Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications
Abstract
1. Introduction
2. Design Strategy of Mussel-Inspired Injectable Adhesive Hydrogels
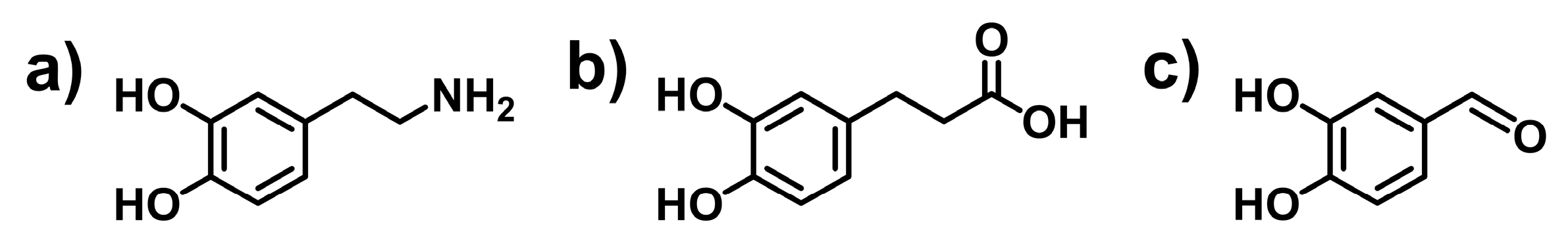
2.1. Incorporation of Catechol Group into Polymers
2.1.1. Incorporation of Catechol Groups by Classic Organic Reactions
2.1.2. Polymerization of Catechol-Based Monomers
2.1.3. Biosynthesis of Catechol-Containing Proteins
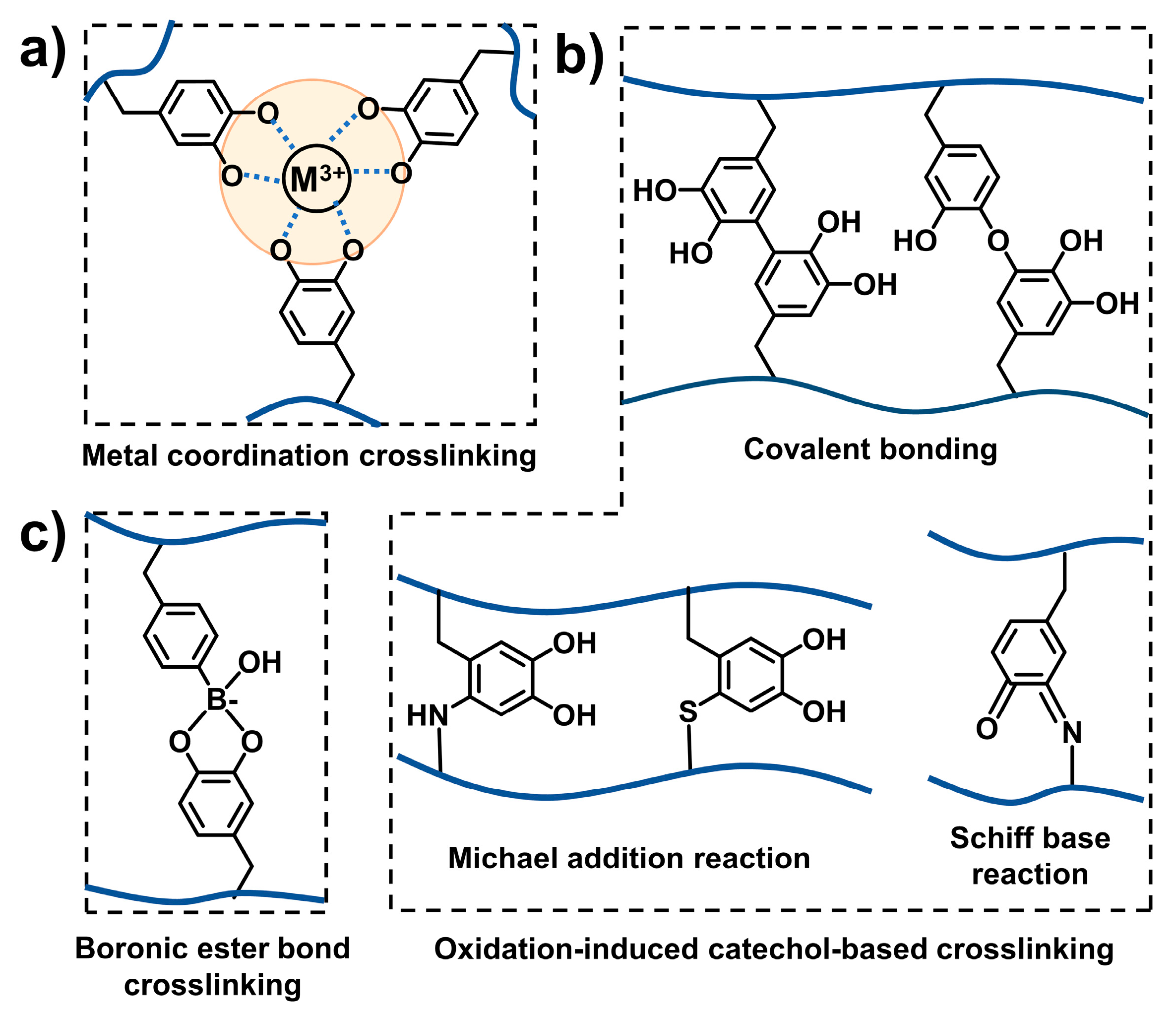
2.2. Crosslinking Strategy of Mussel-Inspired Injectable Adhesive Hydrogels
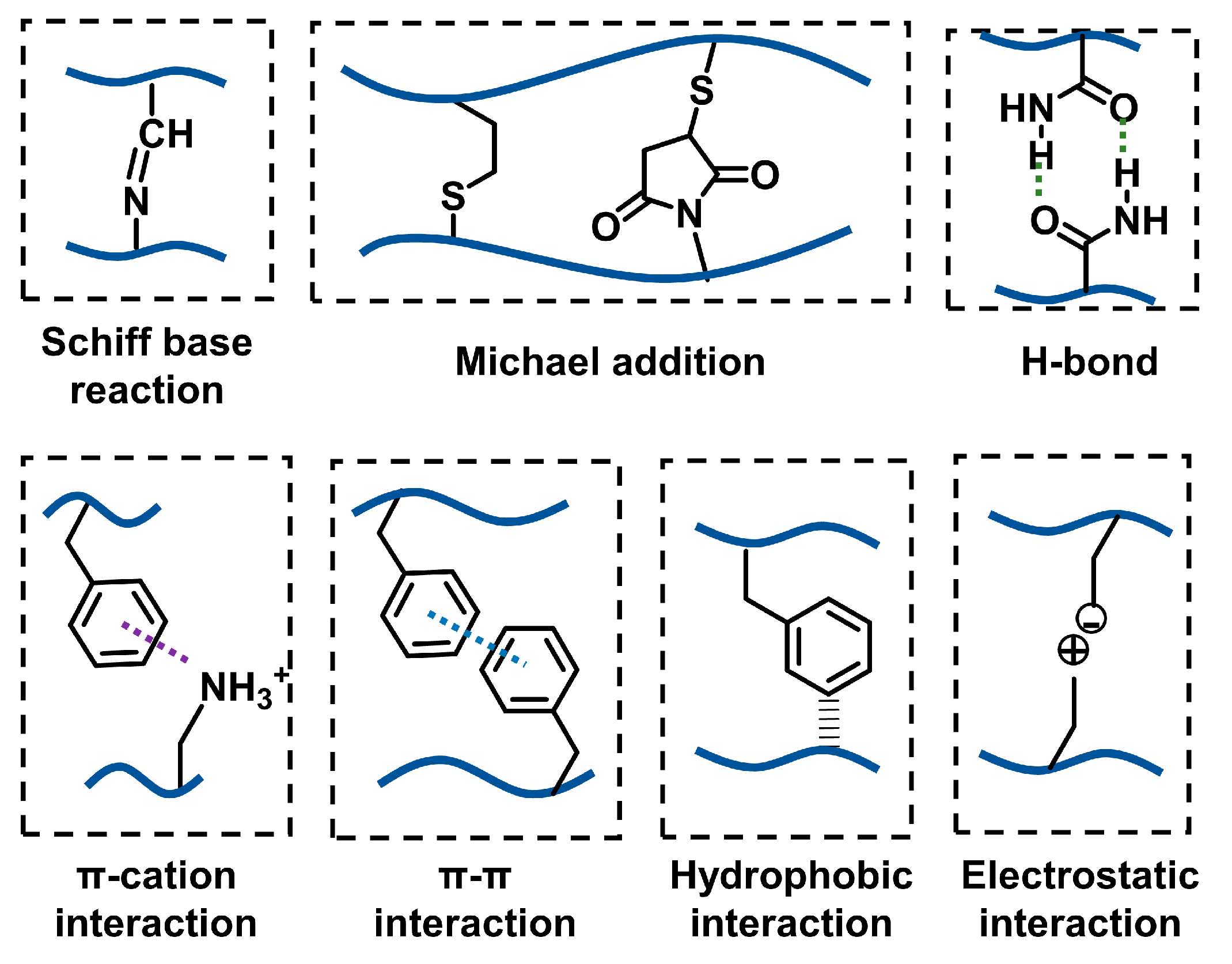
2.2.1. Catechol-Mediated Crosslinking
2.2.2. Other Regular Crosslinking Methods
2.2.3. Combination of Catechol-Mediated and Other Regular Crosslinking Methods
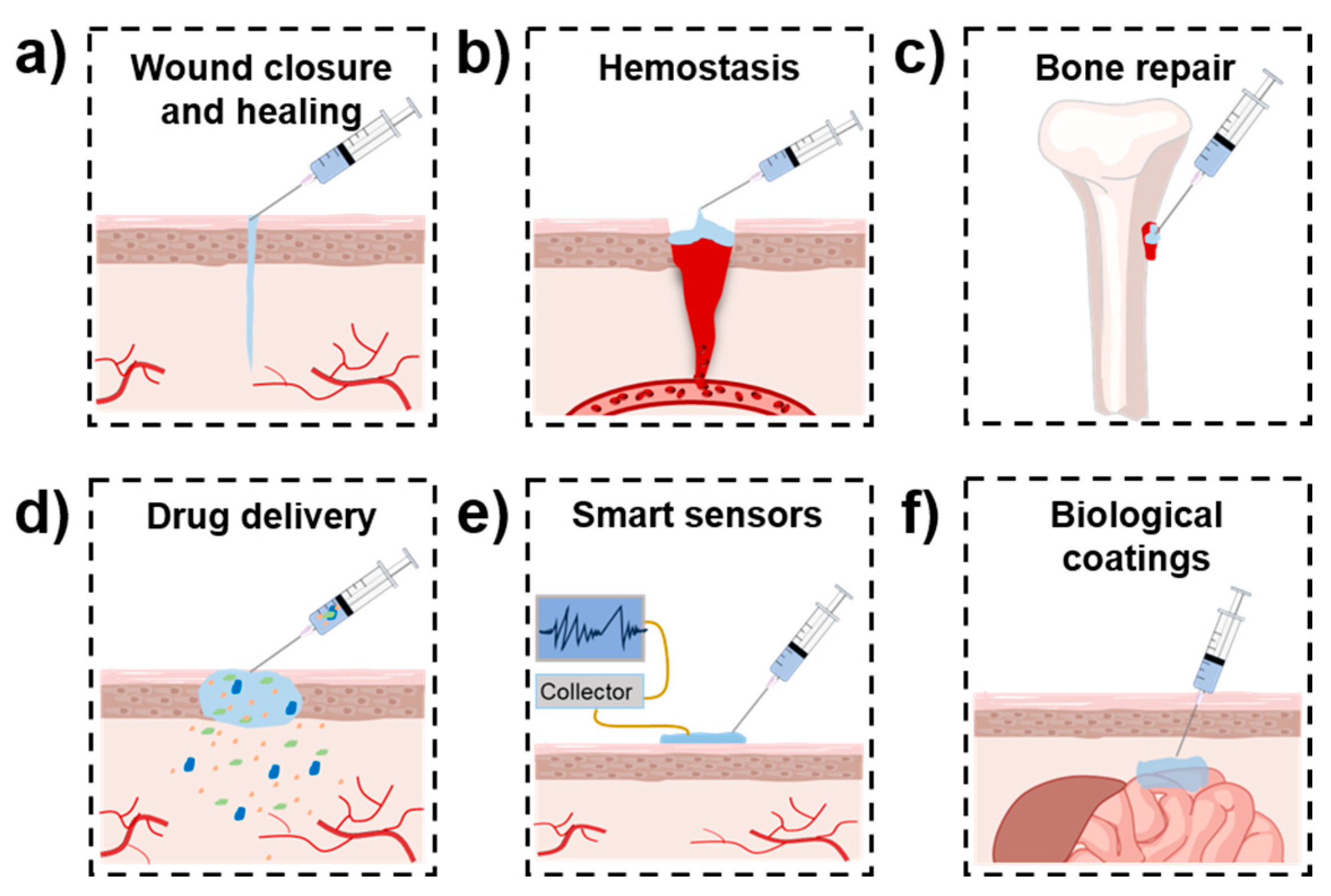
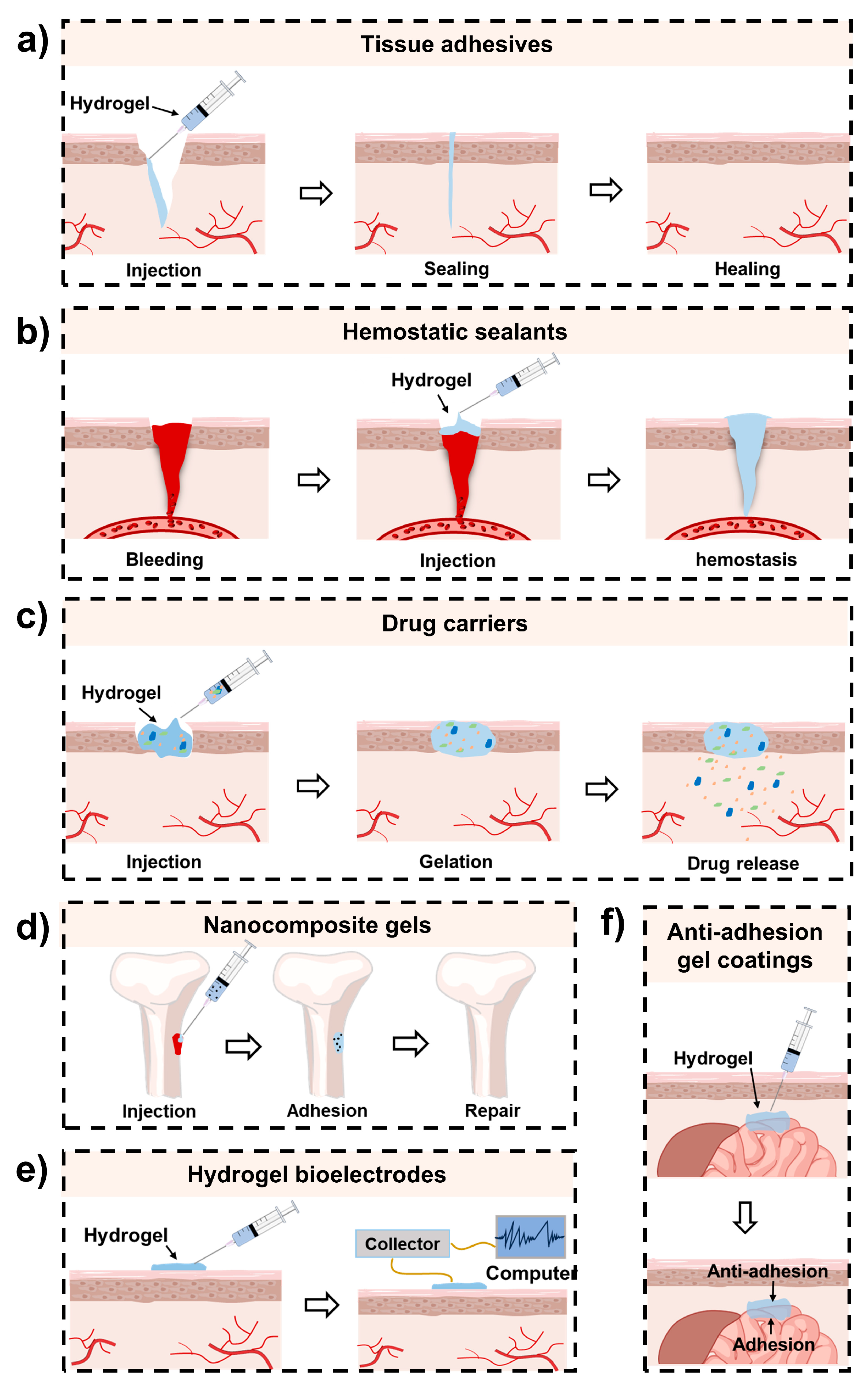
3. Biomedical Applications of Mussel-Inspired Injectable Adhesive Hydrogels
3.1. Wound Closure and Healing
3.2. Hemostasis
3.3. Bone and Cartilage Repair
3.4. Drug Delivery
3.5. Others
4. Challenges and Perspectives of Mussel-Inspired Injectable Adhesive Hydrogels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Name | Abbreviation |
| 3,4-dihydroxyphenylalanine | DOPA |
| Dopamine | DA/Dopa |
| Sodium periodate | NaIO4 |
| Hyaluronic acid | HA |
| Gelatin | Gel/GT |
| Sodium alginate | SA |
| ε-polylysine | PL |
| Polyethylene glycol | PEG |
| Methacrylamide dopamine | DMA |
| Poly (γ-glutamic acid) | PGA |
| Horseradish peroxidase | HRP |
| Hydrogen peroxide | H2O2 |
| Sulfhydryl chitosan | CCS |
| Chitosan quaternary ammonium salt | HTCC |
| Oxidized dextran | OD |
| Copolymer of acrylamide and acrylic acid | PAM |
| Cationized nanofibrillated cellulose | CATNFC |
| Dextran | Dex |
| Oxidized dextran | ODex |
| Oxidized hyaluronic acid | OHA |
| Guar gum | GG |
| Gycol chitosan | GC |
| Reactive oxygen species | ROS |
| Extracellular matrix | ECM |
| Poly (vinyl alcohol) | PVA |
| Polyacrylic acid | PAA |
| Oxidized carboxymethylcellulose | OCMC |
| Tannic acid | TA |
| Nano-hydroxyapatite | nHA |
| Poly (ι-glutamic acid) | PLGA |
| Cod peptides | CPs |
| Laponite | Lap |
| Chondroitin sulfate | CS |
| Regenerated silk fibroin | RSF |
| Dimethyloxalylglycine | DMOG |
| Human umbilical vein endothelial cells | HUVECs |
| Doxorubicin | DOX |
| Proanthocyanin | PC |
| Cellulose nanofiber | CNF |
| Carboxymethyl chitosan | CMCS |
| Polypropylene | PP |
| Polyethylene glycol monomethyl ether-modified glycidyl methacrylate-functionalized chitosan | CSG-PEG |
| Protocatechualdehyde | PA |
| PA-Fe3+-Tris | TPF |
| Oxidized alginate | OA |
| Gelatin–methacryloyl | GelMA |
| Dopamine-grafted alginate | AD |
| Polydopamine-functionalized Laponite | Lap@PDA |
| Dopamine-modified poly(α,β-aspartic acid) derivative | PDAEA |
| Quaternized chitosan | QCS |
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.H.; Zhou, Y.S.; Zhou, F.; Liu, W.M.; Wang, Z.K. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef] [PubMed]
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.X.; Sun, Z.M.; Zhu, X.W.; Zhao, Q.; Zhang, T.F.; Cholewinski, A.; Yang, F.; Zhao, B.X.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, J.; Zeng, L.; Hu, B. An overview on the adhesion mechanisms of typical aquatic organisms and the applications of biomimetic adhesives in aquatic environments. Int. J. Mol. Sci. 2024, 25, 7994. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Cui, C.Y.; Liu, W.G. Recent advances in wet adhesives: Adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021, 116, 101388. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W.M. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, J.S.; Wang, J.L.; Zeng, H.B.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Hofman, A.H.; van Hees, I.A.; Yang, J.; Kamperman, M. Bioinspired underwater adhesives by using the supramolecular toolbox. Adv. Mater. 2018, 30, 1704640. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric tissue adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Du, D.Y.; Chen, X.; Shi, C.; Zhang, Z.Y.; Shi, D.J.; Kaneko, D.; Kaneko, T.; Hua, Z. Mussel-inspired epoxy bioadhesive with enhanced interfacial interactions for wound repair. Acta Biomater. 2021, 136, 223–232. [Google Scholar] [CrossRef]
