Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas
Abstract
1. Introduction
2. Results
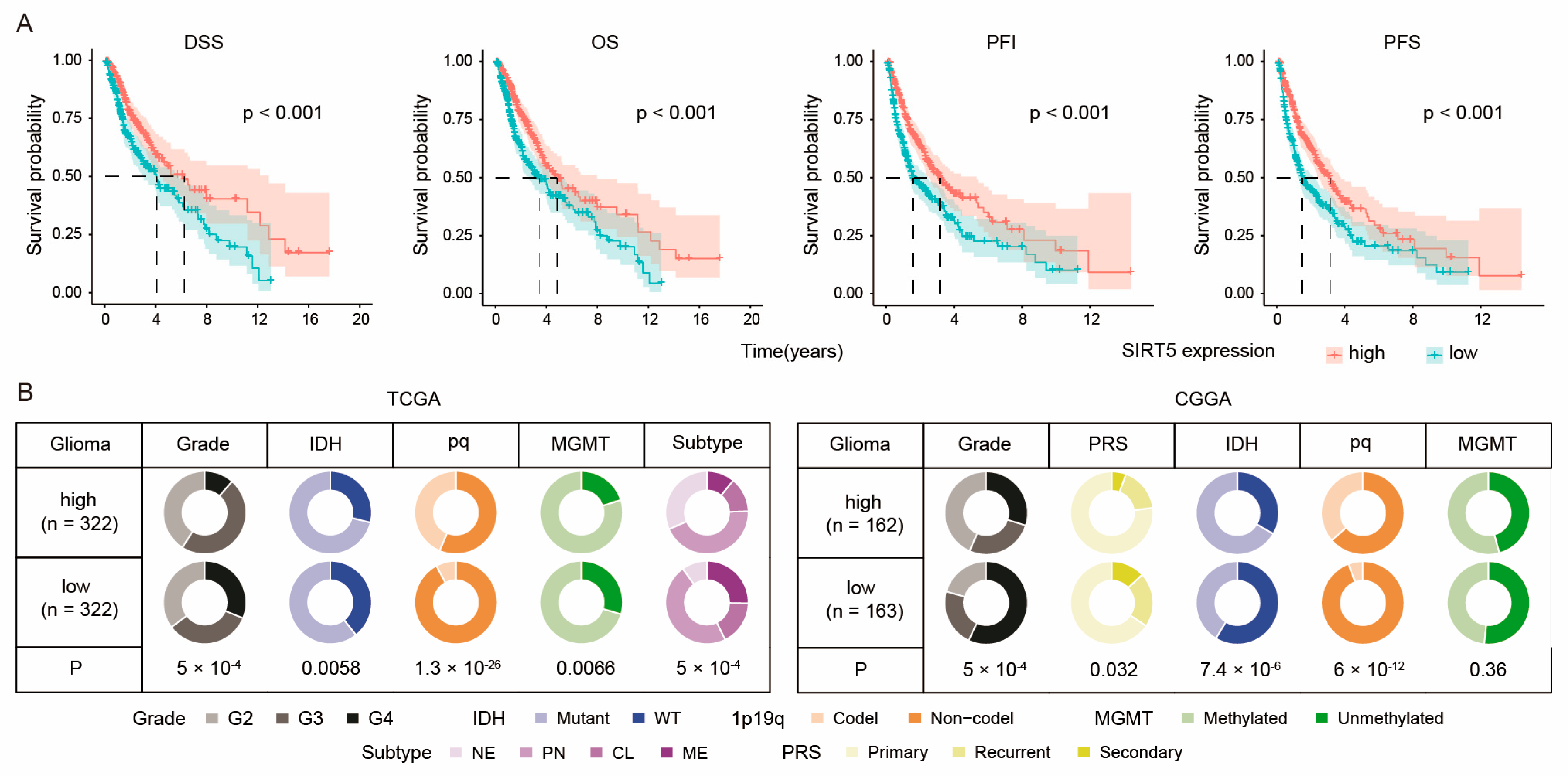
2.1. SIRT5 Expression Correlates with Better Survival in Glioma Patients
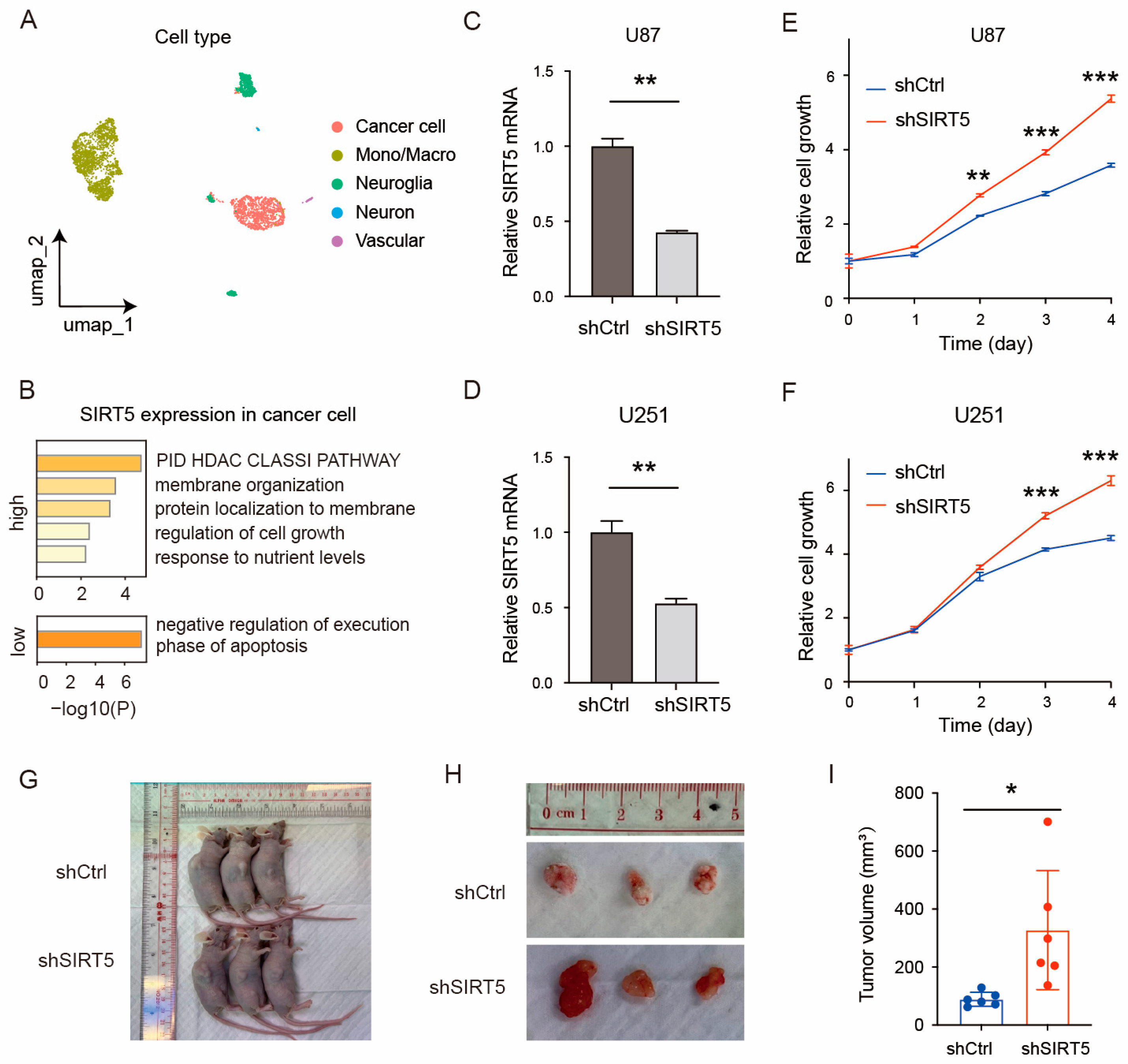
2.2. SIRT5 Knockdown Promotes GBM Cell Growth In Vitro and In Vivo
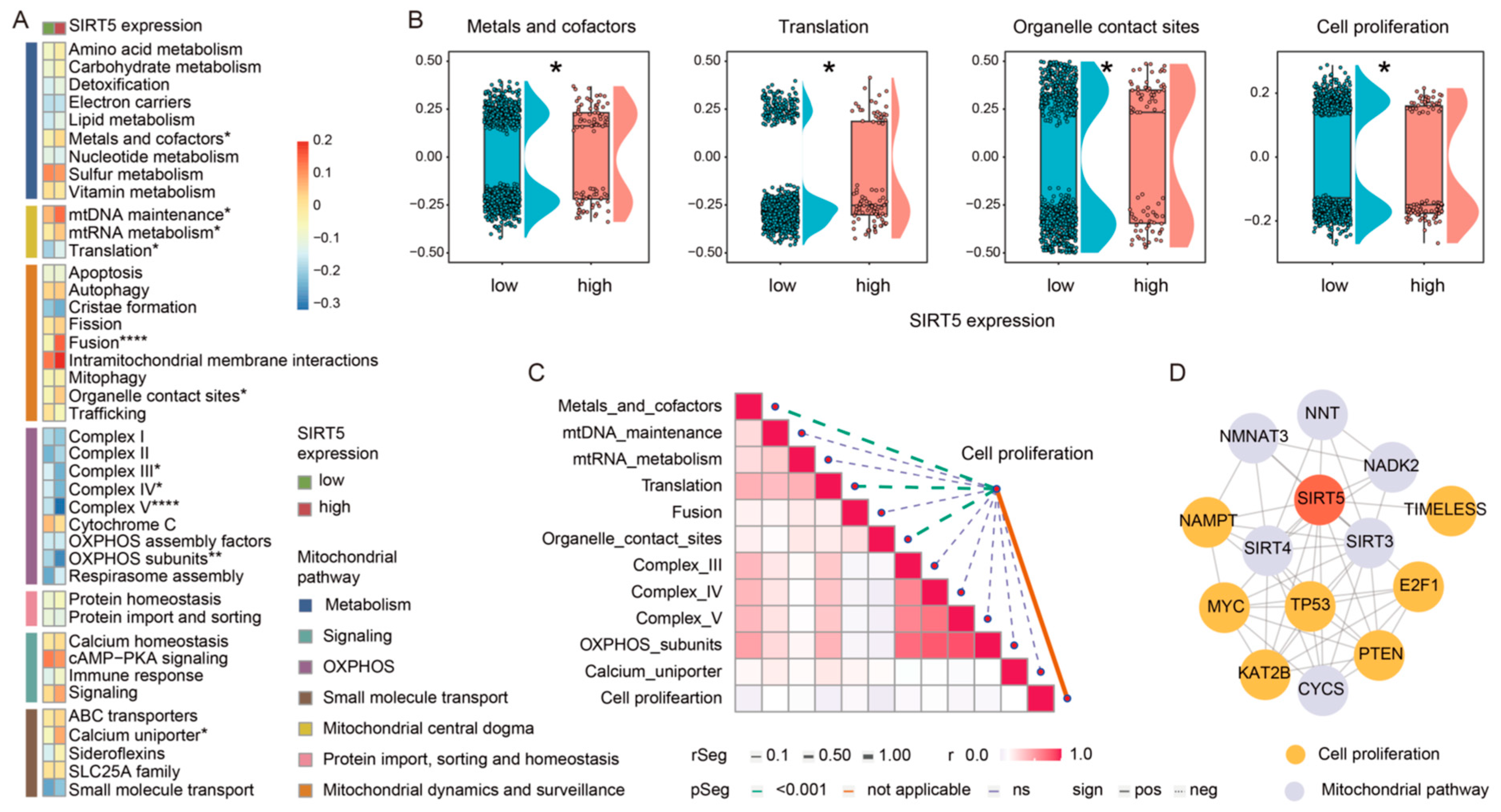
2.3. SIRT5 Potentially Regulates GBM Cell Growth through Modulation of Mitochondrial Pathways
2.4. SIRT5 May Regulate Synapse Function and Immune Response in Gliomas
3. Discussion
4. Materials and Methods
4.1. Sample and Data Collection
4.2. Bulk Transcriptome Data Analysis
4.3. Single-Cell Transcriptome Data Analysis
4.4. Cell Culture
4.5. Transfection of Short Hairpin RNA (shRNA)
4.6. Real-Time Quantitative PCR (RT-qPCR)
4.7. Cell Proliferation Assay
4.8. Subcutaneous Xenograft Model
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Sahm, F.; Brandner, S.; Bertero, L.; Capper, D.; French, P.J.; Figarella-Branger, D.; Giangaspero, F.; Haberler, C.; Hegi, M.E.; Kristensen, B.W.; et al. Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline. Neuro Oncol. 2023, 25, 1731–1749. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, X.; Yang, L.; Zhou, H.; Meng, M.; Wu, H.; Liu, Z.; Zhang, L.; Li, C. Glioma stem cell signature predicts the prognosis and the response to tumor treating fields treatment. CNS Neurosci. Ther. 2022, 28, 2148–2162. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, X.; Yang, L.; Zhou, H.; Meng, M.; Zhang, L.; Li, J. A Cuproptosis Activation Scoring model predicts neoplasm-immunity interactions and personalized treatments in glioma. Comput. Biol. Med. 2022, 148, 105924. [Google Scholar] [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- German, N.J.; Haigis, M.C. Sirtuins