Biological Roles of Lipids in Rice
Abstract
1. Introduction
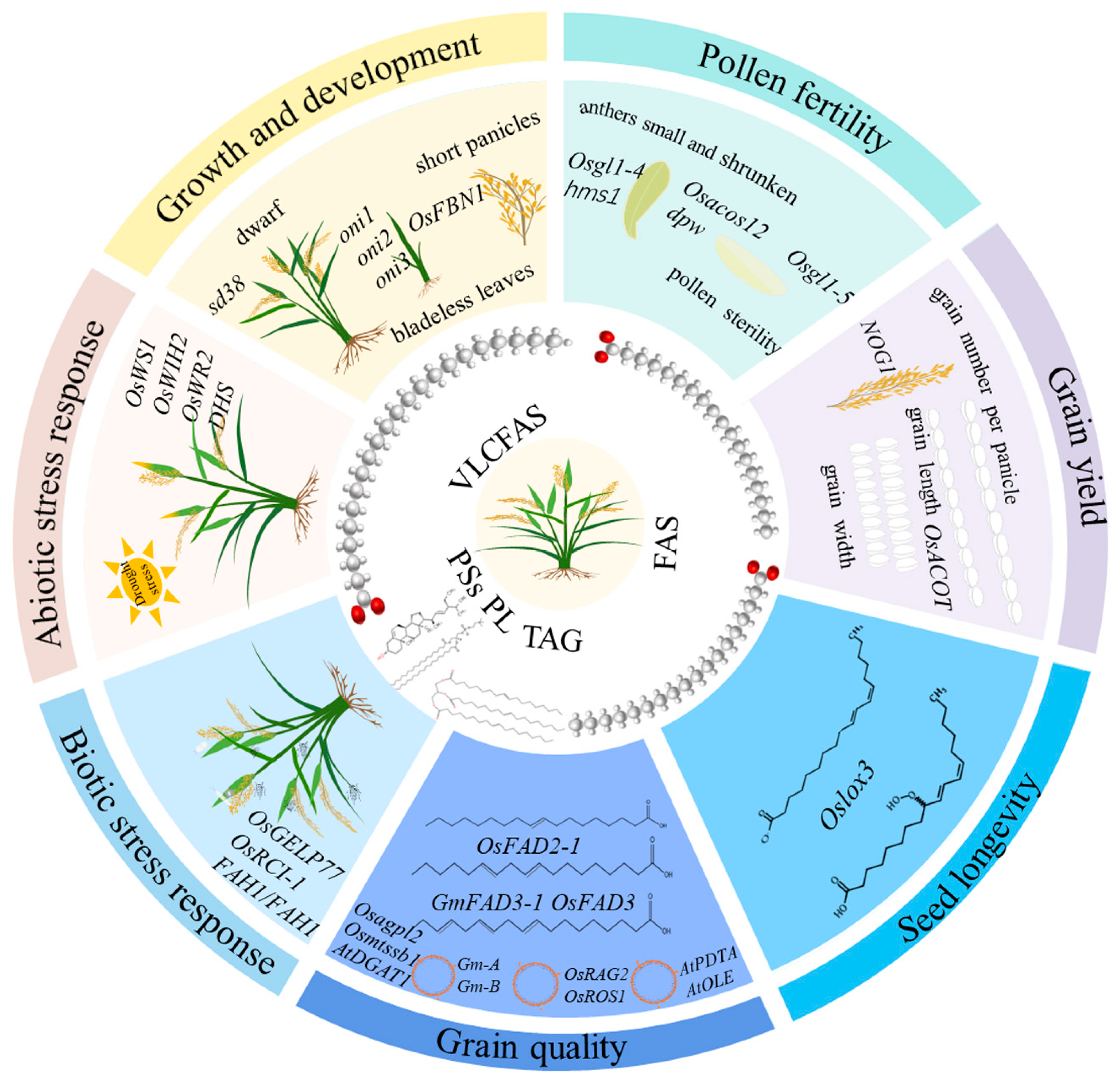
2. Roles of Lipids in Rice Growth and Development
3. Lipids Control Rice Pollen Fertility
4. Lipids Involves in the Regulation of Grain Yield
5. Grain Quality Is Affected by Lipid Contents and Components
6. Lipids Regulate Seed Longevity in Rice
7. Lipids Related to Abiotic Stress Response in Rice
8. Rice Biotic Stress Response Is Mediated by Lipids
9. Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gurr, M.I.; Harwoord, J.L.; Frayn, K.N.; Murphy, D.J.; Michell, R.H. Lipids: Biochemistry, Biotechnology and Health, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Suh, M.C.; Kim, H.U.; Nakamura, Y. Plant lipids: Trends and beyond. J. Exp. Bot. 2022, 73, 2715–2720. [Google Scholar] [CrossRef]
- Tao, B. Industrial Applications for Plant Oils and Lipids. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Harwood, J.L.; Guschina, I.A. Regulation of lipid synthesis in oil crops. FEBS Lett. 2013, 587, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Lu, H.; Zhang, Y.; Cheng, J.; Huang, B.; Lu, X.; Sheteiwy, M.S.; Kuang, S.; Shao, H. Oil crop genetic modification for producing added value lipids. Crit. Rev. Biotechnol. 2020, 40, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Sagun, J.V.; Yadav, U.P.; Alonso, A.P. Progress in understanding and improving oil content and quality in seeds. Front. Plant Sci. 2023, 14, 1116894. [Google Scholar] [CrossRef]
- Zhou, Z.K.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.H.; Lu, J.; Zhang, C.Q.; Liu, Q.Q.; Li, Q.F. Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef]
- Yu, D.M.; Ranathunge, K.; Huang, H.; Pei, Z.Y.; Franke, R.; Schreiber, L.; He, C. Wax Crystal-Sparse Leaf1 encodes a beta-ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kimura, F.; Hirakata, K.; Tsuda, K.; Takasugi, T.; Eiguchi, M.; Nakagawa, K.; Kurata, N. Fatty acid elongase is required for shoot development in rice. Plant J. 2011, 66, 680–688. [Google Scholar] [CrossRef]
- Tsuda, K.; Akiba, T.; Kimura, F.; Ishibashi, M.; Moriya, C.; Nakagawa, K.; Kurata, N.; Ito, Y. ONION2 Fatty Acid Elongase is Required for Shoot Development in Rice. Plant Cell Physiol. 2013, 54, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.X.; Hu, J.; Xu, J.; Yu, H.P.; Shi, Z.Y.; Xiong, G.S.; Zhu, L.; Zeng, D.; Zhang, G.; Gao, Z.; et al. Identification and characterization of Mini1, a gene regulating rice shoot development. J. Integr. Plant Biol. 2015, 57, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Akiba, T.; Hibara, K.I.; Kimura, F.; Tsuda, K.; Shibata, K.; Ishibashi, M.; Moriya, C.; Nakagawa, K.; Kurata, N.; Itoh, J.I.; et al. Organ fusion and defective shoot development in oni3 mutants of rice. Plant Cell Physiol. 2014, 55, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Shanmugabalaji, V.; Zita, W.; Collombat, J.; Kessler, F. Plastoglobules: A hub of lipid metabolism in the chloroplast. Adv. Bot. Res. 2022, 101, 91–119. [Google Scholar]
- Zhang, X.B.; Wang, Y.; Wang, X.W.; Zhu, Z.; Zhang, X.F.; Jia, L.Q.; Li, Y.Y.; Tian, W.J.; Chen, H.Y.; Zhu, X.Y.; et al. A very-long-chain fatty acid synthesis gene, SD38, influences plant height by activating ethylene biosynthesis in rice. Plant J. 2022, 112, 1084–1097. [Google Scholar] [CrossRef]