- Pan, G.X.; Li, F.H.; He, S.H.; Li, W.D.; Wu, Q.M.; He, J.J.; Ruan, R.J.; Xiao, Z.X.; Zhang, J.; Yang, H.H. Mussel- and barnacle cement proteins-inspired dual-bionic bioadhesive with repeatable wet-tissue adhesion, multimodal self-healing, and antibacterial capability for nonpressing hemostasis and promoted wound healing. Adv. Funct. Mate. 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Li, M.J.; Schlaich, C.; Kulka, M.W.; Donskyi, I.S.; Schwerdtle, T.; Unger, W.E.S.; Haag, R. Mussel-inspired coatings with tunable wettability, for enhanced antibacterial efficiency and reduced bacterial adhesion. J. Mater. Chem. B 2019, 7, 3438–3445. [Google Scholar] [CrossRef]
- Imbia, A.S.; Ounkaew, A.; Mao, X.H.; Zeng, H.B.; Liu, Y.; Narain, R. Mussel-inspired polymer-based coating technology for antifouling and antibacterial properties. Langmuir 2024, 40, 10957–10965. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.R.; Guo, J.H.; Zhang, Z.H.; Zhang, X.X.; Zhao, Y.J. Bio-inspired stretchable, adhesive, and conductive structural color film for visually flexible electronics. Adv. Funct. Mater. 2020, 30, 2000151. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.S.; He, J.M.; Bai, Y.P.; Zeng, H.B. Mussel-inspired adhesive and conductive hydrogel with tunable mechanical properties for wearable strain sensors. J. Colloid Interface Sci. 2021, 585, 420–432. [Google Scholar] [CrossRef]
- Xu, J.K.; Tam, M.F.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. [Google Scholar] [CrossRef]
- Kastrup, C.J.; Nahrendorf, M.; Figueiredo, J.L.; Lee, H.; Kambhampati, S.; Lee, T.; Cho, S.W.; Gorbatov, R.; Iwamoto, Y.; Dang, T.T.; et al. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc. Natl. Acad. Sci. USA 2012, 109, 21444–21449. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.I.; Lee, H.; Cho, S.W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.W. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Xing, R.R.; Bai, S.; Yan, X.H. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.G.; Yu, H.J.; Wang, L.; Ni, Z.P.; Liu, X.W.; Shen, D.; Yang, J.; Shi, K.H.; Wang, H.A. Tough adhesion enhancing strategies for injectable hydrogel adhesives in biomedical applications. Adv. Colloid Interface Sci. 2023, 319, 102982. [Google Scholar] [CrossRef]
- Mahmoudi, C.; Tahraoui Douma, N.; Mahmoudi, H.; Iurciuc, C.E.; Popa, M. Hydrogels based on proteins cross-linked with carbonyl derivatives of polysaccharides, with biomedical applications. Int. J. Mol. Sci. 2024, 25, 7839. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Yang, H.; Xu, Y.; Zou, H.J.; Wang, Y.G.; Wang, B.R.; Yu, X.R.; Su, G.S.; Zheng, Y.C. Effect of hydroxy group activity on the hydrogel formation temperature of β-cyclodextrins and its application in profile control. J. Ind. Eng. Chem. 2024, 133, 298–310. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ formation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Sun, Y.N.; Nan, D.; Jin, H.Q.; Qu, X.Z. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Quan, W.Y.; Hu, Z.; Liu, H.Z.; Ouyang, Q.Q.; Zhang, D.Y.; Li, S.D.; Li, P.W.; Yang, Z.M. Mussel-inspired catechol-functionalized hydrogels and their medical applications. Molecules 2019, 24, 2586. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Wang, J.; Yang, G.M.; Pei, X.; Zhang, X. Advances of mussel-inspired hydrogels for bone/cartilage regeneration. Chem. Eng. J. 2024, 487, 150560. [Google Scholar] [CrossRef]
- Yang, Y.T.; Liang, Y.P.; Chen, J.Y.; Duan, X.L.; Guo, B.L. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Xu, H.R.; Li, Z.L.; Zhangji, A.D.; Guo, B.L. Bioinspired injectable self-healing hydrogel sealant with fault-tolerant and repeated thermo-responsive adhesion for sutureless post-wound-closure and wound healing. Nano-Micro Lett. 2022, 14, 185. [Google Scholar] [CrossRef]
- Zhang, W.; Song, S.S.; Huang, J.; Zhang, Z.J. An injectable, robust double network adhesive hydrogel for efficient, real-time hemostatic sealing. Chem. Eng. J. 2023, 476, 146244. [Google Scholar] [CrossRef]
- Song, C.K.; Kim, M.K.; Lee, J.; Davaa, E.; Baskaran, R.; Yang, S.G. Dopa-empowered schiff base forming alginate hydrogel glue for rapid hemostatic control. Macromol. Res. 2019, 27, 119–125. [Google Scholar] [CrossRef]
