Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia
Abstract
1. Introduction
2. Results
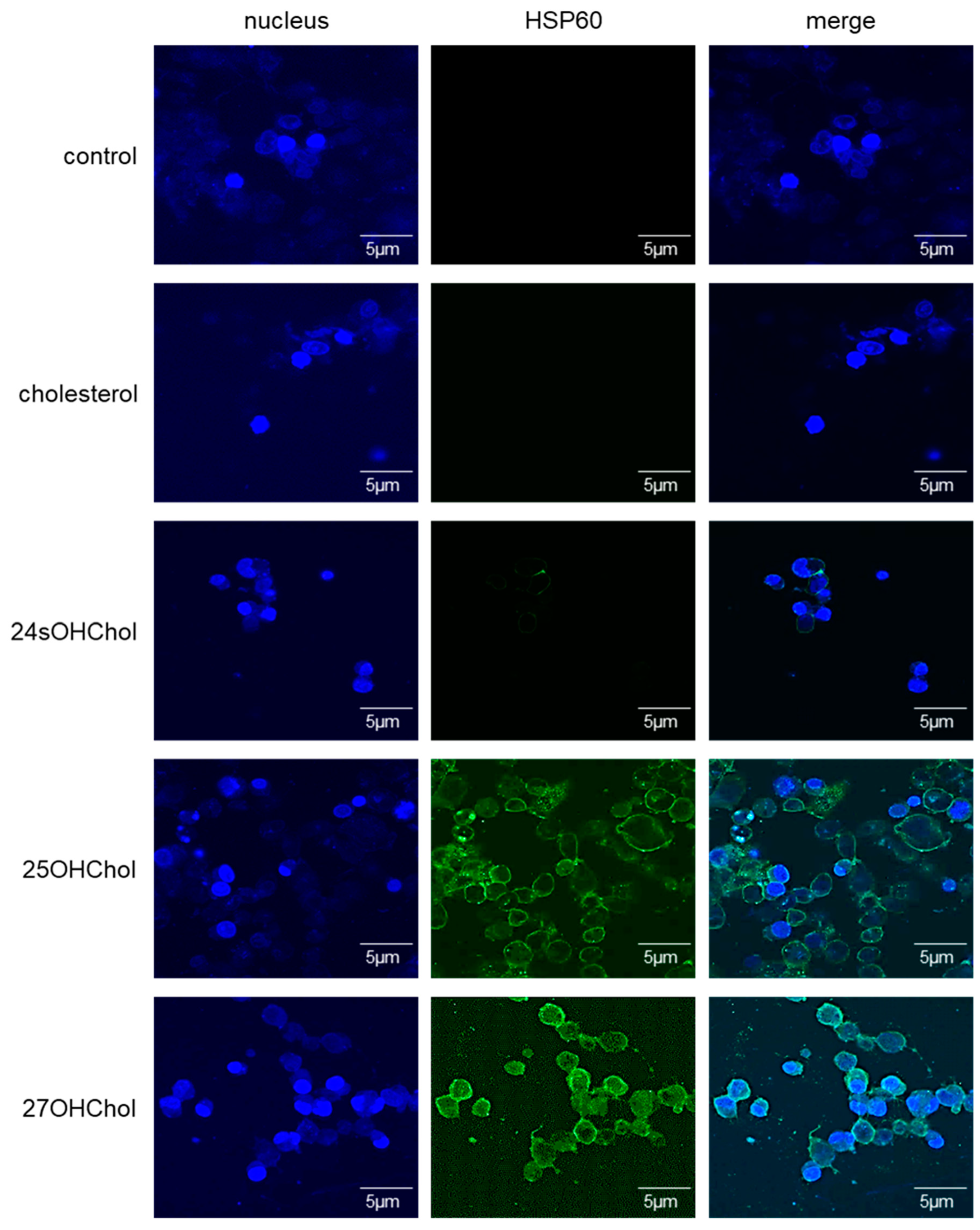
2.1. Induction of HSP60 by the Oxysterols on the Microglia
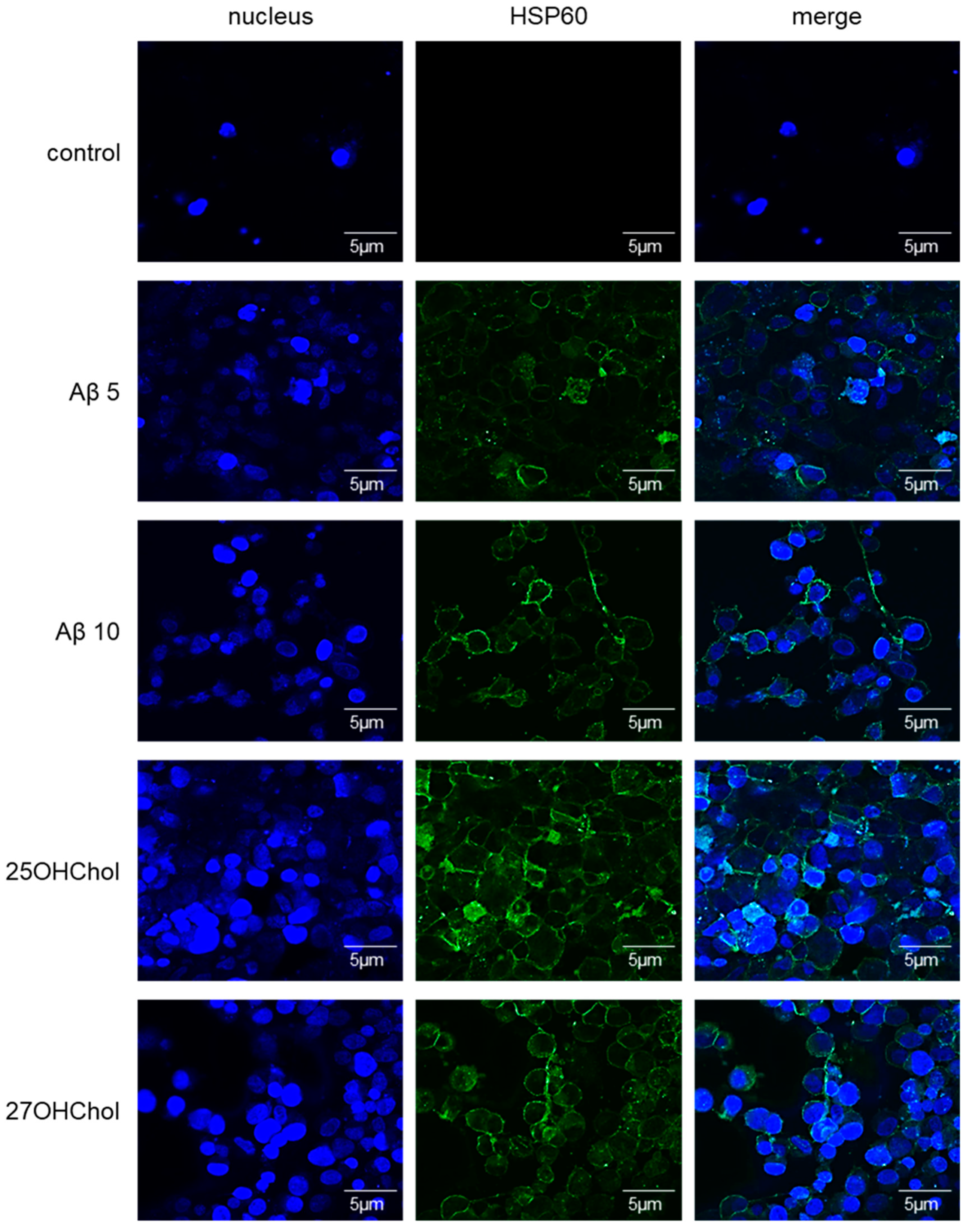
2.2. Roles of aβ in the Induction of HSP60 on Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Isolation of Cell Surface Proteins
4.3. Western Blot Analysis
4.4. Silver Staining
4.5. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (Maldi-Tof) Mass Spectrometry (MS)
4.6. Immunocytofluorescence
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2009, 58, 253–263. [Google Scholar] [CrossRef]
- Casano, A.M.; Peri, F. Microglia: Multitasking Specialists of the Brain. Dev. Cell 2015, 32, 469–477. [Google Scholar] [CrossRef]
- Thameem Dheen, S.; Kaur, C.; Ling, E.-A. Microglial Activation and its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef][Green Version]
- Hayes, G.; Woodroofe, M.; Cuzner, M. Microglia are the major cell type expressing MHC class II in human white matter. J. Neurol. Sci. 1987, 80, 25–37. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Shemer, A.; Levy-Efrati, L.; Gross, M.; Kim, J.; Engel, A.; David, E.; Chappell-Maor, L.; Grozovski, J.; Rotkopf, R.; et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur. J. Immunol. 2018, 48, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem, I.; Diczfalusy, U. Oxysterols. Arter. Thromb. Vasc. Biol. 2002, 22, 734–742. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, B.