Automatic Detection of the EEG Spike–Wave Patterns in Epilepsy: Evaluation of the Effects of Transcranial Current Stimulation Therapy
Abstract
1. Introduction
2. Results
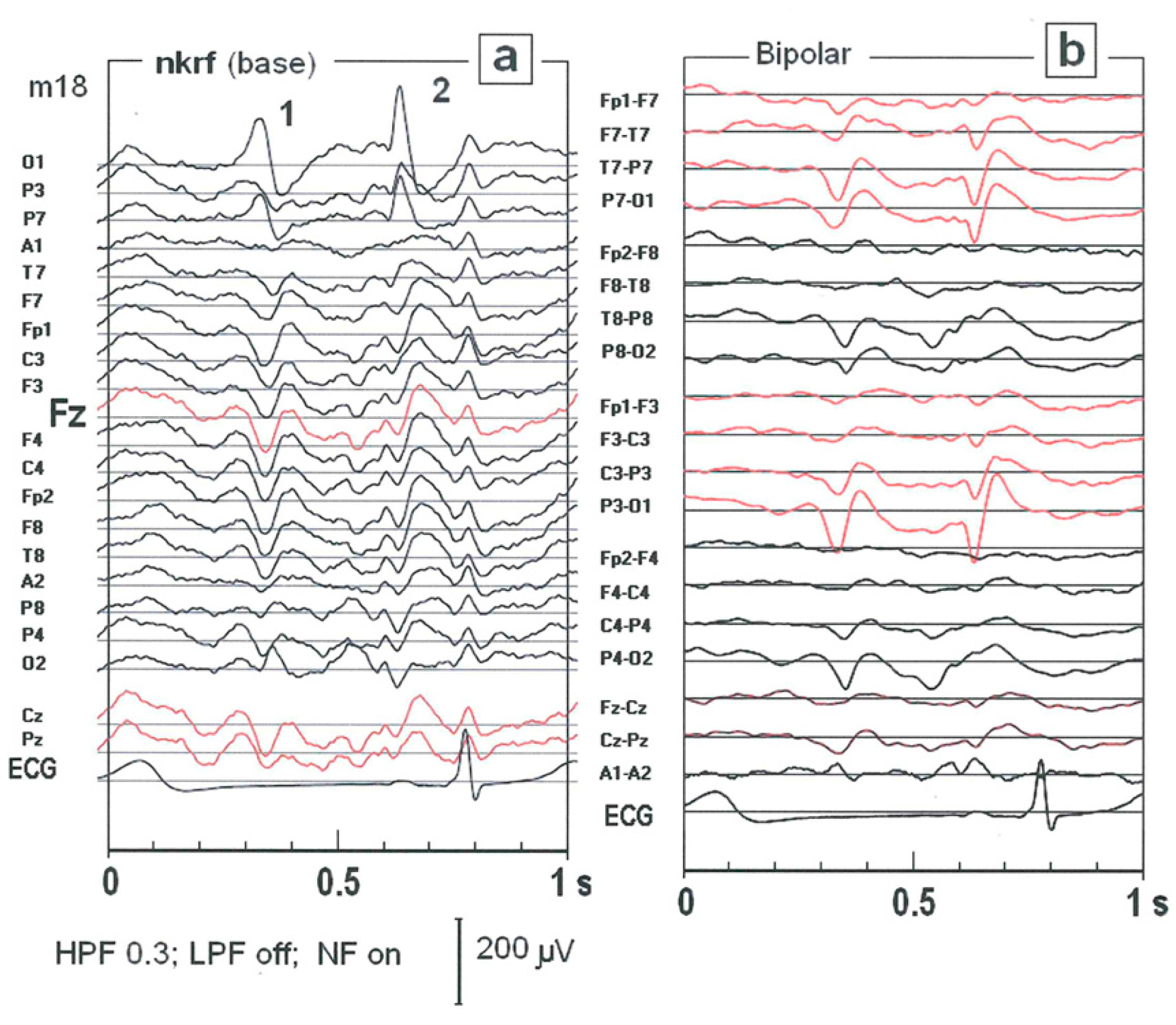
2.1. The Impact of Choice of the Reference Electrode on SW Patterns Identification
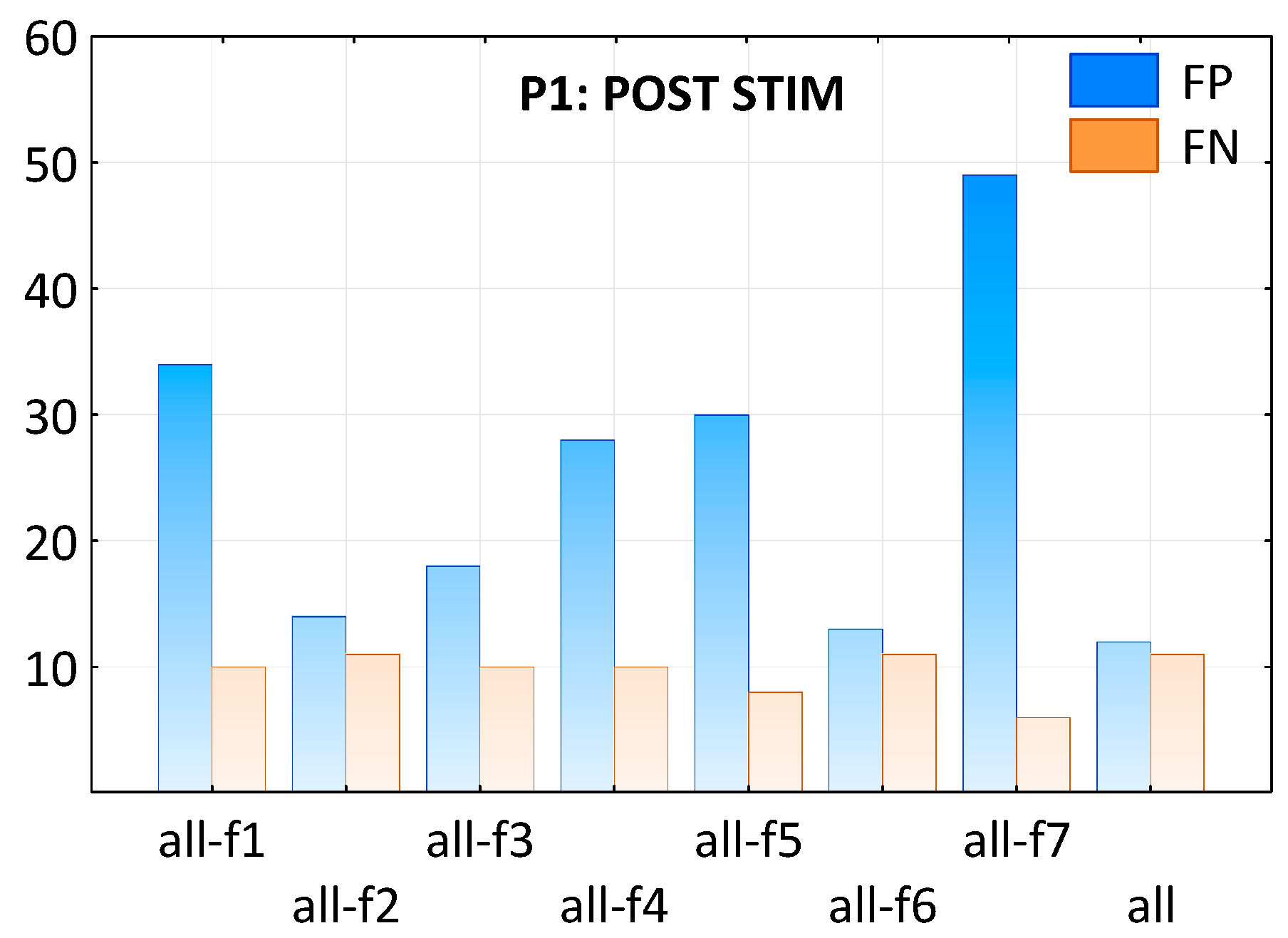
2.2. The Performance of SW Patterns Classification
2.3. Impact of Standardization on Classification Performance
2.4. SW Patterns Similarity Testing
2.5. Impact of Single Features on Classification Performance
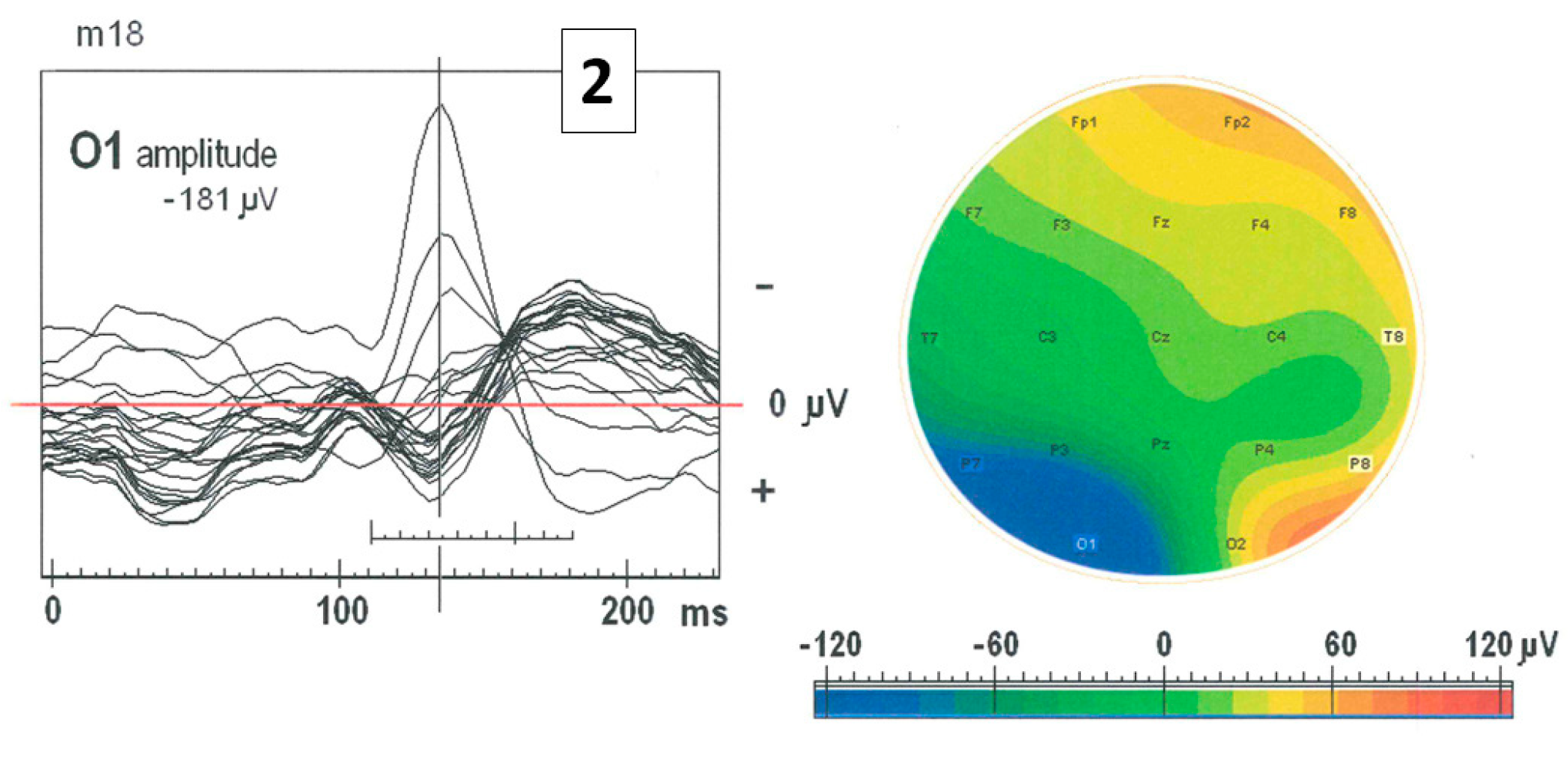
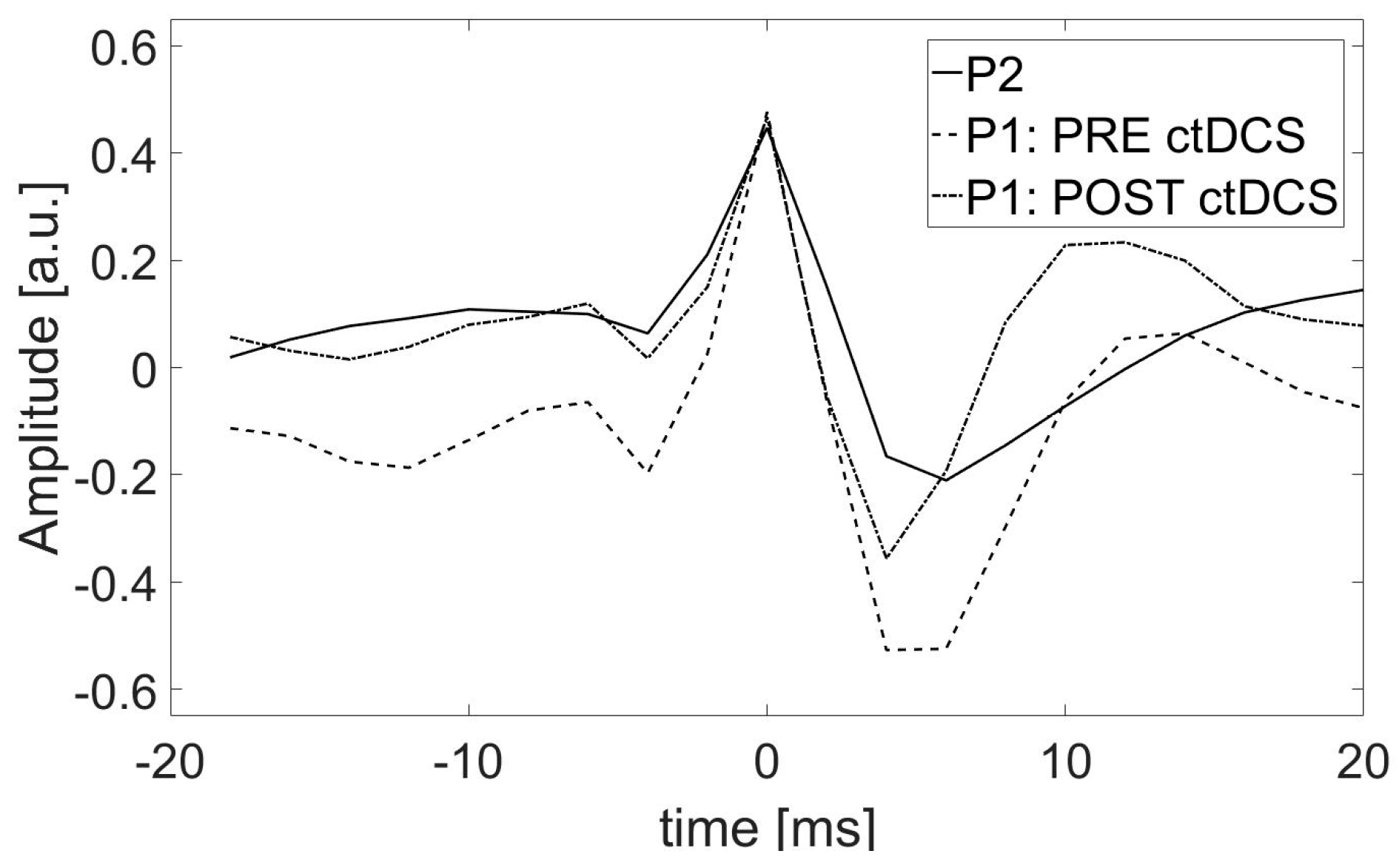
2.6. Impact of the ctDCS on the Morphology of SW Patterns
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. ctDCS Treatment
4.3. EEG Recording
4.4. Automatic Detection of SW Patterns
4.4.1. EEG Preprocessing
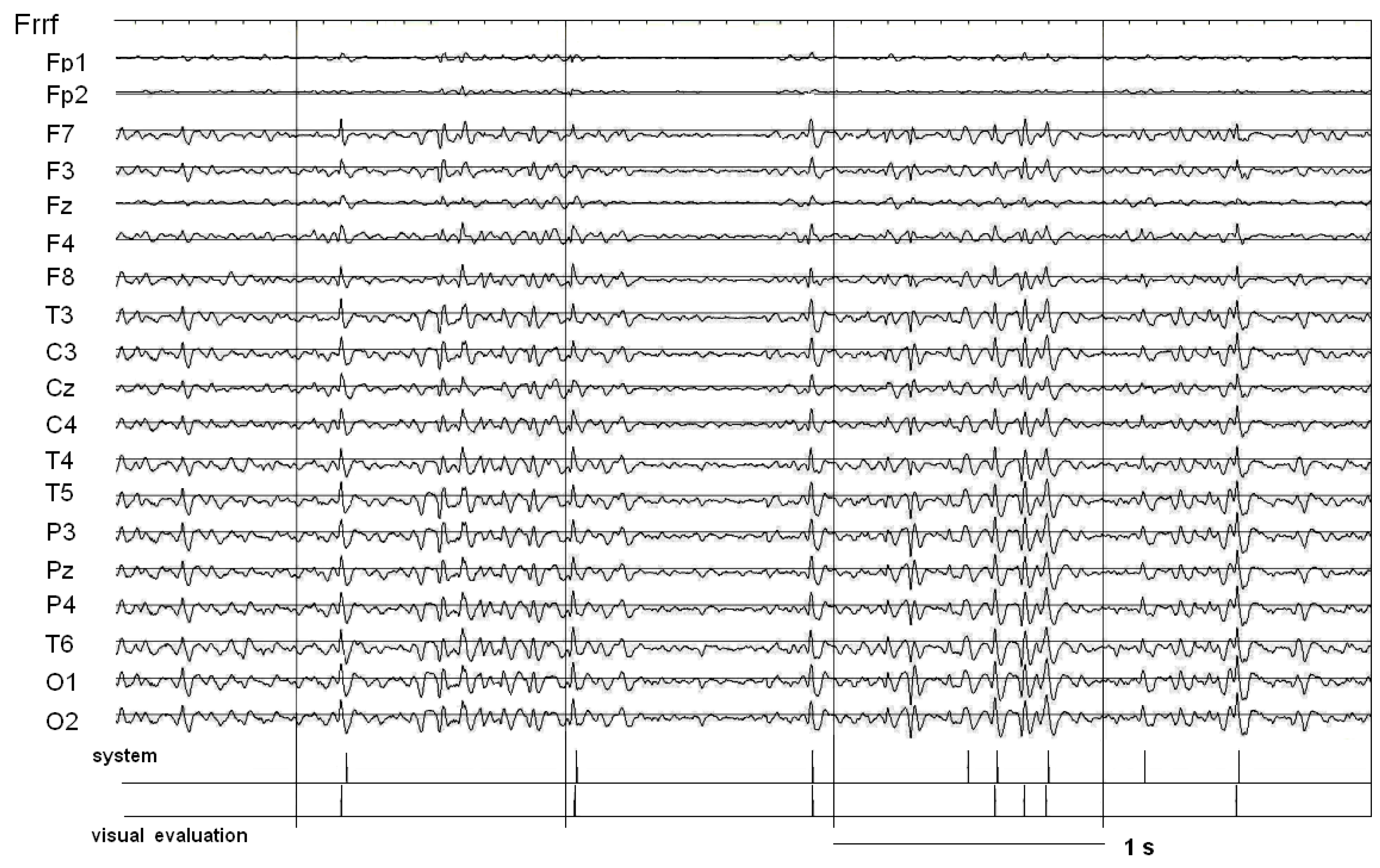
4.4.2. Identification of Potential SW Patterns
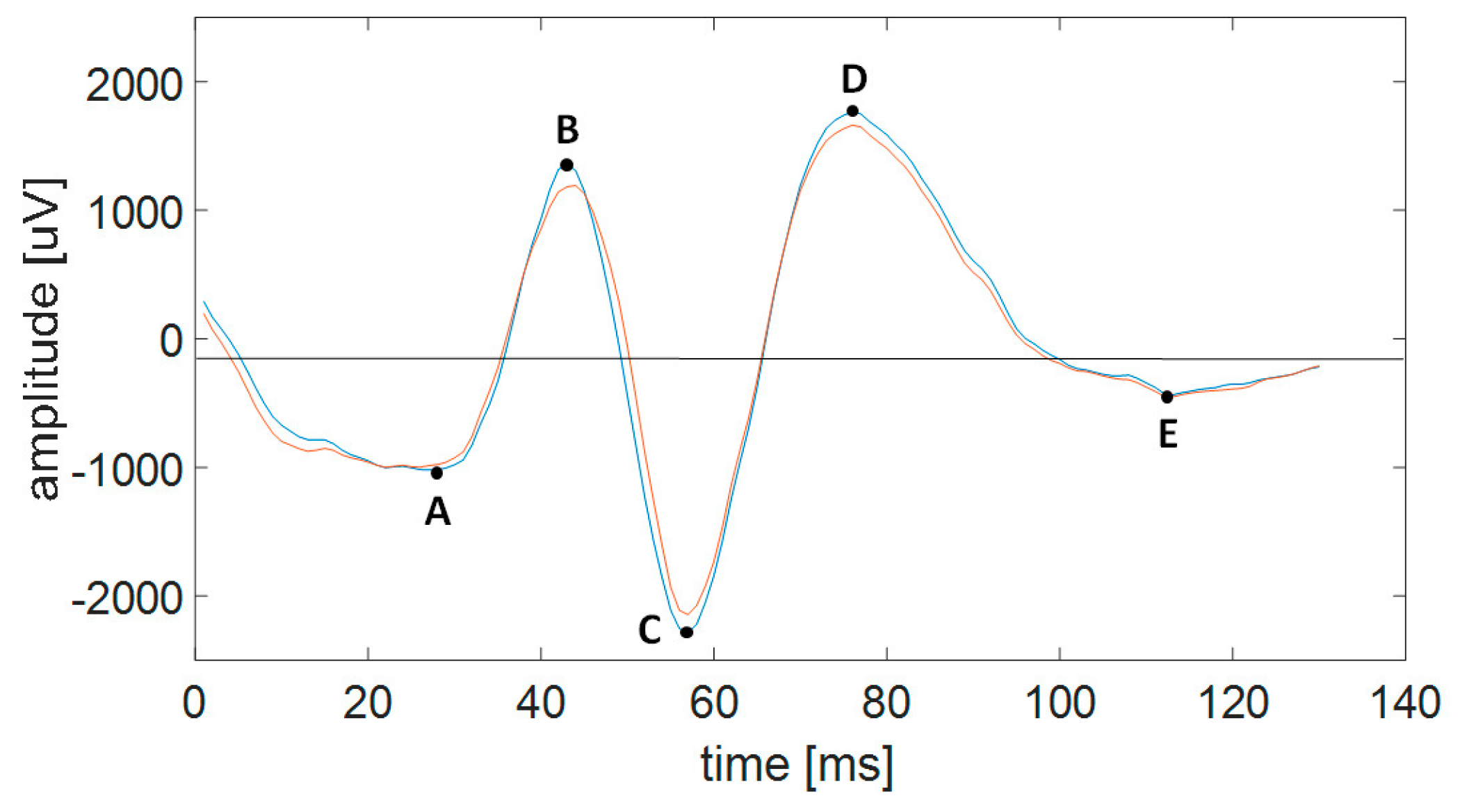
4.4.3. Features of SW Patterns
4.4.4. Selection of SW Patterns
4.5. Similarity Index