- Jung, K.H.; Han, M.J.; Lee, D.Y.; Lee, Y.S.; Schreiber, L.; Franke, R.; Faust, A.; Yephremov, A.; Saedler, H.; Kim, Y.W.; et al. Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 2006, 18, 3015–3032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Liang, W.Q.; Chen, M.J.; Zhang, D.B.; Zhao, X.X.; Shi, J.X. Rice fatty acyl-CoA synthetase OsACOS12 is required for tapetum programmed cell death and male fertility. Planta 2017, 246, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, D.D.; Guo, Z.L.; Shi, Q.S.; Xiong, S.X.; Zhang, C.; Zhu, J.; Yang, Z.N. OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol. 2016, 16, 256. [Google Scholar] [CrossRef]
- Shi, J.; Tan, H.X.; Yu, X.H.; Liu, Y.Y.; Liang, W.Q.; Ranathunge, K.; Franke, R.B.; Schreiber, L.; Wang, Y.; Kai, G.; et al. Defective Pollen Wall Is Required for Anther and Microspore Development in Rice and Encodes a Fatty Acyl Carrier Protein Reductase. Plant Cell 2011, 23, 2225–2246. [Google Scholar] [CrossRef]
- Song, Y.Y.; Tang, Y.Y.; Liu, L.T.; Xu, Y.Y.; Wang, T. The methyl-CpG-binding domain family member PEM1 is essential for Ubisch body formation and pollen exine development in rice. Plant J. 2022, 111, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Zhang, Z.G.; Ni, E.D.; Lin, J.W.; Peng, G.Q.; Huang, J.L.; Zhu, L.Y.; Deng, L.; Yang, F.F.; Luo, Q.; et al. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2020, 225, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Ni, E.D.; Zhou, L.Y.; Li, J.; Jiang, D.G.; Wang, Z.H.; Zheng, S.Y.; Qi, H.; Zhou, Y.; Wang, C.M.; Xiao, S.; et al. OsCER1 Plays a Pivotal Role in Very-Long-Chain Alkane Biosynthesis and Affects Plastid Development and Programmed Cell Death of Tapetum in Rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wu, S.; Zhu, Z.F.; Liu, F.X.; Fu, Y.C.; Cai, H.W.; Sun, X.Y.; Gu, P.; Xie, D.X.; Tan, L.B.; et al. NOG1 increases grain production in rice. Nat. Commun. 2017, 8, 1497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Peng, T.; Sun, H.-Z.; Teotia, S.; Wen, H.-L.; Du, Y.-X.; Zhang, J.; Li, J.Z.; Tnag, G.L.; Xue, H.W.; et al. miR1432-OsACOT (Acyl-CoA thioesterase) module determines grain yield via enhancing grain filling rate in rice. Plant Biotechnol. J. 2019, 17, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Tian, R.C.; Gao, Z.Q.; Yang, H.B. Characterization of AtWRI1 in fatty acids and starch synthesis in rice. Biosci. Biotechnol. Biochem. 2019, 83, 1807–1814. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Zhou, D.; Ouyang, Y.; Yao, J. Overexpression of the 16-kDa α-amylase/trypsin inhibitor RAG2 improves grain yield and quality of rice. Plant Biotechnol. J. 2017, 15, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiao, G.; Lin, H.; Sheng, Z.; Shao, G.; Xie, L.; Tang, S.; Xu, Q.; Hu, P. GRAIN INCOMPLETE FILLING 2 regulates grain filling and starch synthesis during rice caryopsis development. J. Integr. Plant Biol. 2017, 59, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Liu, H.L.; Qu, L.Q. Embryo-specific expression of soybean oleosin altered oil body morphogenesis and increased lipid content in transgenic rice seeds. Theor. Appl. Genet. 2013, 126, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, X.; Yao, X.; Yu, R.; Larkin, P.J.; Liu, C.-M. Mutations in the DNA demethylase OsROS1 result in a thickened aleurone and improved nutritional value in rice grains. Proc. Natl. Acad. Sci. USA 2018, 115, 11327–11332. [Google Scholar] [CrossRef]
