Gestational Diabetes: A 12-Month Randomized Control Trial. Am. J. Clin. Nutr. 2021, 114, 794–803. [Google Scholar] [CrossRef]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of Time-Restricted Feeding in Weight Loss, Metabolic Syndrome and Cardiovascular Risk in Obese Women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent Energy Restriction Is Comparable to Continuous Energy Restriction for Cardiometabolic Health in Adults with Central Obesity: A Randomized Controlled Trial; the Met-IER Study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Häupl, T.; Sörensen, T.; Smiljanovic, B.; Darcy, M.; Scheder-Bieschin, J.; Steckhan, N.; Hartmann, A.M.; Koppold, D.A.; Stuhlmüller, B.; Skriner, K.; et al. Intestinal Microbiota Reduction Followed by Fasting Discloses Microbial Triggering of Inflammation in Rheumatoid Arthritis. J. Clin. Med. 2023, 12, 4359. [Google Scholar] [CrossRef]
- Zouhal, H.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Triki, R.; Hackney, A.C.; Laher, I.; Abderrahman, A. Ben Effects of Ramadan Intermittent Fasting on Inflammatory and Biochemical Biomarkers in Males with Obesity. Physiol. Behav. 2020, 225, 113090. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, C.; Zhang, J.; Zhao, H.; Shi, W.; He, B. Eight-Hour Time-Restricted Feeding Improves Endocrine and Metabolic Profiles in Women with Anovulatory Polycystic Ovary Syndrome. J. Transl. Med. 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, M.; Sellami, M.; Denham, J.; Padulo, J.; Kuvacic, G.; Selmi, W.; Khalifa, R. Time-Restricted Feeding Influences Immune Responses without Compromising Muscle Performance in Older Men. Nutrition 2018, 51–52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wei, J.; Zhang, H.; Luo, P.; Yang, Y.; Jiang, X.; Fei, Z.; Liang, W.; Jiang, J.; Li, X. Intermittent Fasting Reduces Neuroinflammation in Intracerebral Hemorrhage through the Sirt3/Nrf2/HO-1 Pathway. J. Neuroinflammation 2022, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent Fasting Enhanced the Cognitive Function in Older Adults with Mild Cognitive Impairment by Inducing Biochemical and Metabolic Changes: A 3-Year Progressive Study. Nutrients 2020, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Liu, Z.; Wang, J.-Q.; Li, R.-Q.; Ren, J.-Y.; Gao, X.; Lv, S.-S.; Liang, L.-Y.; Zhang, F.; Yin, B.-W.; et al. Randomized Controlled Trial for Time-Restricted Eating in Overweight and Obese Young Adults. iScience 2022, 25, 104870. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of Adipose Tissue Inflammation Are Transiently Elevated during Intermittent Fasting in Women Who Are Overweight or Obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Yshii, L.M.; Viel, T.A.; Buck, H.S.; Mattson, M.P.; Scavone, C.; Kawamoto, E.M. Intermittent Fasting Attenuates Lipopolysaccharide-Induced Neuroinflammation and Memory Impairment. J. Neuroinflammation 2014, 11, 85. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhou, X.; Pan, X.; Lü, J.; Sun, H.; Xie, Z.; Chen, S.; Gao, X. The Anti-Tumor Efficacy of 20(S)-Protopanaxadiol, an Active Metabolite of Ginseng, According to Fasting on Hepatocellular Carcinoma. J. Ginseng Res. 2022, 46, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef]
- Davidson, M.D.; Khetani, S.R. Intermittent Starvation Extends the Functional Lifetime of Primary Human Hepatocyte Cultures. Toxicol. Sci. 2020, 174, 266–277. [Google Scholar] [CrossRef]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef] [PubMed]
- Varaeva, Y.R.; Kirichenko, T.V.; Shaposhnikova, N.N.; Nikityuk, D.B.; Starodubova, A.V. The Role of Diet in Regulation of Macrophages Functioning. Biomedicines 2022, 10, 2087. [Google Scholar] [CrossRef]