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Scharfman, H.E.; DeCurtis, M. How can we identify ictal and interictal abnormal activity? Adv. Exp. Med. Biol. 2014, 813, 3–23. [Google Scholar] [PubMed]
- Kane, N.; Acharya, J.; Benickzy, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J.M.A. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Clin. Neurophysiol. Pract. 2017, 2, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Gotman, J.; Gloor, P. Automatic recognition and quantification of interictal epileptic activity in the human scalp EEG. Electroenceph Clin. Neurophysiol. 1976, 41, 513–529. [Google Scholar] [CrossRef]
- Gotman, J.; Ives, J.R.; Gloor, P. Automatic recognition of inter-ictal epileptic activity in prolonged EEG recordings. Electroenceph Clin. Neurophysiol. 1979, 46, 510–520. [Google Scholar] [CrossRef]
- Gotman, J. Computer analysis of the EEG in epilepsy. In Clinical Applications of Computer Analysis of EEG and Other Neurophysiological Signals; Lopes de Silva, F.H., Storm van Leeuwen, W., Remond, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 171–204. [Google Scholar]
- Gotman, J.; Wang, L.Y. State-dependent spike detection: Concepts and preliminary results. Electroenceph Clin. Neurophysiol. 1991, 79, 11–19. [Google Scholar] [CrossRef]
- Gotman, J.; Wang, L.Y. State dependent spike detection: Validation. Electroenceph Clin. Neurophysiol. 1992, 83, 12–18. [Google Scholar] [CrossRef]
- Wilson, S.B.; Emerson, R. Spike detection: A review and comparison of algorithms. Clin. Neurophysiol. 2002, 113, 1873–1881. [Google Scholar] [CrossRef]
- Harner, R. Automatic EEG spike detection. Clin. EEG Neurosci. 2009, 40, 262–270. [Google Scholar] [CrossRef]
- Gabor, A.J.; Seyal, M. Automated interictal EEG spike detection using artificial neural networks. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 271–280. [Google Scholar] [CrossRef]
- Liu, H.S.; Zhang, T.; Yang, F.S. A multistage, multimethod approach for automatic detection and classification of epileptiform EEG. IEEE Trans. Biomed. Eng. 2002, 49 Pt 2, 1557–1566. [Google Scholar]