- Wu, M.H.; Wang, Y.; Liu, H.F.; Chen, F.X.; Zhang, Y.F.; Wu, P.; Deng, Z.M.; Cai, L. Engineering mussel-inspired multifunctional nanocomposite hydrogels to orchestrate osteoimmune microenvironment and promote bone healing. Mater. Des. 2023, 227, 111705. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.Y.; Wu, N.A.Q.; Yun, W.T.; Wang, W.D.; Zhang, K.X.; Li, G.F.; Yan, S.F.; Xu, G.H.; et al. Mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties for bone regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Lu, C.C.; Li, B.Q.; Shan, M.; Wu, G.L. Injectable dopamine-modified poly(α,β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Z.; Zhao, X.; Liang, X.F.; Ma, P.X.; Guo, B.L. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.F.; Wang, Q.H.; He, P.; Liu, K.; Ni, Y.H.; Ouyang, X.H.; Chen, L.H.; Huang, L.L.; Wang, H.P.; Tan, Y. Mussel-inspired nanocomposite hydrogel-based electrodes with reusable and injectable properties for human electrophysiological signals detection. ACS Sustain. Chem. Eng. 2019, 7, 7918–7925. [Google Scholar] [CrossRef]
- Hu, W.J.; Zhang, Z.G.; Lu, S.L.; Zhang, T.Z.; Zhou, N.Z.; Ren, P.F.; Wang, F.M.; Yang, Y.; Ji, Z.L. Assembled anti-adhesion polypropylene mesh with self-fixable and degradable in situ mussel-inspired hydrogel coating for abdominal wall defect repair. Biomater. Sci. 2018, 6, 3030–3041. [Google Scholar] [CrossRef]
- Hwang, D.S.; Gim, Y.; Yoo, H.J.; Cha, H.J. Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials 2007, 28, 3560–3568. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.Z.; Pei, M.J.; Yang, H.J.; Gu, S.J.; Tao, Y.Z.; Ye, D.Z.; Zhou, Y.S.; Xu, W.L.; Xiao, P. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef]
- Yan, Y.H.; Rong, L.H.; Ge, J.; Tiu, B.D.B.; Cao, P.F.; Advincula, R.C. Mussel-inspired hydrogel composite with multi-stimuli responsive behavior. Macromol. Mater. Eng. 2019, 304, 1800720. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.D.; Yuan, X.B.; Zhao, J.; Hou, X. An in situ catechol functionalized ε-polylysine/polyacrylamide hydrogel formed by hydrogen bonding recombination with high mechanical property for hemostasis. Inter. J. Biol. Macromol. 2021, 191, 714–726. [Google Scholar]
- Guo, Z.; Mi, S.; Sun, W. A facile strategy for preparing tough, self-healing double-network hyaluronic acid hydrogels inspired by mussel cuticles. Macromol. Mater. Eng. 2019, 304, 1800715. [Google Scholar] [CrossRef]
- Hou, M.D.; Wang, X.C.; Yue, O.Y.; Zheng, M.H.; Zhang, H.J.; Liu, X.H. Development of a multifunctional injectable temperature-sensitive gelatin-based adhesive double-network hydrogel. Biomater. Adv. 2022, 134, 112556. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhao, X.; Li, G.; Zhang, D.; Wang, D. Mussel-inspired hydrogels as tissue adhesives for hemostasis with fast-forming and self-healing properties. Eur. Polym. J. 2021, 148, 110361. [Google Scholar] [CrossRef]
- Ham, H.O.; Liu, Z.Q.; Lau, K.H.A.; Lee, H.; Messersmith, P.B. Facile DNA immobilization on surfaces through a catecholamine polymer. Angew. Chem. Int. Ed. 2011, 50, 732–736. [Google Scholar] [CrossRef] [PubMed]
- White, E.M.; Seppala, J.E.; Rushworth, P.M.; Sharma, S.; Locklin, J. Switching the adhesive state of catecholic hydrogels using phototitration. Macromolecules 2013, 46, 8882–8887. [Google Scholar] [CrossRef]
- Yang, B.; Kang, D.G.; Seo, J.H.; Choi, Y.S.; Cha, H.J. A comparative study on the bulk adhesive strength of the recombinant mussel adhesive protein fp-3. Biofouling 2013, 29, 483–490. [Google Scholar] [CrossRef]
- Yang, B.; Ayyadurai, N.; Yun, H.; Choi, Y.S.; Hwang, B.H.; Huang, J.; Lu, Q.Y.; Zeng, H.B.; Cha, H.J. In vivo residue-specific dopa-incorporated engineered mussel bioglue with enhanced adhesion and water resistance. Angew. Chem. Int. Ed. 2014, 53, 13360–13364. [Google Scholar] [CrossRef]
- Lee, J.; Chang, K.; Kim, S.; Gite, V.; Chung, H.; Sohn, D. Phase controllable hyaluronic acid hydrogel with iron(iii) ion catechol induced dual cross-linking by utilizing the gap of gelation kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar] [CrossRef]
- Li, S.D.; Chen, N.; Li, X.P.; Li, Y.; Xie, Z.P.; Ma, Z.Y.; Zhao, J.; Hou, X.; Yuan, X.B. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv. Funct. Mater. 2020, 30, 2000130. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. Ph-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Chen, F.; Wang, T.; Feng, S.G.; Hu, C.L.; Wang, X.L.; Zheng, Z. Effects of dopamine-containing curing agents on the water resistance of epoxy adhesives. J. Mater. Sci. 2016, 51, 4320–4327. [Google Scholar] [CrossRef]
- Li, S.D.; Chen, N.; Li, Y.; Li, X.P.; Zhan, Q.; Ban, J.M.; Zhao, J.; Hou, X.; Yuan, X.B. Metal-crosslinked ε-poly-l-lysine tissue adhesives with high adhesive performance: Inspiration from mussel adhesive environment. Int. J. Biol. Macromol. 2020, 153, 1251–1261. [Google Scholar] [CrossRef]
