Functional Materials from Biomass-Derived Terpyridines: State of the Art and Few Possible Perspectives
Abstract
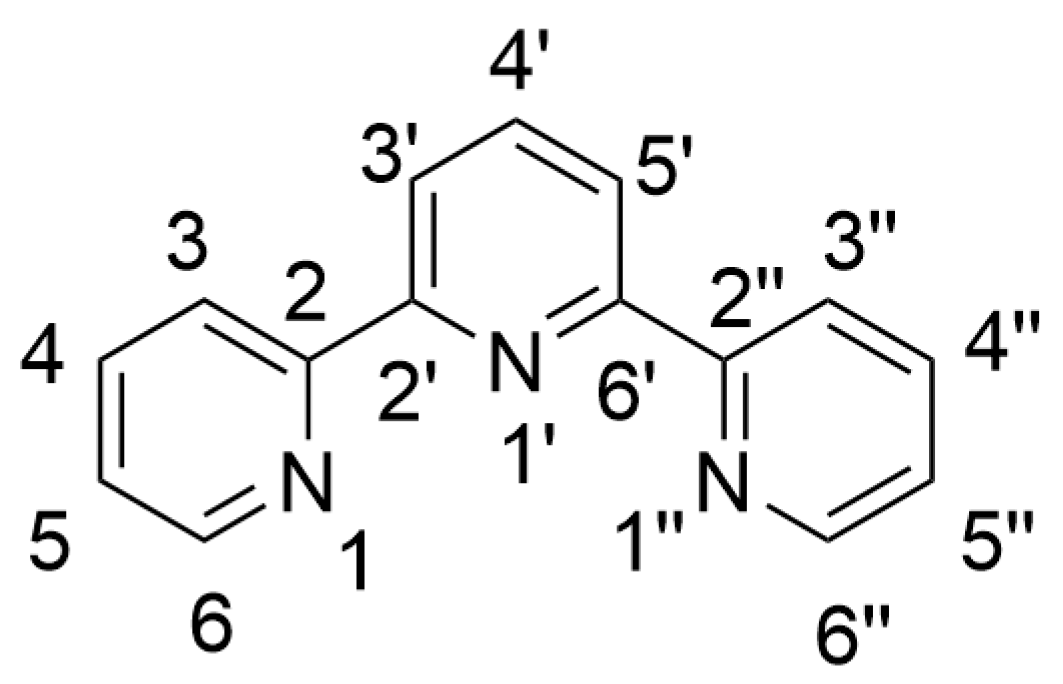
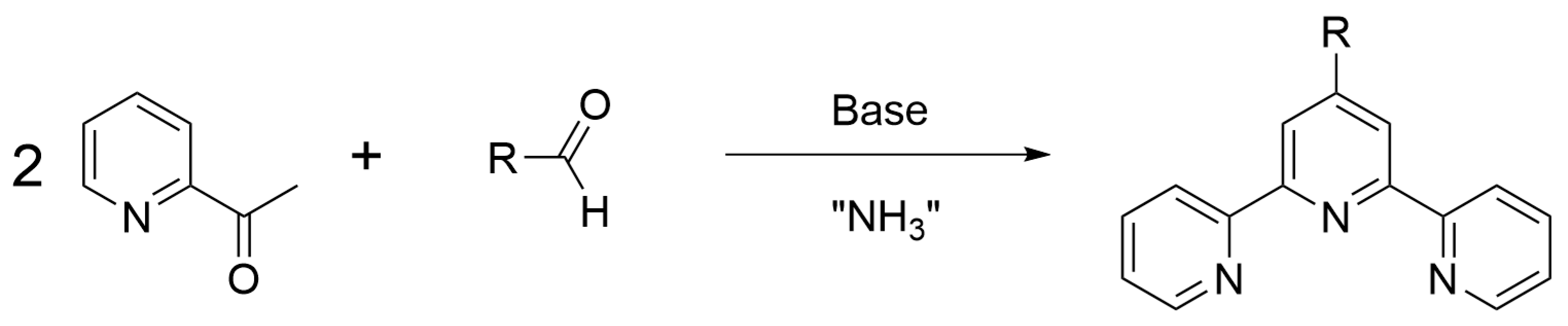
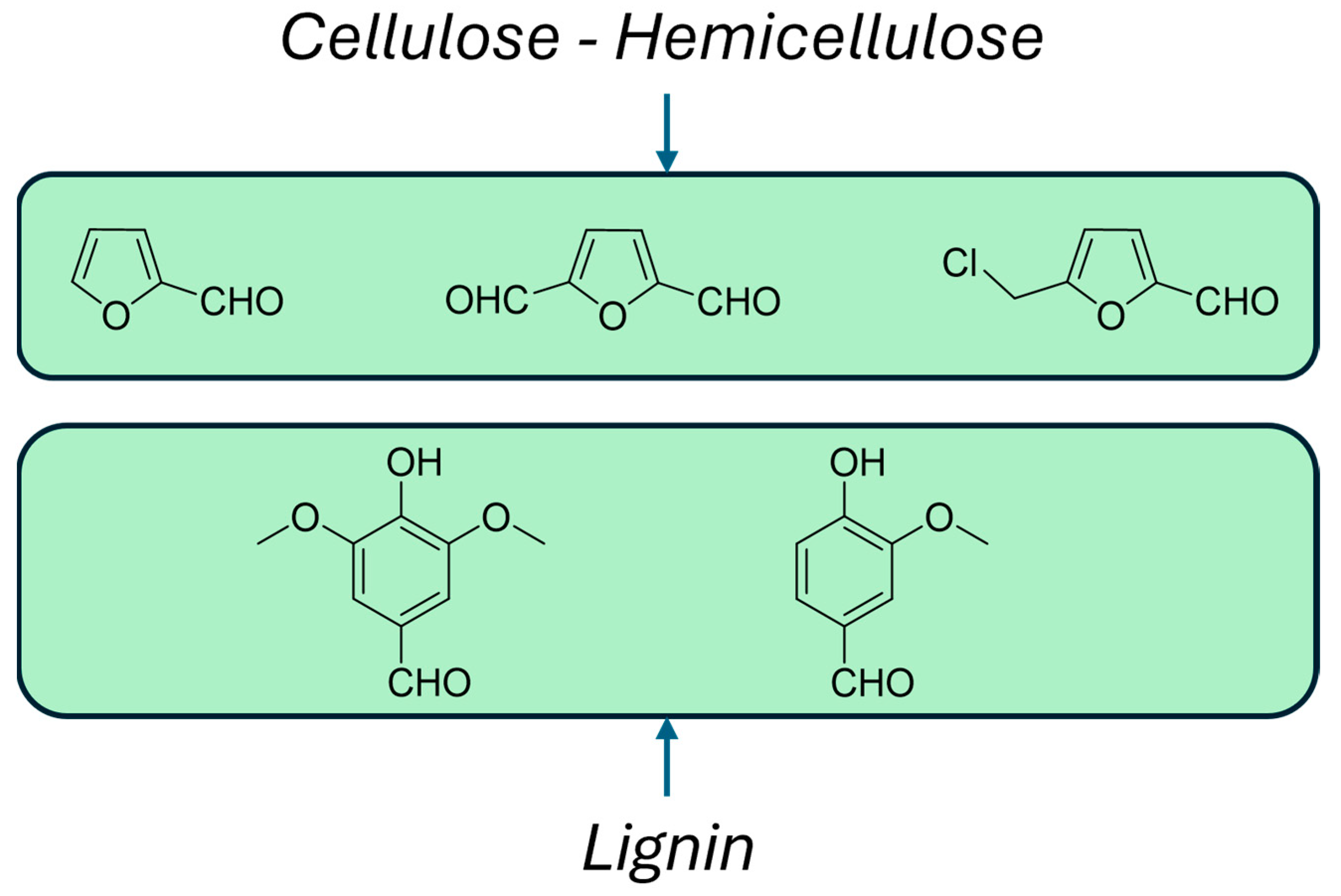
:1. Introduction
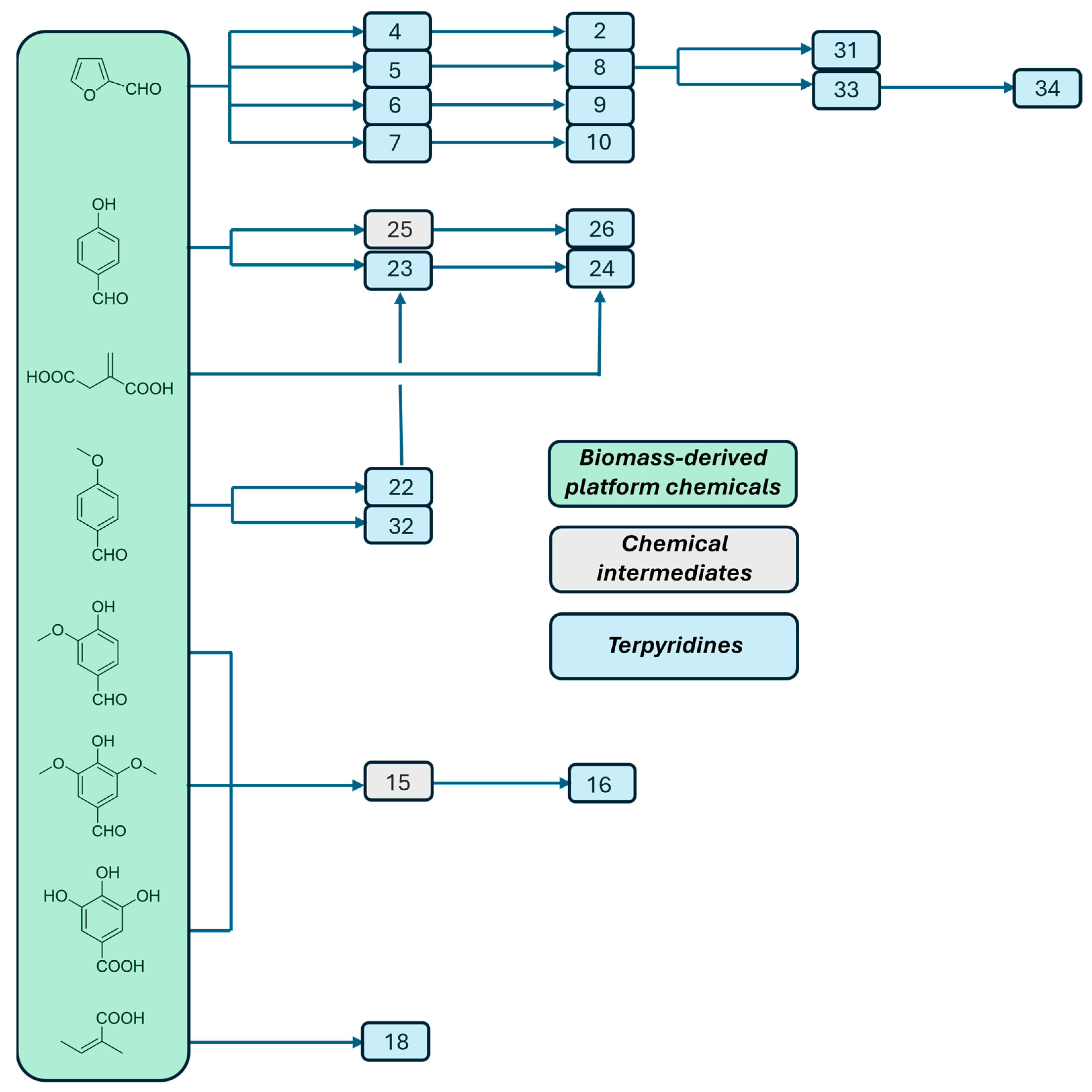
2. Preparation of Functional Materials from Biomass-Derived Terpyridines
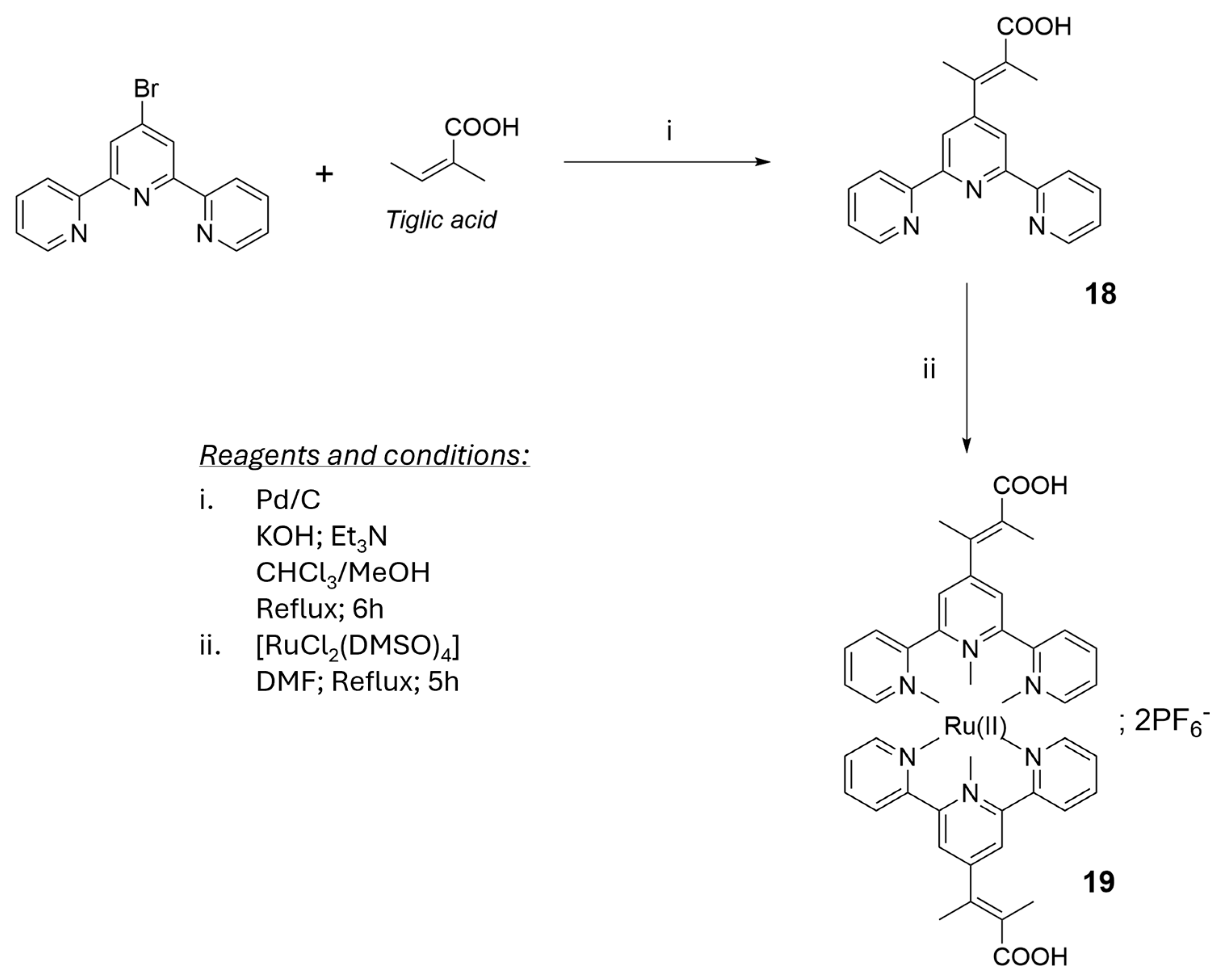
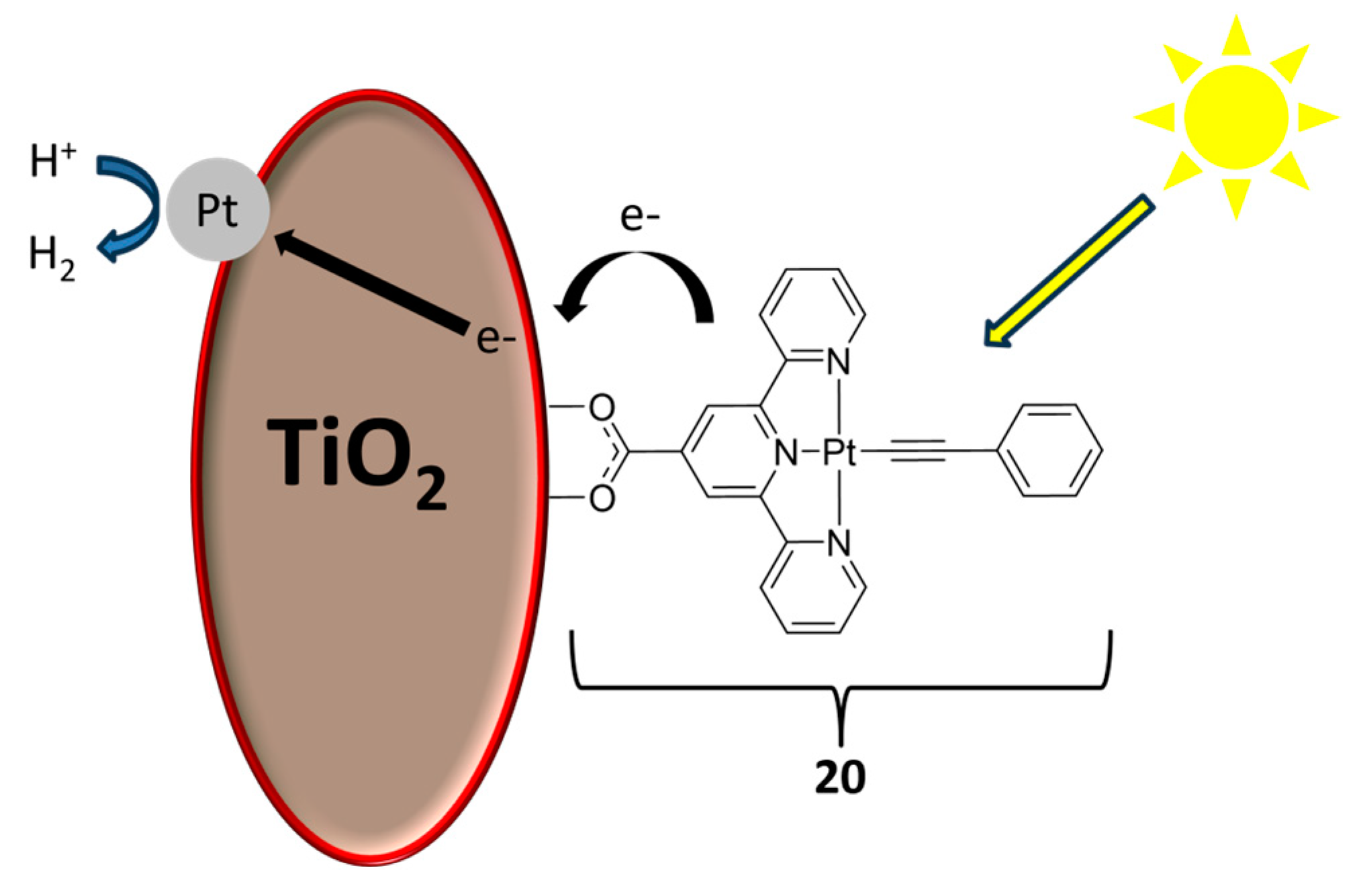
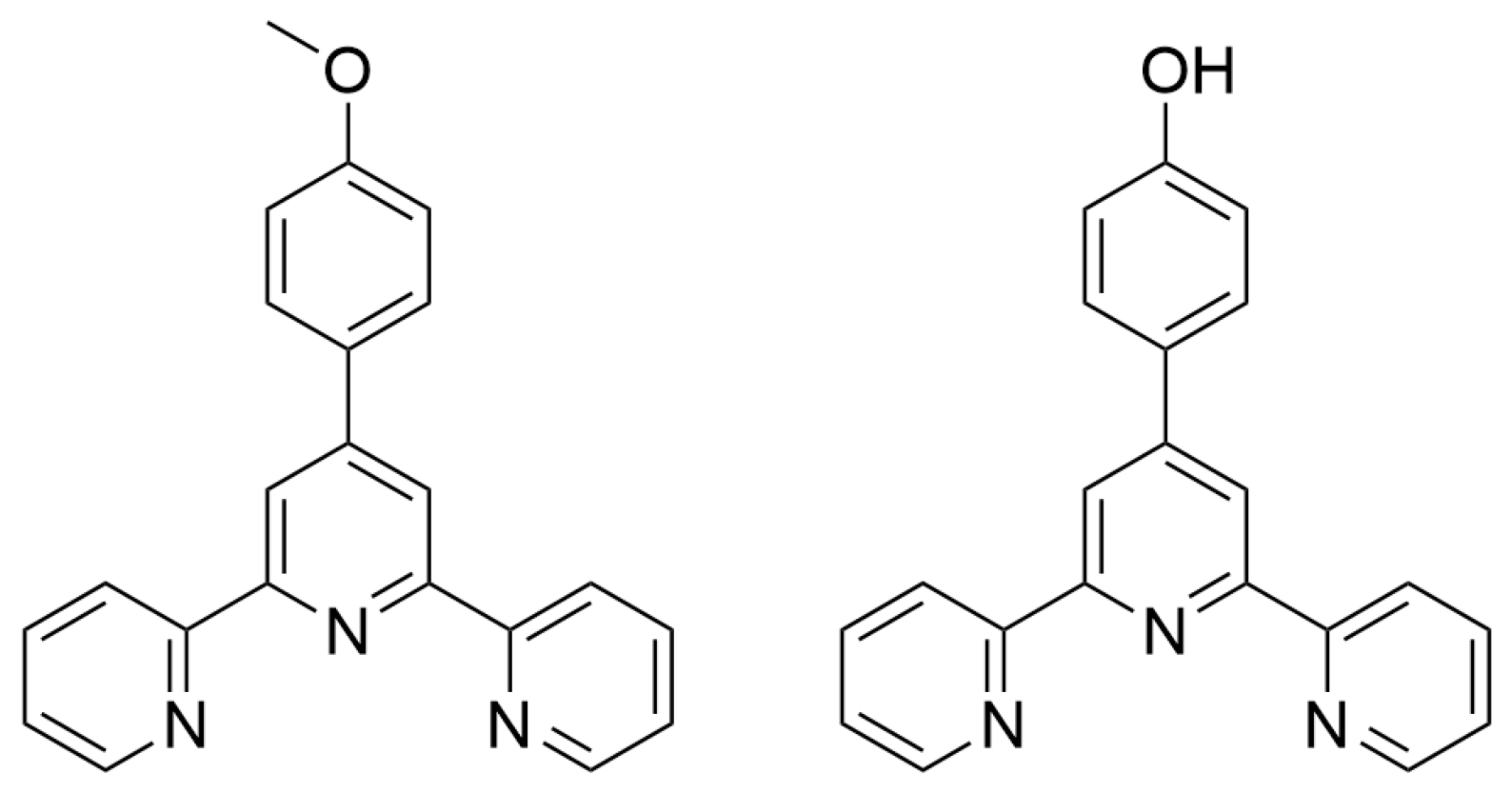
2.1. Materials for Photovoltaic Applications
- -
- 1) Prevention of wastes;
- -
- 3) Less hazardous chemical synthesis;
- -
- 6) Design for energy efficiency;
- -
- 7) Use of renewable feedstock.
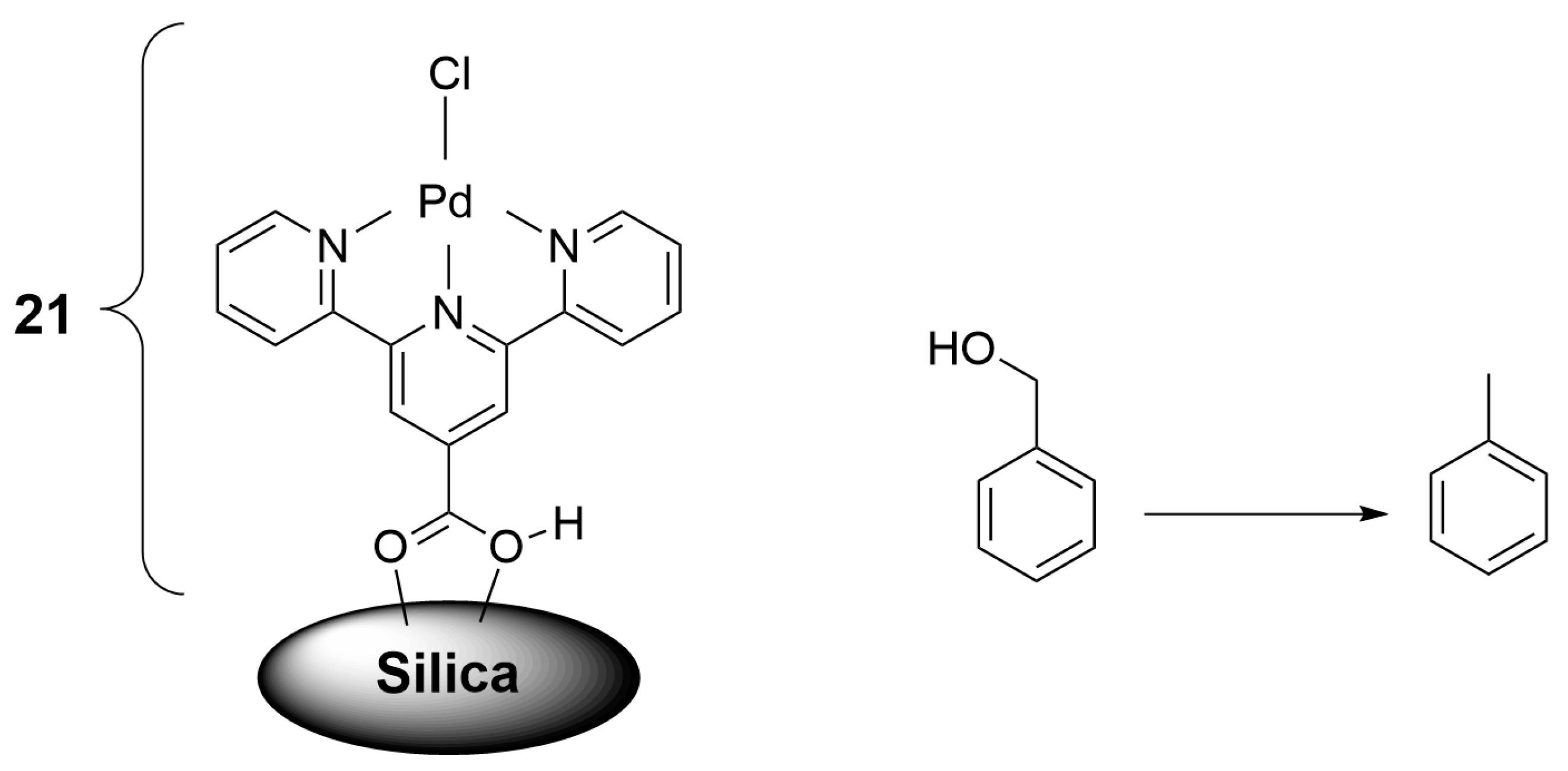
2.2. Materials for Catalysis
2.3. Metallopolymers, Metal–Organic Frameworks (MOFs) and Polyoxometalates (POMs)
3. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, S.G.; Burstall, F.H. Dehydrogenation of pyridine of anhydrous ferric chloride. J. Chem. Soc. 1932, 1, 20–30. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Sciences Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Cummings, S.D. Platinum complexes of terpyridine: Interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- Abhijnakrishna, R.; Magesh, K.; Ayushi, A.; Velmathi, S. Advances in the Biological Studies of Metal-Terpyridine Complexes: An Overview From 2012 to 2022. Coord. Chem. Rev. 2023, 496, 215380. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, P.; Baitalik, S. Design of molecular sensors and switches based on luminescent ruthenium-terpyridine complexes bearing active methylene and triphenylphosphonium motifs as anion recognition sites: Experimental and DFT/TD-DFT investigation. Dalton Trans. 2024, 53, 1307–1321. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Ebralidze, I.I.; Zenkina, O.V. Polypyridine-based architectures for smart electrochromic and energy storage materials. Can. J. Chem. 2023, 101, 400–417. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Conv. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Biorefineries as a driver for sustainability: Key aspects, actual development and future prospects. J. Clean. Prod. 2023, 418, 137925. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2″-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Kröhnke, F. Synthesis using pyridinium salts.5.Specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1, 1–24. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Martin Alonso, D.; Dumesic, J.A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 2014, 16, 4816–4838. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Remzi Becer, C. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Jaswai, A.; Pratap Singh, P.; Mondai, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Gomez Millan, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Sun, Z.; Yuan, T.-Q. Recent Advances in the Catalytic Upgrading of Biomass Platform Chemicals Via Hydrotalcite-Derived Metal Catalysts. Trans. Tianjin Univ. 2022, 28, 89–111. [Google Scholar] [CrossRef]
- Weng, R.; Lu, X.; Ji, N.; Fukuoka, A.; Shrotri, A.; Li, X.; Zhang, R.; Zhang, M.; Xiong, J.; Yu, Z. Taming the butterfly effect: Modulating catalyst nanostructures for better selectivity control of the catalytic hydrogenation of biomass-derived furan platform chemicals. Catal. Sci. Technol. 2021, 11, 7785–7806. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2020, 7, 948. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Irshad, M.; Lee, S.; Choi, E.; Kim, J.W. Efficient Synthetic Routes of Biomass-derived Platform Chemicals. Appl. Chem. Eng. 2019, 30, 280–289. [Google Scholar]