- Senhadji, L.; Wendling, F. Epileptic transient detection: Wavelets and time-frequency approaches. Neurophysiol. Clin. 2002, 32, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.D., Jr. Automatic recognition and characterization of epileptiform discharges in the human EEG. J. Clin. Neurophysiol. 1985, 2, 231–249. [Google Scholar] [CrossRef]
- Woodforth, I.; Hicks, R.; Crawford MStephen, J.; Burke, D. Electroencephalographic evidence of seizure activity under deep sevoflurane anesthesia in a nonepileptic patient. Anesthesiology 1997, 87, 1579–1582. [Google Scholar] [CrossRef]
- Yli-Hankala, A.; Vakkuri, A.; Sarkela, M.; Lindgren, L.; Korttila, K.; Jantti, V. Epileptiform electroencephalogram during mask induction of anesthesia with sevoflurane. Anesthesiology 1999, 91, 1596–1603. [Google Scholar] [CrossRef]
- Jantti, V.; Yli-Hankala, A. Neurophysiology of anaesthesia. Clinical neurophysiology at the beginning of the 21th century. Suppl. Clin. Neurophysiol. 2000, 53, 84–88. [Google Scholar]
- Vakkuri, A.; Yli-Hankala, A.; Särkelä, M.; Lindgren, L.; Mennander, S.; Korttila, K.; Saarnivaara, L.; Jäntti, V. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol. Scand. 2001, 45, 805–811. [Google Scholar] [CrossRef]
- Schultz, A.; Schultz, B.; Grouven, U.; Beger, F.; Korsch, G. Sharp transients in the EEG’s of non-epileptic adult patients receiving sevoflurane. Pharm. World Sci. 2001, 23, 82–85. [Google Scholar] [CrossRef]
- Sato, K.; Shamoto, H.; Kato, M. Effect of sevoflurane on electrocorticogram in normal brain. J. Neurosurg. Anesth. 2002, 14, 63–65. [Google Scholar] [CrossRef]
- Olejarczyk, E.; Jozwik, A.; Zmyslowski, W.; Sobieszek, A.; Marciniak, R.; Byrczek, T.; Jalowiecki, P.; Bem, T. Automatic detection and analysis of the EEG sharp wave-slow wave patterns evoked by fluorinated inhalation anesthetics. Clin. Neurophysiol. 2012, 123, 1512–1522. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, C.C.; Tsai, J.J.; Sun, Y.N. Model-based spike detection of epileptic EEG data. Sensors 2013, 13, 12536–12547. [Google Scholar] [CrossRef]