and the Metabolic Hurdles in Cancer. Curr. Biol. 2015, 25, R569–R583. [Google Scholar] [CrossRef]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Peng, C.; Anderson, K.A.; Chhoy, P.; Xie, Z.; Dai, L.; Park, J.; Chen, Y.; Huang, H.; Zhang, Y.; et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014, 19, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Shukla, S.K.; Vernucci, E.; He, C.; Wang, D.; King, R.J.; Jha, K.; Siddhanta, K.; Mullen, N.J.; Attri, K.S.; et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology 2021, 161, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.F.U.; Hussain, M.Z.; Mahjabeen, I.; Akram, Z.; Saeed, N.; Shafique, R.; Abbasi, S.F.; Kayani, M.A. Oncometabolic role of mitochondrial sirtuins in glioma patients. PLoS ONE 2023, 18, e0281840. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Z.; Zeng, S.; Wang, X.; Liu, W.; Qian, L.; Wei, J.; Yang, X.; Shen, Q.; Gong, Z.; et al. SIRT5 downregulation is associated with poor prognosis in glioblastoma. Cancer Biomark. 2019, 24, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013, 23, 746–758. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: Biological functions and implications. Front. Oncol. 2024, 14, 1384928. [Google Scholar] [CrossRef]
- Yihan, L.; Xiaojing, W.; Ao, L.; Chuanjie, Z.; Haofei, W.; Yan, S.; Hongchao, H. SIRT5 functions as a tumor suppressor in renal cell carcinoma by reversing the Warburg effect. J. Transl. Med. 2021, 19, 521. [Google Scholar] [CrossRef]
- Greene, K.S.; Lukey, M.J.; Wang, X.; Blank, B.; Druso, J.E.; Lin, M.J.; Stalnecker, C.A.; Zhang, C.; Negron Abril, Y.; Erickson, J.W.; et al. SIRT5 stabilizes mitochondrial glutaminase and supports breast cancer tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 26625–26632. [Google Scholar] [CrossRef] [PubMed]