- Serrano-Ruiz, J.C.; Luque, R.; Sepulveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, C.-G.; Chee, P.L.; Qu, C.; Fok, A.Z.; Yong, F.H.; Ong, Z.L.; Kai, D. Strategies for lignin depolymeriztion and reconstruction towards functional polymers. Sustain. Energy Fuels 2023, 7, 2953–2973. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Puglia, D.; Kenny, J.M.; Liu, T.; Guo, J.; Wang, Q.; Ou, R.; Xu, P.; Ma, P.; et al. Bio-renewable polymers based on lignin-derived phenol monomers: Synthesis, applications, and perspectives. SusMat 2022, 2, 535–568. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Vega-Aguilar, C.A.; Rodrigues, A.E. Added-Value Chemicals from Lignin Oxidation. Molecules 2021, 26, 4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Lee, S.; Monnappa, A.K.; Mitchell, R.J. Biological activities of lignin hydrolysate-related compounds. BMB Rep. 2012, 45, 265–274. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, Z. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Dye-Sensitized Solar Cells; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Tontapha, S.; Uppachai, P.; Amornkitbamrung, V. Fabrication of Functional Materials for Dye-sensitized Solar Cells. Front. Energy Res. 2021, 9, 641983. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Rahman, S.; Haleem, A.; Siddiq, M.; Hussain, M.K.; Qamar, S.; Hameed, S.; Waris, M. Research on dye sensitized solar cells: Recent advancement toward the various constituents of dye sensitized solar cells for efficiency enhancement and future prospect. RSC Adv. 2023, 13, 19508–19529. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-S.; Chou, H.-H.; Chen, Y.-C.; Hsu, C.-Y.; Lin, J.T. Recent developments in molecule-based organic materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 8734–8747. [Google Scholar] [CrossRef]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Rees, T.W.; Baranoff, E. Ruthenium complexes with tridentate ligands for dye-sensitized solar cells. Polyhedron 2014, 82, 37–49. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Grätzel, M. Efficient panchromatic sensitization of nanocrystalline TiO2 films by a black dye based on a trithiocyanato-ruthenium complex. Chem. Commun. 1997, 18, 1705–1706. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef]
- Reynal, A.; Forneli, A.; Palomares, E. Dye structure-charge transfer process relationshi^in efficient ruthenium-dye based sensitized solar cells. Energy Environ. Sci. 2010, 3, 805–812. [Google Scholar] [CrossRef]
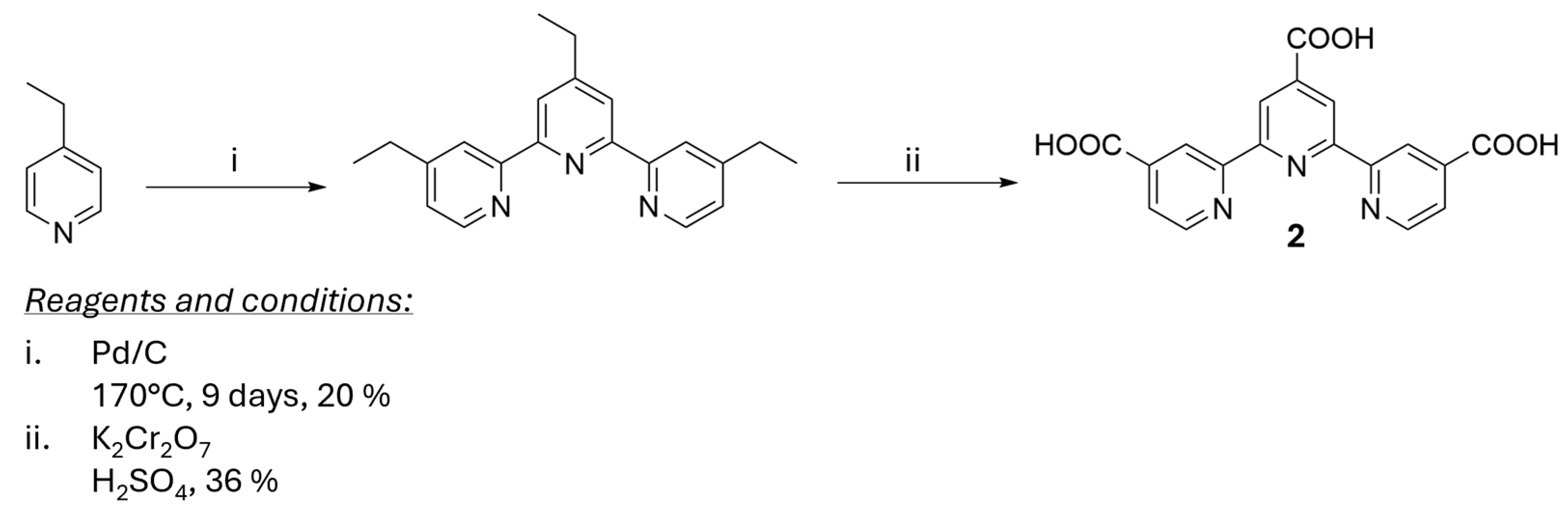
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4′,4″-tricarboxy-2,2′:6′,2″-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Husson, J.; Dehaudt, J.; Guyard, L. Preparation of carboxylate derivatives of terpyridine via the furan pathway. Nat. Protoc. 2014, 9, 21–26. [Google Scholar] [CrossRef]
- Duncton, M.A.J. Minisci reactions: Versatile CH-functionalizations for medicinal chemists. Med. Chem. Commun. 2011, 2, 1135–1161. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Singh, T.; Gadiyaram, S.; Jose, D.A.; Naziruddin, A.R. Heteroleptic ruthenium(II) complexes featuring N-heterocyclic carbene-based C^N donor sets for solar energy conversion. New J. Chem. 2023, 47, 13476–13485. [Google Scholar] [CrossRef]
- Kaneko, R.; Wu, G.; Sugawa, K.; Otsuki, J.; Islam, A.; Han, L.; Bedja, I.; Gupta, R.K. Cyclometalated ruthenium complexes with 6-(ortho-methoxyphenyl)-2,2’-bipyridine as panchromatic dyes for dye-sensitized solar cells. J. Organomet. Chem. 2017, 833, 61–70. [Google Scholar] [CrossRef]