-Y.; Lee, S.-A.; Eo, S.-K.; Yun, Y.; Kim, C.-D.; Kim, K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol. Appl. Pharmacol. 2014, 274, 462–470. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.-M.; Lee, S.-A.; Eo, S.-K.; Kim, K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem. Biophys. Res. Commun. 2013, 438, 161–168. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, I.-J.; Hong, J.-T.; Eo, S.-K.; Lee, D.; Kim, K. Side-Chain Immune Oxysterols Induce Neuroinflammation by Activating Microglia. Int. J. Mol. Sci. 2023, 24, 15288. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflamm. 2018, 15, 177. [Google Scholar] [CrossRef]
- Swaroop, S.; Sengupta, N.; Suryawanshi, A.R.; Adlakha, Y.K.; Basu, A. HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J. Neuroinflammation 2016, 13, 1–19. [Google Scholar] [CrossRef]
- Langer, T.; Neupert, W. Heat shock proteins hsp60 and hsp70: Their roles in folding, assembly and membrane translocation of proteins. Curr. Top. Microbiol. Immunol. 1991, 167, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yi, J.; Wang, F.; Lei, T.; Du, H. Potential application of heat shock proteins as therapeutic targets in Parkinson’s disease. Neurochem. Int. 2023, 162, 105453. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Streit, W.J.; Bechmann, I. Beyond Activation: Characterizing Microglial Functional Phenotypes. Cells 2021, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-Y.; Son, Y.; Choi, J.; Eo, S.-K.; Park, Y.C.; Kim, K. 27-Hydroxycholesterol upregulates the production of heat shock protein 60 of monocytic cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 29–35. [Google Scholar] [CrossRef]