- Abril, Y.L.N.; Fernandez, I.R.; Hong, J.Y.; Chiang, Y.L.; Kutateladze, D.A.; Zhao, Q.; Yang, M.; Hu, J.; Sadhukhan, S.; Li, B.; et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 2021, 40, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Beylerli, O.; Encarnacion Ramirez, M.J.; Shumadalova, A.; Ilyasova, T.; Zemlyanskiy, M.; Beilerli, A.; Montemurro, N. Cell-Free miRNAs as Non-Invasive Biomarkers in Brain Tumors. Diagnostics 2023, 13, 2888. [Google Scholar] [CrossRef]
- Ganau, M.; Bosco, A.; Palma, A.; Corvaglia, S.; Parisse, P.; Fruk, L.; Beltrami, A.P.; Cesselli, D.; Casalis, L.; Scoles, G. A DNA-based nano-immunoassay for the label-free detection of glial fibrillary acidic protein in multicell lysates. Nanomedicine 2015, 11, 293–300. [Google Scholar] [CrossRef]
- Ali, H.; Harting, R.; de Vries, R.; Ali, M.; Wurdinger, T.; Best, M.G. Blood-Based Biomarkers for Glioma in the Context of Gliomagenesis: A Systematic Review. Front. Oncol. 2021, 11, 665235. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, H. Mitochondrial sirtuins: Energy dynamics and cancer metabolism. Mol. Cells 2024, 47, 100029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gibson, G.E. Succinylation Links Metabolism to Protein Functions. Neurochem. Res. 2019, 44, 2346–2359. [Google Scholar] [CrossRef]
- Park, J.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.; Dai, L.; Xie, Z.; Zhang, Y.; Zwaans, B.M.; Skinner, M.E.; et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef]