- Izadi-Darbandi, A.; Younessi-Hamzekhanlu, M.; Sticklen, M. Metabolically engineered rice biomass and grain using genes associated with lipid pathway show high level of oil content. Mol. Biol. Rep. 2020, 47, 7917–7927. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Ying, J.; Shu, Y.; Liu, W.; Li, G.; Chen, L.; Luo, J.; Wang, S.; Wang, Y.; et al. Multi-gene engineering boosts oil content in rice grains. Plant Commun. 2024, 5, 100736. [Google Scholar] [CrossRef]
- Zaplin, E.S.; Liu, Q.; Li, Z.; Butardo, V.M.; Blanchard, C.L.; Rahman, S. Production of high oleic rice grains by suppressing the expression of the OsFAD2-1 gene. Funct. Plant Biol. 2013, 40, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.J.; Liu, Q.; Shreshtha, P.; Li, Z.Y.; Rahman, S. RNAi-mediated down-regulation of the expression of OsFAD2-1: Effect on lipid accumulation and expression of lipid biosynthetic genes in the rice grain. BMC Plant Biol. 2016, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.J.; Liu, H.L.; Dong, X.B.; Tian, L.H.; Xiao, L.; Xu, Y.N.; Qu, L.Q. Increasing α-linolenic acid content in rice bran by embryo-specific expression of ω3/Δ15-desaturase gene. Mol. Breed. 2014, 33, 987–996. [Google Scholar] [CrossRef]
- Xu, H.B.; Wei, Y.D.; Zhu, Y.S.; Lian, L.; Xie, H.G.; Cai, Q.H.; Chen, Q.S.; Lin, Z.P.; Wang, Z.H.; Xie, H.; et al. Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnol. J. 2015, 13, 526–539. [Google Scholar] [CrossRef]
- Xia, K.F.; Ou, X.J.; Gao, C.Z.; Tang, H.D.; Jia, Y.X.; Deng, R.F.; Xu, X.L.; Zhang, M.Y. OsWS1 involved in cuticular wax biosynthesis is regulated by osa-miR1848. Plant Cell Environ. 2015, 38, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Gao, S.X.; Li, J.; Song, P.Y.; Zhang, Q.; Guo, J.F.; Wang, X.Y.; Han, X.Y.; Wang, X.J.; Zhu, Y.; et al. The bHLH transcription factor regulated gene OsWIH2 is a positive regulator of drought tolerance in rice. Plant Physiol. Biochem. 2021, 169, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Tian, X.J.; Zhao, Q.Z.; Liu, Z.Q.; Li, X.F.; Ren, Y.K.; Tang, J.Q.; Fang, J.; Xu, Q.J.; Bu, Q.Y. The E3 Ligase DROUGHT HYPERSENSITIVE Negatively Regulates Cuticular Wax Biosynthesis by Promoting the Degradation of Transcription Factor ROC4 in Rice. Plant Cell 2018, 30, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Jenks, M.A.; Liu, J.; Liu, A.L.; Zhang, X.W.; Xiang, J.H.; Zou, J.; Peng, Y.; Chen, X.B. Overexpression of Transcription Factor OsWR2 Regulates Wax and Cutin Biosynthesis in Rice and Enhances its Tolerance to Water Deficit. Plant Mol. Biol. Rep. 2014, 32, 719–731. [Google Scholar] [CrossRef]
- Nagano, M.; Ishikawa, T.; Fujiwara, M.; Fukao, Y.; Kawano, Y.; Kawai-Yamada, M.; Shimamoto, K. Plasma Membrane Microdomains Are Essential for Rac1-RbohB/H-Mediated Immunity in Rice. Plant Cell 2016, 28, 1966–1983. [Google Scholar] [CrossRef]