- Wu, G.; Kaneko, R.; Islam, A.; Zhang, Y.; Sugawa, K.; Han, L.; Shen, Q.; Bedja, I.; Gupta, R.K.; Otsuki, J. Thiocyanate-free asymmetric ruthenium(II) dye sensitizers containing azole chromophores with near-IR light-harvesting capacity. J. Power Sources 2016, 331, 100–111. [Google Scholar] [CrossRef]
- Wu, G.; Kaneko, R.; Zhang, Y.; Shinozaki, Y.; Sugawa, K.; Islam, A.; Han, L.; Bedja, I.; Gupta, R.K.; Shen, Q.; et al. Neutral and anionic tetrazole-based ligands in designing novel ruthenium dyes for dye-sensitized solar cells. J. Power Sources 2016, 307, 416–425. [Google Scholar] [CrossRef]
- Breivogel, A.; Wooh, S.; Dietrich, J.; Kim, T.Y.; Kang, Y.S.; Char, K.; Heinze, K. Anchor-Functionalized Push-Pull-Substituted Bis(tridentate) Ruthenium(II) Polypyridine Chromophores: Photostability and Evaluation as Photosensitizers. Eur. J. Inorg. Chem. 2014, 16, 2720–2724. [Google Scholar] [CrossRef]
- Raboin, J.C.; Kirsch, G.; Beley, M. On the Way to Unsymmetrical Terpyridines Carrying Carboxylic Acids. J. Heterocycl. Chem. 2000, 37, 1077–1080. [Google Scholar] [CrossRef]
- Woodward, C.P.; Coghlan, C.J.; Rüther, T.; Jones, T.W.; Hebting, Y.; Cordiner, R.L.; Dawson, R.E.; Robinson, D.E.J.E.; Wilson, G.J. Oligopyridine ligands possessing multiple or mixed anchoring functionality for dye-sensitized solar cells. Tetrahedron 2015, 71, 5238–5247. [Google Scholar] [CrossRef]
- Shinpuku, Y.; Inui, F.; Nakai, M.; Nakabayashi, Y. Synthesis and characterization of novel cyclometalated iridium(III) complexes for nanocrystalline TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2011, 222, 203–209. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Stergiopoulos, T.; Kantonis, G.; Kontos, A.G.; Papadopoulos, K.; Stublla, A.; Potvin, P.G.; Falaras, P. Terpyridine- and 2,6-dipyrazinylpyridine-coordinated ruthenium(II) complexes: Synthesis, characterization and application in TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2010, 214, 22–32. [Google Scholar] [CrossRef]
- Ozawa, H.; Fukushima, K.; Sugiura, T.; Urayama, A.; Arakawa, H. Ruthenium sensitizers having an ortho-dicarboxyl group as an anchoring unit for dye-sensitized solar cells: Synthesis, photo- and electrochemical properties, and adsorption behavior to the TiO2 surface. Dalton Trans. 2014, 43, 13208–13218. [Google Scholar] [CrossRef] [PubMed]
- Rosero, W.A.A.; Guimaraes, R.R.; Matias, T.A.; Araki, K. Effect of Push-Pull Ruthenium Complex Adsorption Conformation on the Performance of Dye-Sensitized Solar Cells. J. Braz. Chem. Soc. 2020, 31, 2250–2264. [Google Scholar] [CrossRef]
- Beley, M.; Bignozzi, C.A.; Kirsch, G.; Alebbi, M.; Raboin, J.-C. New ruthenium bisterpyridinyl complexes, as efficient sensitizers of nanocrystalline, TiO2 films. Inorg. Chim. Acta 2001, 318, 197–200. [Google Scholar] [CrossRef]
- da Silva, M.R.E.; Auvray, T.; Hanan, G.S. Synthesis of a novel bipyrimidine dicarboxylic acid ligand for the preparation of panchromatic ruthenium dyes. Inorg. Chim. Acta 2020, 499, 119194. [Google Scholar] [CrossRef]
- Kisserwan, H.; Kamar, A.; Shoker, T.; Ghaddar, T.H. Photophysical properties of new cyclometalated ruthenium complexes and their use in dye sensitized solar cells. Dalton Trans. 2012, 41, 10643–10651. [Google Scholar] [CrossRef]
- Constable, E.C.; Redondo, A.H.; Housecroft, C.E.; Neuburger, M.; Schaffner, S. Copper(I) complexes of 6,6′-disubstituted 2,2′-bipyridine dicarboxylic acids: New complexes for incorporation into copper-based dye sensitized solar cells (DSCs). Dalton Trans. 2009, 33, 6634–6644. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Beley, M.; Kirsch, G. A novel pathway for the synthesis of a carboxylic acid-functionalised Ru(II) terpyridine complex. Tetrahedron Lett. 2003, 44, 1767–1770. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Manjunath, V.; Sakla, R.; Devan, R.S.; Jose, D.A.; Naziruddin, A.R. Ruthenium (II) Complexes Bearing Heteroleptic Terpyridine Ligands: Synthesis, Photophysics and Solar Energy Conversion. Eur. J. Inorg. Chem. 2021, 2021, 5014–5023. [Google Scholar] [CrossRef]