- Asterholm, I.W.; McDonald, J.; Blanchard, P.G.; Sinha, M.; Xiao, Q.; Mistry, J.; Rutkowski, J.M.; Deshaies, Y.; Brekken, R.A.; Scherer, P.E. Lack of “Immunological Fitness” during Fasting in Metabolically Challenged Animals. J. Lipid Res. 2012, 53, 1254. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial Biogenesis and Metabolic Hyperactivation Limits the Application of MTT Assay in the Estimation of Radiation Induced Growth Inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Chan, G.K.Y.; Kleinheinz, T.L.; Peterson, D.; Moffat, J.G. A Simple High-Content Cell Cycle Assay Reveals Frequent Discrepancies between Cell Number and ATP and MTS Proliferation Assays. PLoS ONE 2013, 8, e63583. [Google Scholar] [CrossRef]
- Coyle, J.P.; Johnson, C.; Jensen, J.; Farcas, M.; Derk, R.; Stueckle, T.A.; Kornberg, T.G.; Rojanasakul, Y.; Rojanasakul, L.W. Variation in Pentose Phosphate Pathway-Associated Metabolism Dictates Cytotoxicity Outcomes Determined by Tetrazolium Reduction Assays. Sci. Rep. 2023, 13, 8220. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Horng, T. Lipid Metabolism in Regulation of Macrophage Functions. Trends Cell Biol. 2020, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Prados, J.-C.; Través, P.G.; Cuenca, J.; Rico, D.; Aragonés, J.; Martín-Sanz, P.; Cascante, M.; Boscá, L. Substrate Fate in Activated Macrophages: A Comparison between Innate, Classic, and Alternative Activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying Health Benefits of Fasting. Obesity 2018, 26, 254. [Google Scholar] [CrossRef] [PubMed]
- Bujak, A.L.; Crane, J.D.; Lally, J.S.; Ford, R.J.; Kang, S.J.; Rebalka, I.A.; Green, A.E.; Kemp, B.E.; Hawke, T.J.; Schertzer, J.D.; et al. AMPK Activation of Muscle Autophagy Prevents Fasting-Induced Hypoglycemia and Myopathy during Aging. Cell Metab. 2015, 21, 883. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, M.A.; van der Zon, G.C.; van Dijk, K.W.; Pijl, H.; Guigas, B. Effects of Prolonged Fasting on AMPK Signaling, Gene Expression, and Mitochondrial Respiratory Chain Content in Skeletal Muscle from Lean and Obese Individuals. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1012–E1021. [Google Scholar] [CrossRef]
- Kong, D.; Dagon, Y.; Campbell, J.N.; Guo, Y.; Yang, Z.; Yi, X.; Aryal, P.; Wellenstein, K.; Kahn, B.B.; Sabatini, B.L.; et al. A Postsynaptic AMPK→p21-Activated Kinase Pathway Drives Fasting-Induced Synaptic Plasticity in AgRP Neurons. Neuron 2016, 91, 25–33. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5′-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- Chan, K.L.; Pillon, N.J.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Théret, M.; Chazaud, B.; Klip, A. Palmitoleate Reverses High Fat-Induced Proinflammatory Macrophage Polarization via AMP-Activated Protein Kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef]
- Yan, L.S.; Zhang, S.F.; Luo, G.; Cheng, B.C.Y.; Zhang, C.; Wang, Y.W.; Qiu, X.Y.; Zhou, X.H.; Wang, Q.G.; Song, X.L.; et al. Schisandrin B Mitigates Hepatic Steatosis and Promotes Fatty Acid Oxidation by Inducing Autophagy through AMPK/MTOR Signaling Pathway. Metabolism 2022, 131, 155200. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rius-Bonet, J.; Macip, S.; Massip-Salcedo, M.; Closa, D. Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile. Int. J. Mol. Sci. 2024, 25, 9029. https://doi.org/10.3390/ijms25169029
Rius-Bonet J, Macip S, Massip-Salcedo M, Closa D. Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile. International Journal of Molecular Sciences. 2024; 25(16):9029. https://doi.org/10.3390/ijms25169029
Chicago/Turabian StyleRius-Bonet, Julia, Salvador Macip, Marta Massip-Salcedo, and Daniel Closa. 2024. "Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile" International Journal of Molecular Sciences 25, no. 16: 9029. https://doi.org/10.3390/ijms25169029
APA StyleRius-Bonet, J., Macip, S., Massip-Salcedo, M., & Closa, D. (2024). Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile. International Journal of Molecular Sciences, 25(16), 9029. https://doi.org/10.3390/ijms25169029