- Zhang, M.J.; Chen, D.; Tian, J.J.; Cao, J.B.; Xie, K.B.; He, Y.Q.; Yuan, M. OsGELP77, a QTL for broad-spectrum disease resistance and yield in rice, encodes a GDSL-type lipase. Plant Biotechnol. J. 2024, 22, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.H.; Wang, L.; Li, C.Z.; Cao, M.J.; Wang, J.N.; Yao, Z.L.; Zhou, S.Y.; Zhou, G.X.; Zhang, D.Y.; Lou, Y.G. The lipoxygenase gene is involved in the biosynthesis of herbivore-induced JAs and regulates plant defense and growth in rice. Plant Cell Environ. 2022, 45, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Ito, Y.; Yamaki, S.; Miyao, A.; Hirochika, H.; Kurata, N. Isolation and mapping of three rice mutants that showed ectopic expression of KNOX genes in leaves. Plant Sci. 2009, 177, 131–135. [Google Scholar] [CrossRef]
- Li, J.J.; Yang, J.; Zhu, B.H.; Xie, G.S. Overexpressing OsFBN1 enhances plastoglobule formation, reduces grain-filling percent and jasmonate levels under heat stress in rice. Plant Sci. 2019, 285, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Wu, S.W.; Li, Z.W.; An, X.L.; Tian, Y.H. Lipid Metabolism: Critical Roles in Male Fertility and Other Aspects of Reproductive Development in Plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef] [PubMed]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.G.M.; Hodge, R.; Kalantidis, K.; Florack, D.; Wilson, Z.A.; Mulligan, B.J.; Scott, R.; Pereira, A. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997, 12, 615–623. [Google Scholar] [CrossRef]
- Akoh, C.C.; Lee, G.C.; Liaw, Y.C.; Huang, T.H.; Shaw, J.F. GDSL family of serine esterases/lipases. Prog. Lipid Res. 2004, 43, 534–552. [Google Scholar] [CrossRef]
- Zhao, J.; Long, T.; Wang, Y.F.; Tong, X.H.; Tang, J.; Li, J.L.; Wang, H.M.; Tang, L.Q.; Li, Z.Y.; Shu, Y.Z.; et al. RMS2 Encoding a GDSL Lipase Mediates Lipid Homeostasis in Anthers to Determine Rice Male Fertility. Plant Physiol. 2020, 182, 2047–2064. [Google Scholar] [CrossRef]
- Mondol, P.C.; Xu, D.W.; Duan, L.; Shi, J.X.; Wang, C.H.; Chen, X.F.; Chen, M.J.; Hu, J.P.; Liang, W.Q.; Zhang, D.B. Defective Pollen Wall 3 (DPW3), a novel alpha integrin-like protein, is required for pollen wall formation in rice. New Phytol. 2020, 225, 807–822. [Google Scholar] [CrossRef]
- Yu, B.; Liu, L.T.; Wang, T. Deficiency of very long chain alkanes biosynthesis causes humidity-sensitive male sterility via affecting pollen adhesion and hydration in rice. Plant Cell Environ. 2019, 42, 3340–3354. [Google Scholar] [CrossRef]
- Ni, E.D.; Deng, L.; Chen, H.Q.; Lin, J.W.; Ruan, J.M.; Liu, Z.L.; Zhuang, C.X.; Zhou, H. OsCER1 regulates humidity-sensitive genic male sterility through very-long-chain (VLC) alkane metabolism of tryphine in rice. Funct. Plant Biol. 2021, 48, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, G.; Liu, H.W. Enoyl-CoA hydratase: Reaction, mechanism, and inhibition. Bioorganic Med. Chem. 2003, 11, 9–20. [Google Scholar] [CrossRef]
- Krishna, A.G.G.; Hemakumar, K.H.; Khatoon, S. Study on the composition of rice bran oil and its higher free fatty acids value. J. Am. Oil Chem. Soc. 2006, 83, 117–120. [Google Scholar] [CrossRef]
- Hu, Z.L.; Li, P.; Zhou, M.Q.; Zhang, Z.H.; Wang, L.X.; Zhu, L.H.; Zhu, Y.G. Mapping of quantitative trait loci (QTLs) for rice protein and fat content using doubled haploid lines. Euphytica 2004, 135, 47–54. [Google Scholar] [CrossRef]
- Liu, W.J.; Zeng, J.; Jiang, G.H.; He, Y.Q. QTLs identification of crude fat content in brown rice and its genetic basis analysis using DH and two backcross populations. Euphytica 2009, 169, 197–205. [Google Scholar] [CrossRef]