- Duprez, V.; Biancardo, M.; Krebs, F.C. Characterisation and application of new carboxylic acid-functionalised ruthenium complexes as dye-sensitisers for solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 230–237. [Google Scholar] [CrossRef]
- Koivisto, B.D.; Robson, K.C.D.; Berlinguette, C.P. Systematic Manipulation of the Light-Harvesting Properties for Tridentate Cyclometalated Ruthenium(II) Complexes. Inorg. Chem. 2009, 48, 9644–9652. [Google Scholar] [CrossRef]
- Mongal, B.N.; Bhattacharya, S.; Mandal, T.K.; Datta, J.; Naskar, S. Synthesis, characterization and photovoltaic studies of 2,2′;6′,2″-terpyridine-based ruthenium complexes with phenylamino, anthranyl and furfuryl substitutions at the 4′-position. J. Coord. Chem. 2021, 74, 1382–1398. [Google Scholar] [CrossRef]
- Das, D.; Sutradhar, S.; Singh, A.; Ghosh, B.N. Zinc-Terpyridine Based Chemosensor for Detection of Pyrophosphate Anion in Aqueous Medium. Z. Anorg. Allg. Chem. 2021, 647, 1234–1238. [Google Scholar] [CrossRef]
- Manchand, P.S.; Belica, P.S.; Wong, H. Synthesis of 3,4,5-Trimethoxybenzaldehyde. Synth. Commun. 1990, 20, 2659–2666. [Google Scholar] [CrossRef]
- Rao, D.V.; Stuber, F.A. An Efficient Synthesis of 3,4,5-Trimethoxybenzaldehyde from Vanillin. Synthesis 1983, 4, 308. [Google Scholar] [CrossRef]
- Erofeev, Y.V.; Afanas’eva, V.L.; Glushkov, R.G. Synthetic routes to 3,4,5-trimethoxybenzaldehyde. Pharm. Chem. J. 1990, 24, 501–510. [Google Scholar] [CrossRef]
- Mongal, B.N.; Bhattacharya, S.; Sengupta, S.; Mandal, T.K.; Datta, J.; Naskar, S.A. A novel ruthenium sensitizer with -Ome substituted phenyl-terpyridine ligand for dye sensitized solar cells. Sol. Energy 2016, 134, 107–118. [Google Scholar] [CrossRef]
- Buckles, R.E.; Mock, G.V.; Locatell, L., Jr. Tiglic and Angelic Acids. Chem. Rev. 1955, 55, 659–677. [Google Scholar] [CrossRef]
- Adeloye, A.O.; Olomola, T.O.; Adebayo, A.I.; Ajibade, P.A. A High Molar Extinction Coefficient Bisterpyridyl Homoleptic Ru(II) Complex with trans-2-Methyl-2-butenoic Acid Functionality: Potential Dye for Dye-Sensitized Solar Cells. Int. J. Mol. Sci. 2012, 13, 3511–3526. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Metal-Terpyridine Complexes in Catalytic Application- A Spotlight on the Last Decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar] [CrossRef]
- Jarosz, P.; Du, P.; Schneider, J.; Lee, S.-H.; McCamant, D.; Eisenberg, R. Platinum(II) Terpyridyl Acetylide Complexes on Platinized TiO2: Toward the Photogeneration of H2 in Aqueous Media. Inorg. Chem. 2009, 48, 9653–9663. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, N.A.; Jystad, A.; Laan, K.V.; Tengco, J.M.M.; Caricato, M.; Vannucci, A.K. Silica Supported Molecular Palladium Catalyst for Selective Hydrodeoxygenation of Aromatic Compounds under Mild Conditions. ACS Catal. 2019, 9, 9060–9071. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Sun, W.L.; Shahrajabian, M.H.; Cheng, Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol. 2019, 5, 1673688. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Avila, J.A.; Alejandro, G.J.D.; Franzblau, S.G.; Kouam, S.F.; Hussain, H.; Krohn, K. Villarinol, a new Alkenoyloxyalkenol Derivative from the Endemic Philippine Rubiaceae species Villaria odorata. Nat. Prod. Commun. 2012, 7, 779–780. [Google Scholar] [CrossRef]