- Shibasaki, H.; Nakamura, M.; Sugi, T.; Nishida, S.; Nagamine, T.; Ikeda, A. Automatic interpretation and writing report of the adult waking electroencephalogram. Clin. Neurophysiol. 2014, 125, 1081–1094. [Google Scholar] [CrossRef]
- Chang, W.D.; Cha, H.S.; Lee, C.; Kang, H.C.; Im, C.H. Automatic Identification of Interictal Epileptiform Discharges in Secondary Generalized Epilepsy. Comput. Math Methods Med. 2016, 2016, 8701973. [Google Scholar] [CrossRef]
- Veeranki, Y.R.; McNaboe, R.; Posada-Quintero, H.F. EEG-Based Seizure Detection Using Variable-Frequency Complex Demodulation and Convolutional Neural Networks. Signals 2023, 4, 816–835. [Google Scholar] [CrossRef]
- Olejarczyk, E.; Zappasodi, F.; Ricci, L.; Pascarella, A.; Pellegrino, G.; Paulon, L.; Assenza, G.; Tecchio, f. Functional Source Separation-Identified Epileptic Network: Analysis Pipeline. Brain Sci. 2022, 12, 1179. [Google Scholar] [CrossRef]


| Subject | Condition | Similarity Index | Standardization | TP | FP | TN | FN | Sensitivity | Selectivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | PRE STIM POST STIM | NO NO | NO NO | 139 99 | 13 12 | 16033 63778 | 15 11 | 0.91 0.89 | 0.90 0.90 | 1.00 1.00 |