- Sato, T.; Aoyagi, T.; Ebara, M.; Auzély-Velty, R. Catechol-modified hyaluronic acid: In situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym. Bull. 2017, 74, 4069–4085. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M.; et al. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.N.; Lee, J.; Lee, H.; Park, W.H. Enzymatically cross-linked poly(γ-glutamic acid) hydrogel with enhanced tissue adhesive property. ACS Biomater. Sci. Eng. 2020, 6, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gong, C.; Li, B.Q.; Wu, G.L. A ph, glucose, and dopamine triple-responsive, self-healable adhesive hydrogel formed by phenylborate-catechol complexation. Polym. Chem. 2017, 8, 2997–3005. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhao, J.; Sun, X.L.; Li, S.D.; Hou, X.; Yuan, X.B.; Yuan, X.Y. Rapid gelling chitosan/polylysine hydrogel with enhanced bulk cohesive and interfacial adhesive force: Mimicking features of epineurial matrix for peripheral nerve anastomosis. Biomacromolecules 2016, 17, 622–630. [Google Scholar] [CrossRef]
- Müller-Dethlefs, K.; Hobza, P. Noncovalent interactions: A challenge for experiment and theory. Chem. Rev. 2000, 100, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Wang, M.H.; Gan, D.L.; Deng, W.L.; Wang, K.F.; Fang, L.M.; Liu, K.Z.; Chan, C.W.; Tang, Y.H.; et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.Y.; Cao, J.; Zhang, S.M.; Wang, Y.B. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Chen, X.; Du, D.Y.; Zhang, Z.Y.; Shi, C.; Hua, Z.; Chen, J.H.; Shi, D.J. Injectable dopamine-polysaccharide in situ composite hydrogels with enhanced adhesiveness. ACS Biomater. Sci. Eng. 2023, 9, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, L.; Hong, L.J.; Hu, Q.L. A chitosan hydrogel sealant with self-contractile characteristic: From rapid and long-term hemorrhage control to wound closure and repair. Carbohydr. Polym. 2021, 271, 118428. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ryu, S.B.; Park, K.D. Preparation and characterization of dual-crosslinked gelatin hydrogel via dopa-Fe3+ complexation and fenton reaction. J. Ind. Eng. Chem. 2018, 58, 105–112. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Liu, D.H.; Li, D.J.; Mo, X.M. An injectable double cross-linked hydrogel adhesive inspired by synergistic effects of mussel foot proteins for biomedical application. Colloids Surf. B Biointerfaces 2021, 204, 111782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, S.L.; Ren, X.Z.; Zhang, J.M.; Lin, Q.; Zhao, Y.L. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Yang, Y.; He, G.; Pan, Z.; Zhang, K.W.; Xian, Y.W.; Zhu, Z.R.; Hong, Y.L.; Zhang, C.; Wu, D.C. An injectable hydrogel with ultrahigh burst pressure and innate antibacterial activity for emergency hemostasis and wound repair. Adv. Mater. 2024, 36, 2404811. [Google Scholar] [CrossRef]
- Li, S.D.; Dou, W.G.; Ji, W.J.; Li, X.P.; Chen, N.; Ji, Y.P.; Zeng, X.J.; Sun, P.; Li, Y.S.; Liu, C.; et al. Tissue-adhesive, stretchable and compressible physical double-crosslinked microgel-integrated hydrogels for dynamic wound care. Acta Biomater. 2024, 184, 186–200. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Pal, S.; Kumar, S.; Kar, A.; Awasthi, A.K.; Naaz, A.; Srivastava, A.; Bajaj, A. Injectable, self-healing chimeric catechol-Fe(III) hydrogel for localized combination cancer therapy. ACS Biomater. Sci. Eng. 2017, 3, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.X.; Tian, T.; Wu, S.S.; Xiang, T.; Zhou, S.B. A pH-induced self-healable shape memory hydrogel with metal-coordination cross-links. Polym. Chem. 2019, 10, 1920–1929. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.P.; Huang, Y.; He, J.H.; Han, Y.; Guo, B.L. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhang, Y.; Chang, L.M.; Sun, W.C.; Duan, W.H.; Qin, J.L. Mussel-inspired self-healing hydrogel form pectin and cellulose for hemostasis and diabetic wound repairing. Int. J. Biol. Macromol. 2023, 246, 125644. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Huang, Y.J.; You, S.Y.; Xiang, Y.J.; Cai, E.Y.; Mao, R.T.; Pan, W.H.; Tong, X.Q.; Dong, W.; Ye, F.F.; et al. Engineering robust Ag-decorated polydopamine nano-photothermal platforms to combat bacterial infection and prompt wound healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.D.; Wang, H.Y.; Yao, Y.; Chen, D.Y.; Yang, R.; Wang, Z.Y.; Yang, J.H.; Li, Y.M.; Liu, W.G. An injectable self-crosslinked wholly supramolecular polyzwitterionic hydrogel for regulating microenvironment to boost infected diabetic wound healing. Adv. Funct. Mater. 2023, 33, 2303095. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.P.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef]