- Deng, W.P.; Zhang, H.X.; Wu, X.J.; Li, R.S.; Zhang, Q.H.; Wang, Y. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem. 2015, 17, 5009–5018. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Zhang, Q.-X.; Huang, L.-J.; Xu, Y.-J.; Tang, D.-L. Covalent immobilization of ruthenium polypyridyl complex on multi-walled carbon nanotube supports for oxygen evolution reaction in an alkaline solution. J. Power Sources 2021, 488, 229448. [Google Scholar] [CrossRef]
- Chin, T.; Gao, Z.N.; Lelouche, I.; Shin, Y.G.K.; Purandare, A.; Knapp, S.; Isied, S.S. Molecular Recognition and Reactivity of Ruthenium(II) Bipyridine Barbituric Acid Guests in the Presence of Complementary Hosts: Ruthenium(II) Promoted Enolization of Barbituric Acids in Guest−Host Complexes. J. Am. Chem. Soc. 1997, 119, 12849–12858. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents; A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Meibodi, F.S. Suzuki and Heck cross-coupling reactions using ferromagnetic nanoparticle-supported palladium complex as an efficient and recyclable heterogeneous nanocatalyst in sodium dodecylsulfate micelles. Appl. Organometal. Chem. 2017, 31, c3627. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Bahrami, K.; Meibodi, F.S. Ferromagnetic nanoparticle-supported copper complex: A highly efficient and reusable catalyst for three-component syntheses of 1,4-disubstituted 1,2,3-triazoles and C-S coupling of aryl halides. Appl. Organometal. Chem. 2017, 31, c3714. [Google Scholar] [CrossRef]
- Sarabi, M.F.; Bezaatpour, A.; Mahmoudi, A. Anchoring of a terpyridine-based Mo(VI) complex on manganese ferrite as a recoverable catalyst for epoxidation of olefins under solvent-free conditions. J. Coord. Chem. 2021, 74, 1597–1612. [Google Scholar] [CrossRef]
- Bahrami, K.; Targhan, H. A new strategy to design a graphene oxide supported palladium complex as a new heterogeneous nanocatalyst and application in carbon-carbon and carbon-heteroatom cross-coupling reactions. Appl. Organometal. Chem. 2019, 33, e4842. [Google Scholar] [CrossRef]
- Wan, L.; Sun, X.; Shi, S.; Zhang, J.; Li, X.; Li, Z.; Guo, K. An efficient synthesis of N-substituted phtalimides using SiO2-tpy-Nb as heterogeneous and reusable catalyst. Catal. Commun. 2017, 88, 30–34. [Google Scholar] [CrossRef]
- Targhan, H.; Hassanpour, A.; Bahrami, K. Highly efficient polymer-stabilized palladium heterogeneous catalyst: Synthesis, characterization and application for Suzuki-Miyaura and Mizoroki-Heck coupling reactions. Appl. Organometal. Chem. 2019, 33, e5121. [Google Scholar] [CrossRef]
- Klement, T.; Büchs, J. Itaconic acid—A biotechnological process in change. Bioresour. Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef]
- Targhan, H.; Hassanpour, A.; Sohrabnezhad, S.; Bahrami, K. Palladium Nanoparticles Immobilized with Polymer Containing Nitrogen-Based Ligand: A Highly Efficient Catalyst for Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions. Catal. Lett. 2020, 150, 660–673. [Google Scholar] [CrossRef]
- Yoo, D.-W.; Yoo, S.-K.; Kim, C.; Lee, J.-K. A novel mononuclear Fe(III) mono(terpyridine) complex having labile solvent ligands and its catalytic activity. J. Chem. Soc. Dalton Trans. 2002, 21, 3931–3932. [Google Scholar] [CrossRef]
- Suzuka, T.; Kimura, K.; Nagamine, T. Reusable Polymer-Supported Terpyridine Palladium Complex for Suzuki-Miyaura, Mizoroki-Heck, Sonogashira, and Tsuji-Trost Reaction in Water. Polymers 2011, 3, 621–639. [Google Scholar] [CrossRef]