- Qin, Y.; Kim, S.M.; Zhao, X.; Lee, H.S.; Jia, B.Y.; Kim, K.M.; Eun, M.Y.; Sohn, J.K. QTL detection and MAS selection efficiency for lipid content in brown rice (Oryza sativa L.). Genes Genom. 2010, 32, 506–512. [Google Scholar] [CrossRef]
- Ying, J.Z.; Shan, J.X.; Gao, J.P.; Zhu, M.Z.; Shi, M.; Lin, H.X. Identification of Quantitative Trait Loci for Lipid Metabolism in Rice Seeds. Mol. Plant 2012, 5, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xia, D.; Li, P.B.; Ao, Y.T.; Xu, X.D.; Wan, S.S.; Li, Y.H.; Wu, B.; Shi, H.; Wang, K.Y.; et al. Genetic architecture and key genes controlling the diversity of oil composition in rice grains. Mol. Plant 2021, 14, 456–469. [Google Scholar] [CrossRef]
- Sun, R.; Ye, R.; Gao, L.; Zhang, L.; Wang, R.; Mao, T.; Zheng, Y.S.; Li, D.D.; Lin, Y.J. Characterization and Ectopic Expression of CoWRI1, an AP2/EREBP Domain-Containing Transcription Factor from Coconut (Cocos nucifera L.) Endosperm, Changes the Seeds Oil Content in Transgenic Arabidopsis thaliana and Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 63. [Google Scholar] [CrossRef]
- Li, Y.T.; Han, D.X.; Hu, G.R.; Sommerfeld, M.; Hu, Q.A. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef]
- Li, D.Q.; Wu, X.B.; Wang, H.F.; Feng, X.; Yan, S.J.; Wu, S.Y.; Liu, J.X.; Yao, X.F.; Bai, A.N.; Zhao, H.; et al. Defective mitochondrial function by mutation in THICK ALEURONE 1 encoding a mitochondrion-targeted single-stranded DNA-binding protein leads to increased aleurone cell layers and improved nutrition in rice. Mol. Plant 2022, 15, 1638–1639. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Ageing of stored rice: Changes in chemical and physical attributes. J. Cereal. Sci. 2002, 35, 65–78. [Google Scholar] [CrossRef]
- Stoutjesdijk, P.A.; Singh, S.P.; Liu, Q.; Hurlstone, C.J.; Waterhouse, P.A.; Green, A.G. hpRNA-mediated targeting of the Arabidopsis FAD2 gene gives highly efficient and stable silencing. Plant Physiol. 2002, 129, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Singh, S.P.; Green, A.G. High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Yin, Z.J.; Xiao, L.; Xu, Y.N.; Qu, L.Q. Identification and evaluation of ω-3 fatty acid desaturase genes for hyperfortifying α-linolenic acid in transgenic rice seed. J. Exp. Bot. 2012, 63, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ise, K.; Li, C.Y.; Honda, I.; Iwai, Y.; Matsukura, U. Volatile components in stored rice [Oryza sativa (L.)] of varieties with and without lipoxygenase-3 in seeds. J. Agric. Food Chem. 1999, 47, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Gayen, D.; Ali, N.; Sarkar, S.N.; Datta, S.K.; Datta, K. Down-regulation of lipoxygenase gene reduces degradation of carotenoids of golden rice during storage. Planta 2015, 242, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Water transport in plant cuticles: An update. J. Exp. Bot. 2006, 57, 2493–2499. [Google Scholar] [CrossRef]
- Jenks, M.A.; Tuttle, H.A.; Eigenbrode, S.D.; Feldmann, K.A. Leaf Epicuticular Waxes of the Eceriferum Mutants in Arabidopsis. Plant Physiol. 1995, 108, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.Y.; Xiong, L.Z. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef]