- Qiao, Z.W.; Lv, X.L.; He, S.H.; Bai, S.M.; Liu, X.C.; Hou, L.X.; He, J.J.; Tong, D.M.; Ruan, R.J.; Zhang, J.; et al. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioact. Mater. 2021, 6, 2829–2840. [Google Scholar] [CrossRef]
- Ma, W.C.; Yang, M.L.; Wu, C.; Wang, S.Y.; Du, M. Bioinspired self-healing injectable nanocomposite hydrogels based on oxidized dextran and gelatin for growth-factor-free bone regeneration. Int. J. Biol. Macromol. 2023, 251, 126145. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Lu, W.X.; Shen, S.Z.; Wei, L. Chitosan derivative-based mussel-inspired hydrogels as the dressings and drug delivery systems in wound healing. Cellulose 2021, 28, 11429–11450. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Z.; Cheng, X.L.; Zou, Z.; Chen, X.S.; He, C.L. Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure. Sci. Adv. 2023, 9, eadh4327. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Li, Y.C.E.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue adhesives: From research to clinical translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, V.; Becker, M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dou, W.; Zhu, S.; Zeng, X.; Ji, W.; Li, X.; Chen, N.; Li, Y.; Liu, C.; Fan, H.; et al. Epidermal growth factor-loaded, dehydrated physical microgel-formed adhesive hydrogel enables integrated care of wet wounds. Int. J. Biol. Macromol. 2024, 275, 133655. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.Y.; Lei, X.X.; Hu, J.J.; Jiang, Y.L.; Li, Q.J.; Song, Y.T.; Zhang, Q.Y.; Li-Ling, J.; Xie, H.Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.F.; Bu, Y.Z.; Chen, Y.R.; Yang, F.; Yu, J.K.; Wu, D.C. An injectable and instant self-healing medical adhesive for wound sealing. ACS Appl. Mater. Interfaces 2020, 12, 9132–9140. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.S.; Pereira, M.J.N.; Liu, S.H.S.; Hu, Y.S.; Karp, J.M.; Artzi, N.; Lee, Y.H. Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Li, Q.; Song, W.; Li, J.H.; Ma, C.Y.; Zhao, X.X.; Jiao, J.L.; Mrowczynski, O.; Webb, B.S.; Rizk, E.B.; Lu, D.; et al. Bioinspired super-strong aqueous synthetic tissue adhesives. Matter 2022, 5, 933–956. [Google Scholar] [CrossRef]
- Deng, X.Y.; Huang, B.X.; Wang, Q.H.; Wu, W.L.; Coates, P.; Sefat, F.; Lu, C.H.; Zhang, W.; Zhang, X.M. A mussel-inspired antibacterial hydrogel with high cell affinity, toughness, self-healing, and recycling properties for wound healing. ACS Sustain. Chem. Eng. 2021, 9, 3070–3082. [Google Scholar] [CrossRef]
- Li, S.D.; Chen, N.; Li, Y.; Zhao, J.; Hou, X.; Yuan, X.B. Environment-dependent adhesive behaviors of mussel-inspired coordinate-crosslinked bioadhesives. Macromol. Mater. Eng. 2020, 305, 1900620. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhong, K.J.; Zong, Y.; Wang, S.H.; Yang, H.Y.; Zhen, L.; Tao, S.Y.; Sun, L.Z.; Yang, J.J.; Li, J.Y. A mussel-inspired wet-adhesion hydrogel with hemostasis and local anti-inflammation for managing the development of acute wounds. Mater. Des. 2022, 213, 110347. [Google Scholar] [CrossRef]
- Maier, G.P.; Rapp, M.V.; Waite, J.H.; Israelachvili, J.N.; Butler, A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science 2015, 349, 628–632. [Google Scholar] [CrossRef]
- Rapp, M.V.; Maier, G.P.; Dobbs, H.A.; Higdon, N.J.; Waite, J.H.; Butler, A.; Israelachvili, J.N. Defining the catechol-cation synergy for enhanced wet adhesion to mineral surfaces. J. Am. Chem. Soc. 2016, 138, 9013–9016. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.Z.; Chen, W.; Xu, T.T.; Yun, S.F.; Xu, Z.; Xu, Z.Q.; Sato, T.; Chi, B.; Xu, H. A biomimetic mussel-inspired ε-poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv. Funct. Mater. 2017, 27, 1604894. [Google Scholar] [CrossRef]
- Wang, W.; Jia, B.; Xu, H.; Li, Z.; Qiao, L.; Zhao, Y.; Huang, H.; Zhao, X.; Guo, B. Multiple bonds crosslinked antibacterial, conductive and antioxidant hydrogel adhesives with high stretchability and rapid self-healing for mrsa infected motion skin wound healing. Chem. Eng. J. 2023, 468, 143362. [Google Scholar] [CrossRef]