- Kaskel, S. The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Schubert, U.S.; Eschbaumer, C. Macromolecules containing bipyridine and terpyridine metal complexes: Towards metallosupramolecular polymers. Angew. Chem. Int. Ed. 2002, 41, 2892–2926. [Google Scholar] [CrossRef]
- Etienne, S.; Beley, M. Preparation and study of ruthenium(II) 2,2′:6′,2″-terpyridine complexes linked by an O-CH2 spacer to thiophene moieties. Inorg. Chem. Commun. 2006, 9, 68–71. [Google Scholar] [CrossRef]
- Manca, P.; Pilo, M.I.; Sanna, G.; Bergamini, G.; Ceroni, P.; Boaretto, R.; Caramori, S. Heteroleptic Ru(II)-terpyridine complex and its metal-containing conducting polymer: Synthesis and characterization. Synth. Met. 2015, 200, 109–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, H.; Kang, Y.; Li, X.; Jiang, H.; Xue, B.; Wang, Y.; Lü, X.; Zhu, X. Water soluble Ln(III)-based metallopolymer with AIE-active and ACQ-effect lanthanide behaviors for detection of nanomolar pyrophosphate. Sens. Actuator B-Chem. 2019, 282, 999–1007. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, W.; Zhou, Z.; Zheng, H.; Zhao, Y.; Yan, H.; Lü, X. Tetraphenylethylene-based Eu3+-metallopolymers with aggregation-enhanced white emission for self-calibrating temperature sensing and white light-emitting diodes (WLEDs). J. Mater. Chem. C 2022, 10, 7586–7593. [Google Scholar] [CrossRef]
- Friese, V.A.; Kurth, D.G. Soluble dynamic coordination polymers as a paradigm for materials science. Coord. Chem. Rev. 2008, 252, 199–211. [Google Scholar] [CrossRef]
- Robb, M.G.; Brooker, S. Incorporation of Switchable Inorganic Building Blocks into Heterometallic Coordination Polymers. Cryst. Growth Des. 2023, 23, 1848–1859. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Lin, H.; Wang, R.; Zhang, L.; Sun, D. Synthesis, structure, and properties of a 3D porous Zn(II) MOF constructed from a terpyridine-based ligand. RSC Adv. 2016, 6, 16575–16580. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Wu, X.-Y.; Zhang, L.; Lu, C.-Z. Synthesis, Photoluminescence, and Gas Adsorption Properties of a New Furan-Functionalized MOF and Direct Carbonization for Synthesis of Porous Carbon. Cryst. Growth Des. 2016, 16, 475–482. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Coronado, E.; Housecroft, C.E.; Neuburger, M. Not just size and shape: Spherically symmetrical d5 and d10 metal ions give different coordination nets with 4,2′:6′,4″-terpyridines. CrystEngComm 2010, 12, 2139–2145. [Google Scholar] [CrossRef]
- Nijs, T.; Malzner, F.J.; Fatayer, S.; Wäckerlin, A.; Nowakowska, S.; Constable, E.C.; Housecroft, C.E.; Jung, T.A. Programmed assembly of 4,2′:6′,4″-terpyridine derivatives into porous, on-surface networks. Chem. Commun. 2015, 51, 12297–12300. [Google Scholar] [CrossRef] [PubMed]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures. Materials 2017, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Goswami, S.; Biswas, S.; Tomar, K.; Jena, H.S.; Konar, S. Stable Multiresponsive Luminescent MOF for Colorimetric Detection of Small Molecules in Selective and Reversible Manner. Chem. Mater. 2015, 27, 5349–5360. [Google Scholar] [CrossRef]
- Kharwar, A.K.; Konar, S. Exchange coupled Co(II) based layered and porous metal-organic frameworks: Structural diversity, gas adsorption, and magnetic properties. Dalton Trans. 2020, 49, 4012–4021. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z. Polyoxometalate-based functional nanostructured films: Current progress and future prospects. Nano Today 2010, 5, 267–281. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Walsh, J.J.; Bond, A.M.; Forster, R.J.; Keyes, T.E. Hybrid polyoxometalate materials for photo(electro-) chemical applications. Coord. Chem. Rev. 2016, 306, 217–234. [Google Scholar] [CrossRef]