- Qin, B.X.; Tang, D.; Huang, J.; Li, M.; Wu, X.R.; Lu, L.L.; Wang, K.J.; Yu, H.X.; Chen, J.M.; Gu, M.H.; et al. Rice OsGL1-1 is involved in leaf cuticular wax and cuticle membrane. Mol. Plant 2011, 4, 985–995. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, L.Z.; Xiang, J.H.; Gao, G.F.; Xu, F.X.; Liu, A.L.; Zhang, X.W.; Peng, Y.; Chen, X.B.; Wan, X.Y. OsGL1-3 is Involved in Cuticular Wax Biosynthesis and Tolerance to Water Deficit in Rice. PLoS ONE 2015, 10, e116676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Ni, E.D.; Yang, J.W.; Zhou, H.; Liang, H.; Li, J.; Jiang, D.G.; Wang, Z.H.; Liu, Z.L.; Zhuang, C.X. Rice OsGL1-6 is involved in leaf cuticular wax accumulation and drought resistance. PLoS ONE 2013, 8, e65139. [Google Scholar] [CrossRef] [PubMed]
- Lolle, S.J.; Hsu, W.; Pruitt, R.E. Genetic analysis of organ fusion in. Genetics 1998, 149, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Li, S.B.; He, S.; Wassmann, F.; Yu, C.H.; Qin, G.J.; Schreiber, L.; Qu, L.J.; Gu, H.Y. CFL1, a WW Domain Protein, Regulates Cuticle Development by Modulating the Function of HDG1, a Class IV Homeodomain Transcription Factor, in Rice and Arabidopsi. Plant Cell 2011, 23, 3392–3411. [Google Scholar] [CrossRef] [PubMed]
- Mawlong, I.; Ali, K.; Kurup, D.; Yadav, S.; Tyagi, A. Isolation and characterization of an AP2/ERF-type drought stress inducible transcription factor encoding gene from rice. J. Plant Biochem. Biotechnol. 2014, 23, 42–51. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xiong, L.Z. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.H.; Yu, J.J.; Liao, B.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Kan, Y.; Zhang, H.; Yang, Y.B.; et al. An a/f3 hydrolase family member negatively regulates salt tolerance but promotes flowering through three distinct functions in rice. Mol. Plant 2022, 15, 1908–1930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Li, X.M.; Lin, H.X.; Chong, K. Crop Improvement Through Temperature Resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mongrand, S.; Stanislas, T.; Bayer, E.M.F.; Lherminier, J.; Simon-Plas, F. Membrane rafts in plant cells. Trends Plant Sci. 2010, 15, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhou, Z.Q.; Li, Q.; Zhai, C.; Liu, L.J.; Quilichini, T.D.; Gao, P.; Kessler, S.A.; Jaillais, Y.; Datla, R.; et al. Specific Recruitment of Phosphoinositide Species to the Plant-Pathogen Interfacial Membrane Underlies Arabidopsis Susceptibility to Fungal Infection. Plant Cell 2020, 32, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Sha, G.; Sun, P.; Kong, X.J.; Han, X.Y.; Sun, Q.P.; Fouillen, L.; Zhao, J.; Li, Y.; Yang, L.; Wang, Y.; et al. Genome editing of a rice CDP-DAG synthase confers multipathogen resistance. Nature 2023, 618, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Tang, P.; Zuo, R.B.; Chen, H.F.; Zhao, T.Q.; Yang, S.M.; Yang, J. Exogenous Jasmonic Acid Alleviates Blast Resistance Reduction Caused by LOX3 Knockout in Rice. Biomolecules 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Ouyang, B.; Wang, F.; Lan, D.; Wang, Y. Lipidomics analysis of rice bran during storage unveils mechanisms behind dynamic changes in functional lipid molecular species. Food Chem. 2024, 447, 138946. [Google Scholar] [CrossRef] [PubMed]
| Gene | LOC ID | Annotation | Phenotype Description | References |
|---|---|---|---|---|
| WSL1 | LOC_Os06g39750 | β–ketoacyl CoA synthase | The mutants had reduced growth, leaf fusion, sparse wax crystals, and low fertility. | [9] |