| PRE STIM POST STIM | YES YES | NO NO | 58 62 | 94 49 | 15952 63741 | 96 48 | 0.38 0.56 | 0.38 0.56 | 0.99 1.00 | |
| PRE STIM POST STIM | NO NO | YES YES | 145 104 | 62 41 | 15984 63749 | 9 6 | 0.70 0.72 | 0.94 0.95 | 1.00 1.00 | |
| PRE STIM POST STIM | YES YES | YES YES | 114 110 | 93 35 | 15953 63755 | 40 0 | 0.55 0.76 | 0.74 1.00 | 0.99 1.00 | |
| P2 | - | NO | YES | 112 | 9 | 29868 | 11 | 0.93 | 0.91 | 1.00 |
| YES | YES | 114 | 7 | 29870 | 9 | 0.94 | 0.93 | 1.00 |
| Feature | PRE_STIM | POST_STIM | p-Value |
|---|---|---|---|
| f1 | 0.48±0.15 | 0.47±0.11 | p < 0.100 |
| f2 | 4.63 ± 1.02 | 4.19 ± 1.08 | p < 0.010 |
| f3 | 1.06 ± 0.24 | 0.80 ± 0.19 | p < 0.001 |
| f4 | 1.14 ± 0.23 | 0.92 ± 0.19 | p < 0.001 |
| f5 | 0.66 ± 0.13 | 0.59 ± 0.13 | p < 0.001 |
| f6 | 1.24 ± 0.21 | 1.33 ± 0.23 | p < 0.040 |
| f7 | 7.60 ± 1.45 | 7.20 ± 1.27 | p < 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejarczyk, E.; Sobieszek, A.; Assenza, G. Automatic Detection of the EEG Spike–Wave Patterns in Epilepsy: Evaluation of the Effects of Transcranial Current Stimulation Therapy. Int. J. Mol. Sci. 2024, 25, 9122. https://doi.org/10.3390/ijms25169122
Olejarczyk E, Sobieszek A, Assenza G. Automatic Detection of the EEG Spike–Wave Patterns in Epilepsy: Evaluation of the Effects of Transcranial Current Stimulation Therapy. International Journal of Molecular Sciences. 2024; 25(16):9122. https://doi.org/10.3390/ijms25169122
Chicago/Turabian StyleOlejarczyk, Elzbieta, Aleksander Sobieszek, and Giovanni Assenza. 2024. "Automatic Detection of the EEG Spike–Wave Patterns in Epilepsy: Evaluation of the Effects of Transcranial Current Stimulation Therapy" International Journal of Molecular Sciences 25, no. 16: 9122. https://doi.org/10.3390/ijms25169122
APA StyleOlejarczyk, E., Sobieszek, A., & Assenza, G. (2024). Automatic Detection of the EEG Spike–Wave Patterns in Epilepsy: Evaluation of the Effects of Transcranial Current Stimulation Therapy. International Journal of Molecular Sciences, 25(16), 9122. https://doi.org/10.3390/ijms25169122