- Verissimo, M.I.S.; Evtuguin, D.V.; Gomes, M.T.S.R. Polyoxometalate Functionalized Sensors: A Review. Front. Chem. 2022, 10, 840657. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.-P.; Pal, A.K.; Hanan, G.S.; Proust, A.; Hasenknoph, B. Discrete Covalent Organic-Inorganic Hybrids: Terpyridine Functionalized Polyoxometalates Obtained by a Modular Strategy and Their Metal Complexation. Inorg. Chem. 2011, 50, 6737–6745. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, W.; Meng, Z.; Wong, W.-Y. A Tetrakis(terpyridine) Ligand Based Cobalt(II) Complex Nanosheet as a Stable Dual Ion Battery Cathode Material. Small 2020, 16, 1905204. [Google Scholar] [CrossRef]
- Zhu, H.; Liao, S.; Bian, R.; Su, B.; Ding, X.; Li, M.; Ge, S.; Zhang, H.; Liu, Q. An iron supramolecular compound containing terpyridine polycarboxylic acid for high performance lithium-ion batteries. Inorg. Chim. Acta 2022, 535, 120848. [Google Scholar] [CrossRef]
- Gu, C.; Li, J.; Liu, J.-P.; Wang, H.; Peng, Y.; Liu, C.-S. Conferring supramolecular guanosine gel nanofiber with ZIF-67 for high-performance oxygen reduction catalysis in rechargeable zinc-air batteries. Appl. Catal. B-Environ. 2021, 286, 119888. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, H.; Wang, B.; Qiu, M.; Liu, Z.; Wu, X.; Zhong, S.; Tong, G.; Chen, Z.; Zhang, J.; et al. Coordination polymer-reinforced composite polymer electrolyte for all-solid-state Li-metal batteries. Chem. Eng. J. 2024, 487, 150489. [Google Scholar] [CrossRef]
- Mondal, S.; Yoshida, T.; Maji, S.; Ariga, K.; Higuchi, M. Transparent Supercapacitor Display with Redox-Active Metallo-Supramolecular Polymer Films. ACS Appl. Mater. Interfaces 2020, 12, 16342–16349. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Arabatzis, G. Electrical energy storage systems in electricity generation: Energy policies, innovative technologies, and regulatory regimes. Renew. Sust. Energ. Rev. 2016, 56, 1044–1067. [Google Scholar] [CrossRef]
- Zhang, H.D.; Zhu, H.T.; Bian, R.R.; Liu, Q.; Liao, S.Y. Heterometallic-organic frameworks containing Fe(II) nodes as high-performance anode materials for Li-ion batteries. J. Solid State Electrochem. 2024. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chiang, P.-C.; Pan, W.; Kim, H. Advances in state-of-art valorization technologies for captured CO2 toward sustainable carbon cycle. Crit. Rev. Environ. Sci. Technol. 2018, 48, 471–534. [Google Scholar] [CrossRef]
- Arana, C.; Keshavarz, M.; Potts, K.T.; Abruna, H.D. Electrocatalytic reduction of CO2 and O2 with electropolymerized films of vinyl-terpyridine complexes of Fe, Ni and Co. Inorg. Chim. Acta 1994, 225, 285–295. [Google Scholar] [CrossRef]
- Gautam, P.; Srivastava, V. Synthesis and Catalytic Application of Ru-Deposited Magnetic Nanoparticles for the Selective Hydrogenation of CO2 Gas. Lett. Org. Chem. 2022, 19, 705–710. [Google Scholar] [CrossRef]
- Lam, E.; Reisner, E. A TiO2-Co(terpyridine)2 Photocatalyst for the Selective Oxidation of Cellulose to Formate Coupled to the Reduction of CO2 to Syngas. Angew. Chem. Int. Ed. 2021, 60, 23306–23312. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Rehman, Z.U.; ul Aein, Q.; Saeed, A. Terpyridine-Pr-Fe3O4@boehmite nanoparticles; a novel and highly effective magnetic nanocatalyst for preparation of cyclic carbonates from carbon dioxide and epoxides under solventless conditions. Mater. Chem. Phys. 2019, 231, 272–280. [Google Scholar] [CrossRef]