- Sun, A.; Hu, D.R.; He, X.Y.; Ji, X.; Li, T.; Wei, X.W.; Qian, Z.Y. Mussel-inspired hydrogel with injectable self-healing and antibacterial properties promotes wound healing in burn wound infection. NPG Asia Mater. 2022, 14, 86. [Google Scholar] [CrossRef]
- He, X.Y.; Sun, A.; Li, T.; Qian, Y.J.; Qian, H.; Ling, Y.F.; Zhang, L.H.; Liu, Q.Y.; Peng, T.; Qian, Z.Y. Mussel-inspired antimicrobial gelatin/chitosan tissue adhesive rapidly activated in situ by H2O2/ascorbic acid for infected wound closure. Carbohydr. Polym. 2020, 247, 116692. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable adhesive self-healing multiple-dynamic-bond crosslinked hydrogel with photothermal antibacterial activity for infected wound healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Zhu, S.Z.; Dou, W.G.; Zeng, X.J.; Chen, X.C.; Gao, Y.L.; Liu, H.L.; Li, S.D. Recent advances in the degradability and applications of tissue adhesives based on biodegradable polymers. Int. J. Mol. Sci. 2024, 25, 5249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Ning, X.Q.; Chen, Y.W.; Chang, H.X.; Lu, D.H.; Pei, D.T.; Geng, Z.J.; Zeng, Z.W.; Guo, C.P.; Huang, J.; et al. Dual glucose/ros-sensitive injectable adhesive self-healing hydrogel with photothermal antibacterial activity and modulation of macrophage polarization for infected diabetic wound healing. ACS Mater. Lett. 2023, 5, 3142–3155. [Google Scholar] [CrossRef]
- Fu, M.M.; Zhao, Y.T.; Wang, Y.; Li, Y.; Wu, M.; Liu, Q.; Hou, Z.G.; Lu, Z.H.; Wu, K.K.; Guo, J.S. On-demand removable self-healing and pH-responsive europium-releasing adhesive dressing enables inflammatory microenvironment modulation and angiogenesis for diabetic wound healing. Small 2023, 19, 2205489. [Google Scholar] [CrossRef]
- Dong, Q.; Liang, X.; Chen, F.X.; Ke, M.F.; Yang, X.D.; Ai, J.J.; Cheng, Q.Q.; Zhou, Y.; Chen, Y. Injectable shape memory hydroxyethyl cellulose/soy protein isolate based composite sponge with antibacterial property for rapid noncompressible hemorrhage and prevention of wound infection. Int. J. Biol. Macromol. 2022, 217, 367–380. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Deng, Y.R.; Xie, X.; Xu, L.M.; Xu, X.Y.; Yuan, W.H.; Yang, B.G.; Yang, X.F.; Xia, X.F.; et al. Ultrafast self-gelling and wet adhesive powder for acute hemostasis and wound healing. Adv. Funct. Mater. 2021, 31, 2102583. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.Y.; Gao, X.; Zhuang, Y.; Fan, C.X.; Shen, H.; Chen, Y.Y.; Dai, J.W. Biomimetic natural biopolymer-based wet-tissue adhesive for tough adhesion, seamless sealed, emergency/nonpressing hemostasis, and promoted wound healing. Adv. Funct. Mater. 2023, 33, 2211340. [Google Scholar] [CrossRef]
- Wei, C.Y.; Shi, W.L.; Zhao, C.Q.; Yang, S.; Zheng, J.J.; Zhong, J.P.; Zhao, T.Y.; Kong, S.M.; Gong, X.; Liu, M.J. Superwetting injectable hydrogel with ultrastrong and fast tissue adhesion for minimally invasive hemostasis. Adv. Healthcare Mater. 2023, 12, 2201799. [Google Scholar] [CrossRef]
- Cui, C.Y.; Fan, C.C.; Wu, Y.H.; Xiao, M.; Wu, T.L.; Zhang, D.F.; Chen, X.Y.; Liu, B.; Xu, Z.Y.; Qu, B.; et al. Water-triggered hyperbranched polymer universal adhesives: From strong underwater adhesion to rapid sealing hemostasis. Adv. Mater. 2019, 31, 1905761. [Google Scholar] [CrossRef]
- Lu, S.C.; Zhang, X.H.; Tang, Z.W.; Xiao, H.; Zhang, M.; Liu, K.; Chen, L.H.; Huang, L.L.; Ni, Y.H.; Wu, H. Mussel-inspired blue-light-activated cellulose-based adhesive hydrogel with fast gelation, rapid haemostasis and antibacterial property for wound healing. Chem. Eng. J. 2021, 417, 129329. [Google Scholar] [CrossRef]
- Cui, G.H.; Guo, X.Y.; Su, P.; Zhang, T.S.; Guan, J.; Wang, C.A. Mussel-inspired nanoparticle composite hydrogels for hemostasis and wound healing. Front. Chem. 2023, 11, 1154788. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, C.H.; Park, M.R.; Song, S.C. Development of an injectable dopamine-conjugated poly(organophophazene) hydrogel for hemostasis. Bull. Korean Chem. Soc. 2016, 37, 372–377. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Yang, Z.X.; Zhai, Q.M.; Li, D.Z.; Zhu, X.Y.; He, Q.Q.; Li, L.J.; Cannon, R.D.; Wang, H.A.; Tang, H.; et al. An all-in-one “4a hydrogel”: Through first-aid hemostatic, antibacterial, antioxidant, and angiogenic to promoting infected wound healing. Small 2023, 19, 2207437. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.M.; Cui, J.W.; Hao, J.C. Mussel-inspired hydrogels for tissue healing. Acta Chim. Sinica 2020, 78, 105–113. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, Z.Q.; Pan, Y.Y.; Chen, S.S.; Fan, X.; Li, X.L.; Chen, G.Q.; Ma, Y.H.; Cai, Y.J.; Zhang, J.X.; et al. Polycatechol-derived mesoporous polydopamine nanoparticles for combined ros scavenging and gene interference therapy in inflammatory bowel disease. ACS Appl. Mater. Interfaces 2022, 14, 19975–19987. [Google Scholar] [CrossRef]