- Son, H.; Choi, H.-S.; Baek, S.E.; Kim, Y.-H.; Hur, J.; Han, J.-H.; Moon, J.H.; Lee, G.S.; Park, S.G.; Woo, C.-H.; et al. Shear stress induces monocyte/macrophage-mediated inflammation by upregulating cell-surface expression of heat shock proteins. Biomed. Pharmacother. 2023, 161, 114566. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Cho, H.-r.; Kim, B.-y.; Kim, J.; Park, D.; Kwon, R.J.; Son, Y. Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia. Int. J. Mol. Sci. 2024, 25, 9073. https://doi.org/10.3390/ijms25169073
Kim K, Cho H-r, Kim B-y, Kim J, Park D, Kwon RJ, Son Y. Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia. International Journal of Molecular Sciences. 2024; 25(16):9073. https://doi.org/10.3390/ijms25169073
Chicago/Turabian StyleKim, Koanhoi, Hyok-rae Cho, Bo-young Kim, Jaesung Kim, Dongha Park, Ryuk Jun Kwon, and Yonghae Son. 2024. "Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia" International Journal of Molecular Sciences 25, no. 16: 9073. https://doi.org/10.3390/ijms25169073
APA StyleKim, K., Cho, H.-r., Kim, B.-y., Kim, J., Park, D., Kwon, R. J., & Son, Y. (2024). Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia. International Journal of Molecular Sciences, 25(16), 9073. https://doi.org/10.3390/ijms25169073