| ONI1 | LOC_Os03g08360 | β–ketoacyl CoA synthase | The mutants produced small shoots and aberrant outer epidermal cells and ceased to grow after germination. | [10] |
| ONI2 | LOC_Os10g28060 | β–ketoacyl CoA synthase | The mutants had tiny shoots, fused leaves and ceased growing after germination. | [11] |
| ONI3 | LOC_Os09g19930 | Long-chain fatty acid ω-alcohol dehydrogenase | The mutants had severe growth defects with fused neighboring organs and finally became lethal. | [12,13] |
| OsFBN1 | LOC_Os09g04790 | Probable plastid-lipid-associated protein | Over-expression of OsFBN1 exhibited more tillers, short panicles, poor grain-filling percentage, and JA levels under heat stress. | [14] |
| SD38 | LOC_Os10g33370 | β–ketoacyl CoA synthase | The mutants’ cell length, 1000-grain weight, and seed setting rate were significantly reduced. | [15] |
| OsGL1-5 | LOC_Os10g33250 | Glossy1-homologous gene | The mutants showed pollen sterility and significant defects in the biosynthesis of VLCFAs. | [16] |
| OsACOS12 | LOC_Os04g24530 | Acyl-CoA synthetase | The mutants had normal vegetative growth but white anthers that were shorter and smaller and produced no mature pollen grains | [17,18] |
| DPW | LOC_Os03g07140 | Fatty Acyl Carrier Protein Reductase | The mutants display defected anther and degenerated pollen grains with an irregular exine | [19] |
| PEM1 | LOC_Os02g09920 | Methyl-CpG-binding domain family member | pem1 anthers became small and shrunken, with 30% lower viable pollen grains | [20] |
| HMS1 | LOC_Os03g12030 | β-ketoacyl-CoA synthase | The mutants that exhibited male sterility at low humidity but were fully fertile at high humidity | [21] |
| OsGL1-4 | LOC_Os02g40784 | Glossy1-homologous gene | The mutants significantly reduced the content of VLC alkanes (C25 and C27), finally leading to sterile pollen grains | [22] |
| NOG1 | LOC_Os01g54860 | Enoyl-CoA hydratase/isomerase | NOG1 can significantly increase the grain yield of commercial high-yield varieties. | [23] |
| OsACOT | LOC_Os04g35590 | Acyl-CoA thioesterase | Over-expression of the miR1432-resistant form of OsACOT resulted in an increased yield of approximately 50%. | [24] |
| AtWRI1 | AT3G54320 | AP2/EREBP transcription factor | Over-expression of AtWRI1 in rice increased fatty acid content by 30–40% in vegetative organs but decreased fatty acid content in the endosperm. | [25] |
| RAG2 | LOC_Os07g11380 | α-amylase/trypsin inhibitor | Over-expression of RAG2 gave rise to a 10–30% increase in lipid content and higher storage protein levels. | [26] |
| OsAGPL2 | LOC_Os01g44220 | ADP-glucose pyrophosphorylase large subunit | The oil content of mutant grains reached over 10%. | [27] |
| Gm-A Gm-B | U09118 U09119 | 24 kDa oleosin | Transgenic rice showed greater numbers of smaller oil bodies, and lipid content increased by 36.93% and 46.06%, respectively | [28] |
| OsROS1 | LOC_Os01g11900 | DNA demethylase | The number of cell layers in the anthers increased significantly, while the oil content of the rice increased by 48%, and the vitamin E content doubled | [29] |
| AtPDAT AtDGAT1 AtWRI1 AtOLE | AT5G13640 AT2G19450 AT3G54320 AT4G25140 | Phospholipid:diacylglycerol acyltransferase Diacylglycerol acyltransferase AP2/EREBP transcription factor Oleosin1 | Increased TAG, oleic acid, palmitic acid, and total oil contents by 26%, 28%, 27%, and 70% | [30] |