- Li, X.F.; Lu, P.P.; Jia, H.R.; Li, G.F.; Zhu, B.F.; Wang, X.; Wu, F.G. Emerging materials for hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Xu, L.; Jiao, G.H.; Huang, Y.L.; Ren, P.F.; Liang, M.; Wei, D.D.; Zhang, T.Z. Laponite nanoparticle-crosslinked carboxymethyl cellulose-based injectable hydrogels with efficient underwater-specific adhesion for rapid hemostasis. Inter. J. Biol. Macromol. 2024, 255, 128288. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.X.; Zou, C.Y.; Hu, J.J.; Fan, M.H.; Jiang, Y.L.; Xiong, M.; Han, C.; Zhang, X.Z.; Li, Y.X.; Zhao, L.M.; et al. A self-assembly pro-coagulant powder capable of rapid gelling transformation and wet adhesion for the efficient control of non-compressible hemorrhage. Adv. Sci. 2024, 11, 2306289. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Deng, Y.H.; Su, J.C. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Senarath-Yapa, K.; Li, S.L.; Walmsley, G.G.; Zielins, E.; Paik, K.; Britto, J.A.; Grigoriadis, A.E.; Wan, D.C.; Liu, K.J.; Longaker, M.T.; et al. Small molecule inhibition of transforming growth factor beta signaling enables the endogenous regenerative potential of the mammalian calvarium. Tissue Eng. Part A 2016, 22, 707–720. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Fabrication and biomedical application of alginate composite hydrogels in bone tissue engineering: A review. Int. J. Mol. Sci. 2024, 25, 7810. [Google Scholar] [CrossRef]
- Kofron, M.D.; Laurencin, C.T. Bone tissue engineering by gene delivery. Adv. Drug Del. Rev. 2006, 58, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Di Bella, C.; Myers, D.E.; Choong, P.F.M. The osteochondral dilemma: Review of current management and future trends. ANZ J. Surg. 2014, 84, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.Z.; Syed, S.; Zhuang, J.; Xu, X.Y.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Gan, D.L.; Li, Z.Q.; Shen, J.; Tang, P.F.; Luo, S.Y.; Li, P.F.; Lu, X.; Zheng, W. The characteristics of mussel-inspired nha/osa injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.C.; Chen, H.R.; Cheng, S.Z.; Wu, C.; Wang, L.S.; Du, M. Gelatin hydrogel reinforced with mussel-inspired polydopamine-functionalized nanohydroxyapatite for bone regeneration. Int. J. Biol. Macromol. 2023, 240, 124287. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.L.; Xiao, M.; Yang, Z.Z.; Peng, M.Z.; Li, C.D.; Zhou, X.J.; Wang, J.W. Impact of bone marrow mesenchymal stem cell immunomodulation on the osteogenic effects of laponite. Stem Cell. Res. Ther. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.J.; Xia, H.T.; Jia, L.T.; Zhao, J.Z.; Zhao, D.D.; Yan, X.Y.; Zhang, Y.Q.; Tang, S.J.; Zhou, G.D.; Zhu, L.Y.; et al. Ultrafast, tough, and adhesive hydrogel based on hybrid photocrosslinking for articular cartilage repair in water-filled arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O.; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Bernhard, S.; Tibbitt, M.W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Del. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Zhang, A.Q.; Cheng, S.X.; Rong, L.; Zhang, X.Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.T.; Wei, H.; Yu, C.Y. Injectable hydrogels as emerging drug-delivery platforms for tumor therapy. Biomater. Sci. 2024, 12, 1151–1170. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Umesh; Sarkar, S.; Bera, S.; Moitra, P.; Bhattacharya, S. A self-healable and injectable hydrogel for pH-responsive doxorubicin drug delivery in vitro and in vivo for colon cancer treatment. Mater. Today Chem. 2023, 30, 101554. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and ph-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Qiu, Y.Y.; Wang, Q.Q.; Li, D.W.; Hussain, T.; Ke, H.Z.; Wei, Q.F. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem. Eng. J. 2020, 399, 125668. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 41–49. [Google Scholar] [CrossRef]