- Kumar, S.; Lombard, D.B. Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.; Wang, Y.; Duan, Y.; Zhan, H. Targeting succinylation-mediated metabolic reprogramming as a potential approach for cancer therapy. Biomed. Pharmacother. 2023, 168, 115713. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ruan, H.; Lin, S.; Liu, B.; Song, H.; Li, L.; Ma, T. Lysine succinylation, the metabolic bridge between cancer and immunity. Genes. Dis. 2023, 10, 2470–2478. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Z.; Bao, R.; Guo, X.; Gu, Y.; Yang, W.; Wei, J.; Chen, X.; Tong, L.; Meng, J.; et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J. Hepatol. 2022, 77, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Wang, D.; Wu, Q.; Shang, Q.; Gao, C.; Wang, H.; Wang, H.; Du, M.; Peng, P.; et al. SIRT5-related desuccinylation modification of AIFM1 protects against compression-induced intervertebral disc degeneration by regulating mitochondrial homeostasis. Exp. Mol. Med. 2023, 55, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus Encarnacion, M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef]
- Huang-Hobbs, E.; Cheng, Y.T.; Ko, Y.; Luna-Figueroa, E.; Lozzi, B.; Taylor, K.R.; McDonald, M.; He, P.; Chen, H.C.; Yang, Y.; et al. Remote neuronal activity drives glioma progression through SEMA4F. Nature 2023, 619, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Li, Y.; Chou, M.M.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Choi, J.E.; Han, D.; Koh, S.M.; Kim, H.S.; Kaang, B.K. Decreased Neuron Number and Synaptic Plasticity in SIRT3-Knockout Mice with Poor Remote Memory. Neurochem. Res. 2019, 44, 676–682. [Google Scholar] [CrossRef]
- Lin, C.W.; Chang, L.C.; Tseng, G.C.; Kirkwood, C.M.; Sibille, E.L.; Sweet, R.A. VSNL1 Co-Expression Networks in Aging Include Calcium Signaling, Synaptic Plasticity, and Alzheimer’s Disease Pathways. Front. Psychiatry 2015, 6, 30. [Google Scholar] [CrossRef]