| OsAGPL2 OsMTSSB1 AtDGAT1 | LOC_Os01g44220 LOC_Os05g43440 AT2G19450 | ADP-glucose pyrophosphorylase large subunit Mitochondrion-targeted single-stranded DNA-binding protein Diacylglycerol acyltransferase | The lipid content increased from approximately 2% to around 12%, | [31] |
| OsFAD2-1 | LOC_Os02g48560 | ω-6 fatty acid desaturase | Mutant showed an increase in oleic acid and a reduction in linoleic and palmitic acids in the grains | [32,33] |
| GmFAD3-1 OsFAD3 | GLYMA_14G194300v4 LOC_Os12g01370 | microsomal omega-3-fatty acid desaturase ω-3 fatty acid desaturase gene | GmFAD3-1 and OsFAD3 over-expression lines consistently increased 23.8- and 27.9-fold across multiple generations. | [34] |
| OsLOX3 | LOC_Os03g49260 | lipoxygenase gene | Suppressing LOX3 expression in rice endosperm successfully extended rice seed longevity | [35] |
| OsWS1 | LOC_Os04g40590 | Membrane-bound O-acyl transferase gene | OsWS1 over-expression lines displayed 35% higher VLCFA contents, denser wax papillae around the stoma, and a higher survival rate upon water-deficit treatment. | [36] |
| OsWIH2 | LOC_Os03g31679 | cysteine-rich and transmembrane domain-containing protein WIH2 | Over-expression of OsWIH2 positively regulate rice drought resistance by alleviating water loss, reducing reactive oxygen species (ROS) accumulation, and altering wax content | [37] |
| DHS | LOC_Os02g45780 | RING-type E3 ubiquitin ligase | Over-expression of DHS significantly reduced the cuticular wax contents and conferred drought-hypersensitive. | [38] |
| OsWR2 | LOC_Os06g40150 | Ethylene response factor | Over-expression of OsWR2 increased the total cuticular wax in leaves and panicles, decreased water loss, and enhanced drought resistance. | [39] |
| OsFAH1 OsFAH2 | LOC_Os12g43363 LOC_Os03g56820 | fatty acid 2-hydroxylase | fah1fah2 harbors fewer microdomains and higher susceptibility to rice blast fungus infection | [40] |
| OsGELP77 | LOC4340180 | GDSL esterase/lipase gene | Elevated expression of OsGELP77 or pyramiding of a natural elite haplotype significantly increased resistance to various pathogens | [41] |
| OsLOX11/OsRCI-1 | LOC_Os12g37260 | lipoxygenase gene | Over-expression of OsRCI-1 elevated the levels of JA, jasmonate-isoleucine, and trypsin protease inhibitors, which decreased colonization, fecundity, and mass of the BHP insects | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Luo, Z.; Huang, W.; Liu, Z.; Miao, X.; Tao, S.; Wang, J.; Zhang, J.; Wang, S.; Zeng, X. Biological Roles of Lipids in Rice. Int. J. Mol. Sci. 2024, 25, 9046. https://doi.org/10.3390/ijms25169046
Zhou K, Luo Z, Huang W, Liu Z, Miao X, Tao S, Wang J, Zhang J, Wang S, Zeng X. Biological Roles of Lipids in Rice. International Journal of Molecular Sciences. 2024; 25(16):9046. https://doi.org/10.3390/ijms25169046
Chicago/Turabian StyleZhou, Kun, Zhengliang Luo, Weidong Huang, Zemin Liu, Xuexue Miao, Shuhua Tao, Jiemin Wang, Jian Zhang, Shiyi Wang, and Xiaoshan Zeng. 2024. "Biological Roles of Lipids in Rice" International Journal of Molecular Sciences 25, no. 16: 9046. https://doi.org/10.3390/ijms25169046
APA StyleZhou, K., Luo, Z., Huang, W., Liu, Z., Miao, X., Tao, S., Wang, J., Zhang, J., Wang, S., & Zeng, X. (2024). Biological Roles of Lipids in Rice. International Journal of Molecular Sciences, 25(16), 9046. https://doi.org/10.3390/ijms25169046