- Wu, D.; Shi, X.G.; Zhao, F.L.; Chilengue, S.T.F.; Deng, L.D.; Dong, A.J.; Kong, D.L.; Wang, W.W.; Zhang, J.H. An injectable and tumor-specific responsive hydrogel with tissue-adhesive and nanomedicine-releasing abilities for precise locoregional chemotherapy. Acta Biomater. 2019, 96, 123–136. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yang, L.; Wang, L.L.; Li, Z.M.; Yu, Y.K.; Zheng, Y.; Lian, D.Z.; Li, X.M.; Chen, H.Z.; Mei, L.; et al. An injectable hydrogel based on Bi2Se3 nanosheets and hyaluronic acid for chemo-photothermal synergistic therapy. Int. J. Biol. Macromol. 2023, 244, 125064. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Li, X.Y.; Zhang, R.Z.; Zhang, L.B. Stimuli-responsive hydrogels for antibacterial applications. Adv. Healthcare Mater. 2024, 2400513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, B.P.; Zhang, Y.X.; Xu, L.J.; Huang, Q.Y.; He, Y.; Li, X.F.; Wu, J.; Yang, J.T.; Chen, Q.; et al. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mater. Chem. B 2020, 8, 3171–3191. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef]
- Qian, Y.A.; Xu, K.J.; Shen, L.L.; Dai, M.L.; Zhao, Z.L.; Zheng, Y.J.; Wang, H.O.; Xie, H.L.; Wu, X.; Xiao, D.C.; et al. Dopamine-based high-transparent hydrogel as bioadhesive for sutureless ocular tissue repair. Adv. Funct. Mater. 2023, 33, 2300707. [Google Scholar] [CrossRef]



| Hydrogel Types | Advantages | Drawbacks |
|---|---|---|
| Preformed hydrogels | High physical properties Stable physicochemical properties | Invasive application Fail to fill irregular shapes |
| Injectable hydrogels | Adaption to irregular shapes Minimally invasive application | Low mechanical properties Weak adhesion properties |
| Applications | Examples | Ref. |
|---|---|---|
| Wound closure and healing | CSG-PEG/DMA/Zn hydrogel GT-SA-TPFx | [36] [37] |
| Hemostasis | DNAH 1 Dopa-OA glue | [38] [39] |
| Bone repair | GMAD/LP 2 nHA/PLGA-Dex hydrogel | [40] [41] |
| Drug delivery | PDAEA-Fe3+ hydrogel QCS/GT/DA | [42] [43] |
| Smart sensors | PC-CNF-GG-glycerol hydrogel | [44] |
| Biological coatings | OCMC-DA/CMCS hydrogel | [45] |
| Main Crosslinking Methods | Ref. |
|---|---|
| Schiff base reaction and catechol–Fe coordination | [61] |
| Schiff base reaction and catechol–catechol adducts | [73] |
| Schiff base reaction and catechol–Fe coordination/oxidation-induced catechol-based crosslinking | [74] |
| Schiff base reaction/catechol-based Michael addition and Schiff base reaction | [75] |
| Fenton reaction and Dopa–Fe3+ complexation | [76] |
| Michael addition and catechol–Fe coordination/oxidation-induced catechol-based crosslinking | [77] |
| Methods | Advantages | Drawbacks |
|---|---|---|
| Traditional suturing | Stable wound closure Desirable mechanical features | Time-consuming Secondary tissue damage Risk of wound infection Prone to leaving scars |
| Tissue adhesives | Easy to manipulation Sealing of air/fluid leakage Minimal tissue damage Less pain and scars | Weak mechanical and adhesive strength Relatively high cost |
| Challenges | Strategies |
|---|---|
| Catechol group is prone to oxidation | Utilizing specific DOPA derivative Utilizing reductive group, such as thiol group |
| Difficulty in polymerization of catechol-based monomers | Protection of catechol groups by alkylsilanes or nitrobenzyl group Tuning the polymerization conditions |
| Balancing interfacial adhesion and crosslinking | Tuning the ratio of catechol group to crosslinkers Crosslinking by other functional groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, W.; Zeng, X.; Zhu, S.; Zhu, Y.; Liu, H.; Li, S. Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 9100. https://doi.org/10.3390/ijms25169100
Dou W, Zeng X, Zhu S, Zhu Y, Liu H, Li S. Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications. International Journal of Molecular Sciences. 2024; 25(16):9100. https://doi.org/10.3390/ijms25169100
Chicago/Turabian StyleDou, Wenguang, Xiaojun Zeng, Shuzhuang Zhu, Ye Zhu, Hongliang Liu, and Sidi Li. 2024. "Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications" International Journal of Molecular Sciences 25, no. 16: 9100. https://doi.org/10.3390/ijms25169100
APA StyleDou, W., Zeng, X., Zhu, S., Zhu, Y., Liu, H., & Li, S. (2024). Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications. International Journal of Molecular Sciences, 25(16), 9100. https://doi.org/10.3390/ijms25169100