- Barbagallo, D.; Condorelli, A.; Ragusa, M.; Salito, L.; Sammito, M.; Banelli, B.; Caltabiano, R.; Barbagallo, G.; Zappala, A.; Battaglia, R.; et al. Dysregulated miR-671-5p/CDR1-AS/CDR1/VSNL1 axis is involved in glioblastoma multiforme. Oncotarget 2016, 7, 4746–4759. [Google Scholar] [CrossRef]
- Heuer, S.; Winkler, F. Glioblastoma revisited: From neuronal-like invasion to pacemaking. Trends Cancer 2023, 9, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, L.; Zhou, H.; Ye, W.; Luo, C.; Yang, L.; Fang, N.; Tang, A. HMOX1 promotes lung adenocarcinoma metastasis by affecting macrophages and mitochondrion complexes. Front. Oncol. 2022, 12, 978006. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Guthrie, E.H.; Huang, M.T.; Taxman, D.J. Short hairpin RNA (shRNA): Design, delivery, and assessment of gene knockdown. Methods Mol. Biol. 2010, 629, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chen, B.; Leung, G.K.-K.; Kiang, K.M. Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas. Int. J. Mol. Sci. 2024, 25, 9125. https://doi.org/10.3390/ijms25169125
Tang W, Chen B, Leung GK-K, Kiang KM. Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas. International Journal of Molecular Sciences. 2024; 25(16):9125. https://doi.org/10.3390/ijms25169125
Chicago/Turabian StyleTang, Wanjun, Bo Chen, Gilberto Ka-Kit Leung, and Karrie M. Kiang. 2024. "Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas" International Journal of Molecular Sciences 25, no. 16: 9125. https://doi.org/10.3390/ijms25169125
APA StyleTang, W., Chen, B., Leung, G. K.-K., & Kiang, K. M. (2024). Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas. International Journal of Molecular Sciences, 25(16), 9125. https://doi.org/10.3390/ijms25169125