- Xang, X.; Li, J.; Kou, M.; Dou, W.; Bai, D.; Tang, X.; Tang, Y.; Liu, W. Dual-Function Precious-Metal-Free Metal-Organic Framework for Photocatalytic Conversion and Chemical Fixation of Carbon Dioxide. Inorg. Chem. 2023, 62, 19015–19024. [Google Scholar]
- Zhou, P.; Zhou, X.; Song, Z.; Shao, D.; Wang, D. Metal-organic frameworks with Lewis acid-base sites as highly efficient catalyst for the cycloaddition of carbon dioxide. J. Solid State Chem. 2024, 331, 124500. [Google Scholar] [CrossRef]
- Kim, D.; Bhattacharjee, S.; Lam, E.; Casadevall, C.; Rodriguez-Jimenez, S.; Reisner, E. Photocatalytic CO2 Reduction Using Homogeneous Carbon Dots with a Molecular Cobalt Catalyst. Small 2024, 2400057. [Google Scholar] [CrossRef]
- Verma, P.; Singh, A.; Rahimi, F.A.; Sarkar, P.; Nath, S.; Pati, S.K.; Maji, T.K. Charge-transfer regulated visible light driven photocatalytic H2 production and CO2 reduction in tetrathiafulvalene based coordination polymer gel. Nat. Commun. 2021, 12, 7313. [Google Scholar] [CrossRef]
- Zhao, J.; Ziaratti, A.; Rosspeintner, A.; Bürgi, T. Anchoring of Metal Complexes on Au25 Nanocluster for Enhanced Photocoupled Electrocatalytic CO2 Reduction. Angew. Int. Ed. 2024, 63, e202316649. [Google Scholar] [CrossRef]
- Wang, R.; Both, S.K.; Geven, M.; Calucci, L.; Forte, C.; Dijkstra, P.J.; Karpieren, M. Kinetically stable metal ligand charge transfer complexes as crosslinks in nanogels/hydrogels: Physical properties and cytotoxicity. Acta Biomater. 2015, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Pica, A.; Petruk, G.; Pane, F.; Amoresano, A.; Cilibrizzi, A.; Vilar, R.; Monti, D.M.; Merlino, A. Preparation, structure, Cytotoxicity and Mechanism of Action of ferritin-Pt(II) Terpyridine Compound Nanocomposites. Nanomedicine 2018, 13, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Babiuch, K.; König, M.; Winter, A.; Hager, M.D.; Gottschaldt, M.; Prokon, A.; Schubert, U.S. Synthesis of a glycopolymeric PtII carrier and its induction of apoptosis in resistant cancer cells. Chem. Commun. 2012, 48, 6357–6359. [Google Scholar] [CrossRef]
- Viale, M.; Bertole, N.; Panebianco, R.; Vecchio, G. Terpyridine Functionalized Cyclodextrin Nanoparticles as Carriers for Doxorubicin: Analysis of In Vitro Antiproliferative Activity. Anticancer Res. 2023, 43, 5409–5414. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.A.; Hassan, M.L. Rice straw nanofibrillated cellulose films with antimicrobial properties via supramolecular route. Ind. Crop Prod. 2016, 93, 142–151. [Google Scholar] [CrossRef]
- Gao, L.; Hou, Y.; Wang, H.; Li, M.; Ma, L.; Chu, Z.; Donskyi, I.S.; Haag, R. A Metal-Ion-Incorporated Mussel-Inspired Poly(Vinyl Alcohol)-Based Polymer Coating Offers Improved Antibacterial Activity and Cellular Mechanoresponse Manipulation. Angew. Chem. Int. Ed. 2022, 61, e202201563. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, M.; Izzo, M.; Osella, S.; Kozdra, S.; Michalowski, P.P.; Golowicz, D.; Kazimierczuk, K.; Gorkowski, M.T.; Lewera, A.; Teodorczyk, M.; et al. Development of a universal conductive platform for anchoring photo- and electroactive proteins using organometallic terpyridine molecular wires. Nanoscale 2021, 13, 9773–9787. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, G.; Wang, X.; Zhen, S.; Huang, C.; Zhan, L.; Li, Y. Facile synthesis of dual-ligand europium-metal organic gels for ratiometric electrochemiluminescence detecting I27L gene. Biosens. Bioelectron. 2024, 246, 115863. [Google Scholar] [CrossRef] [PubMed]



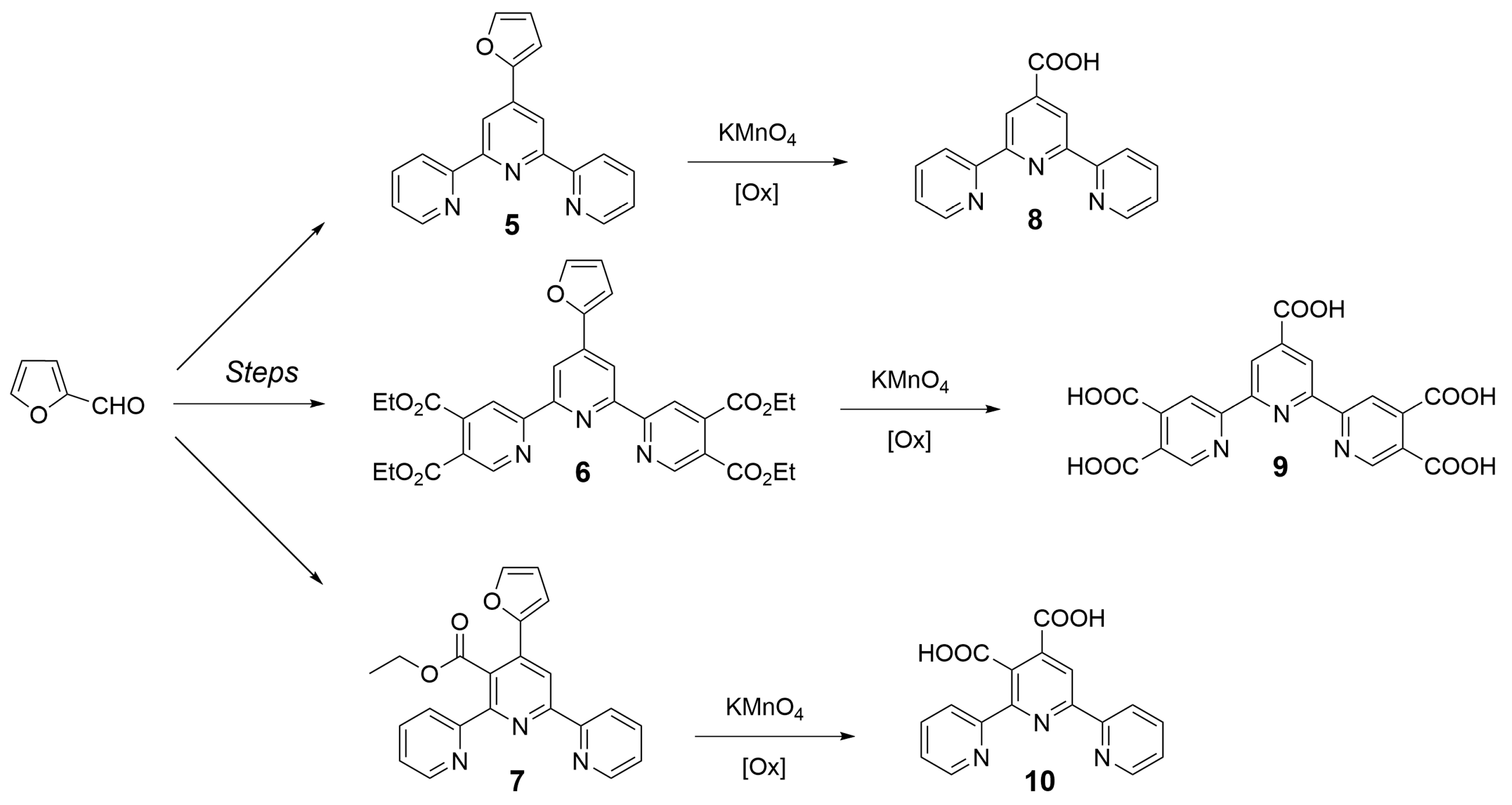
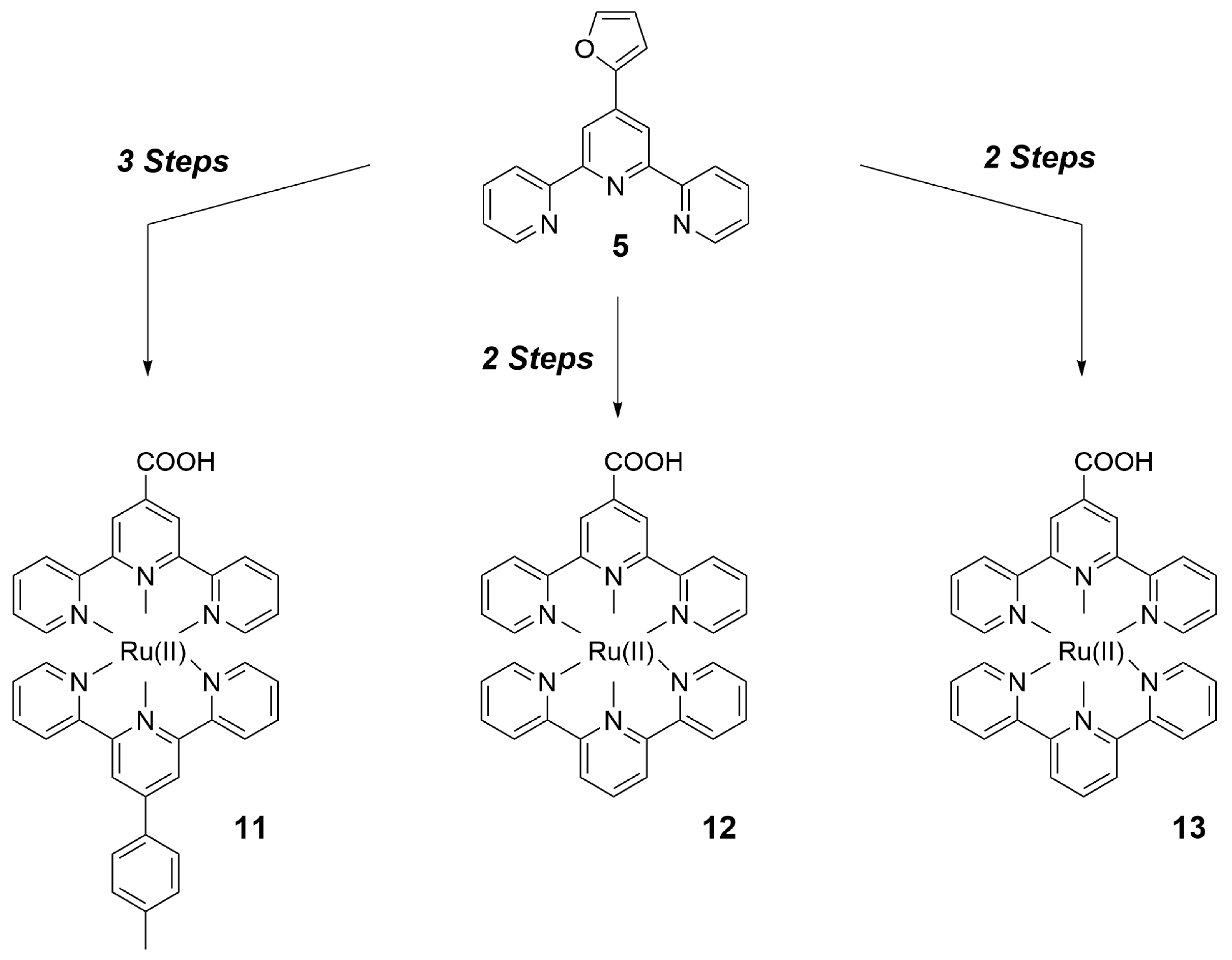

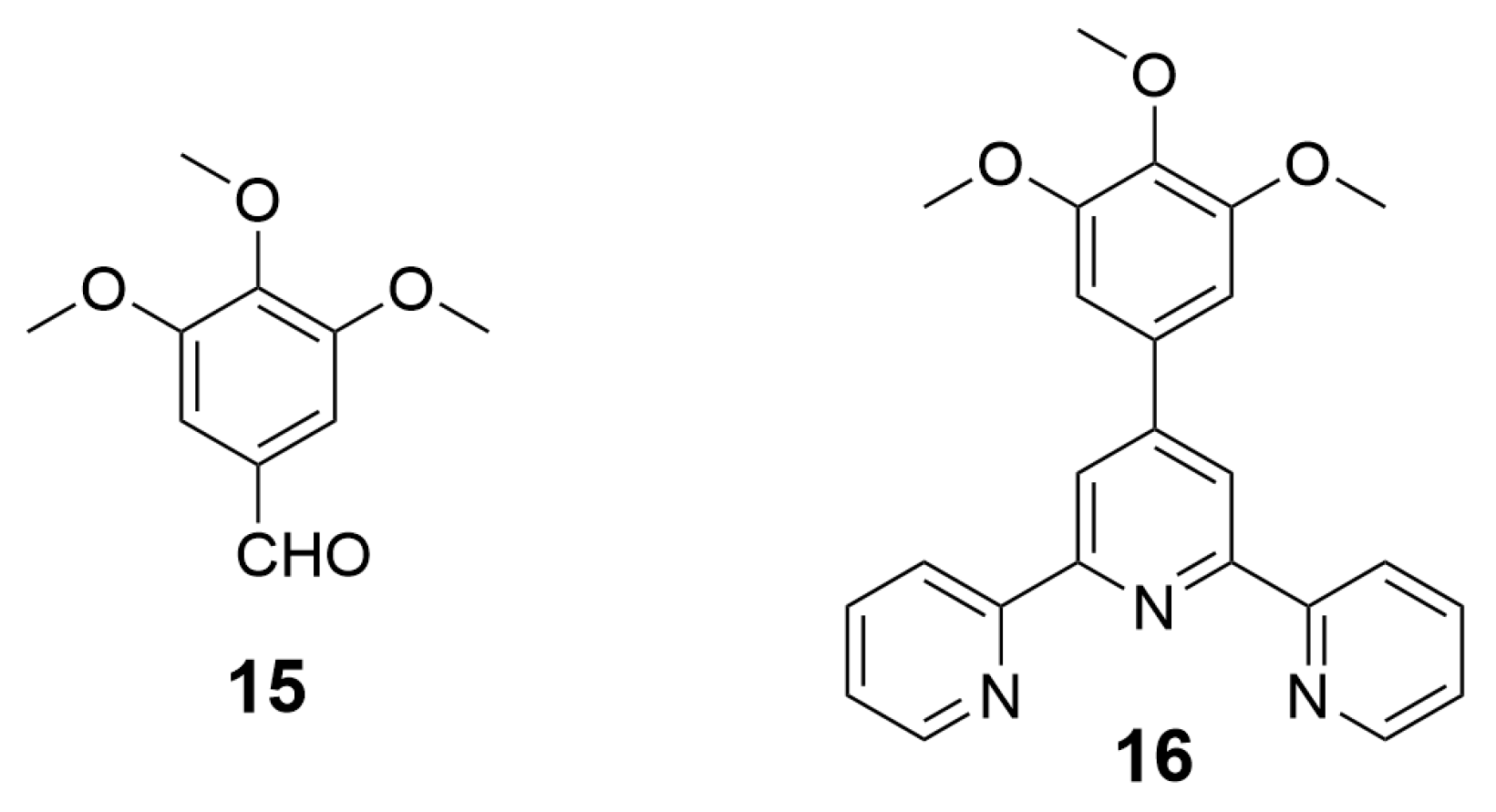
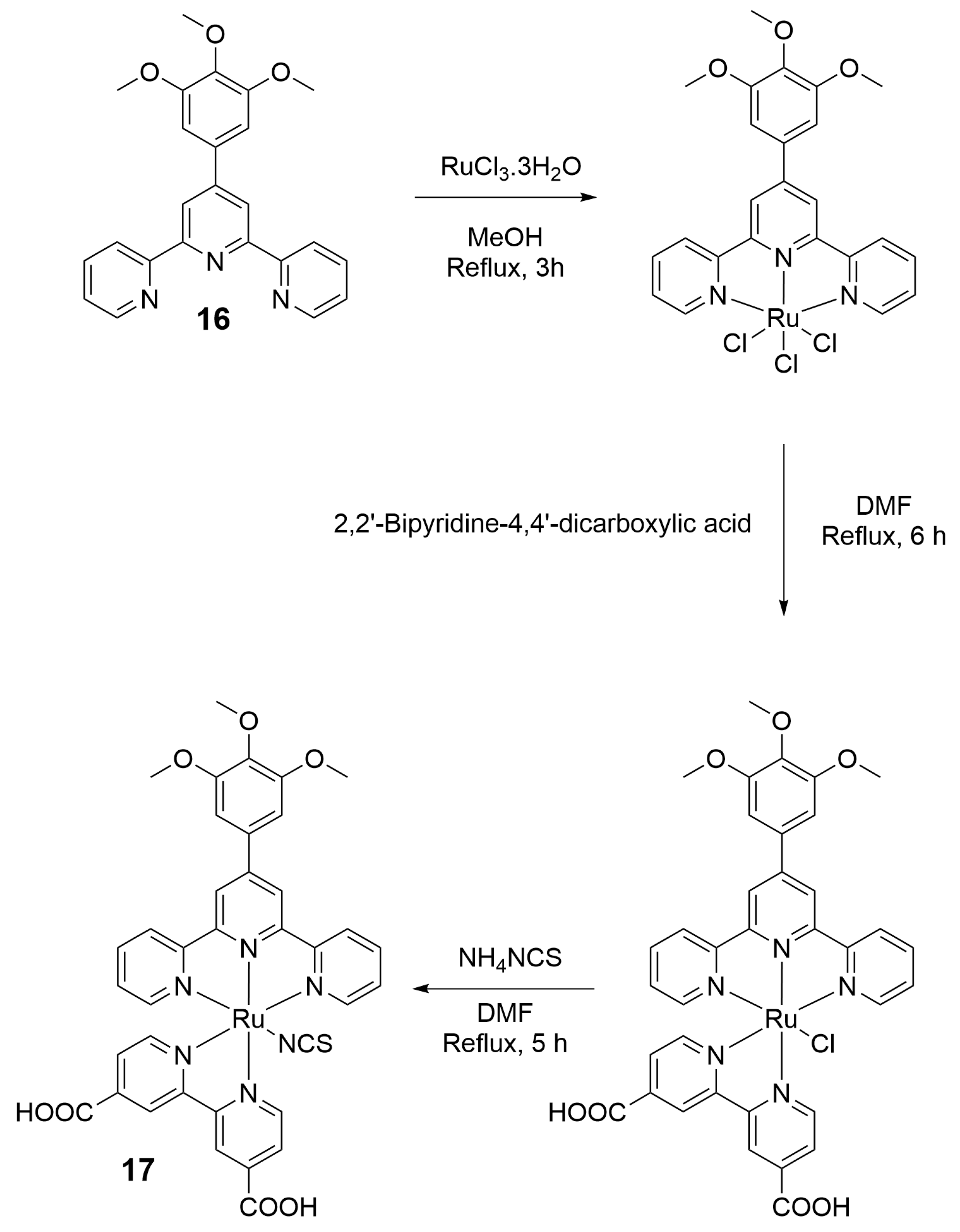
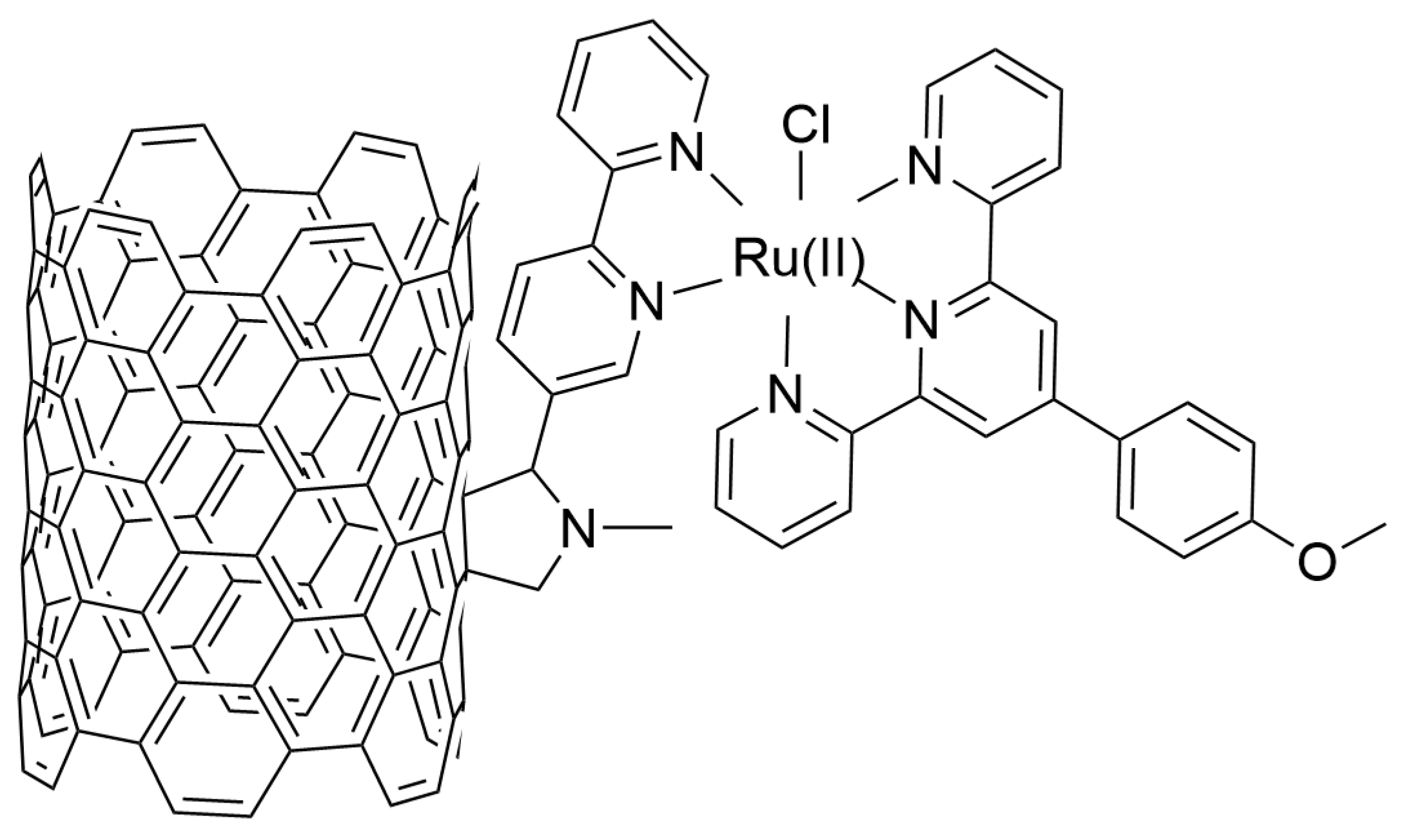
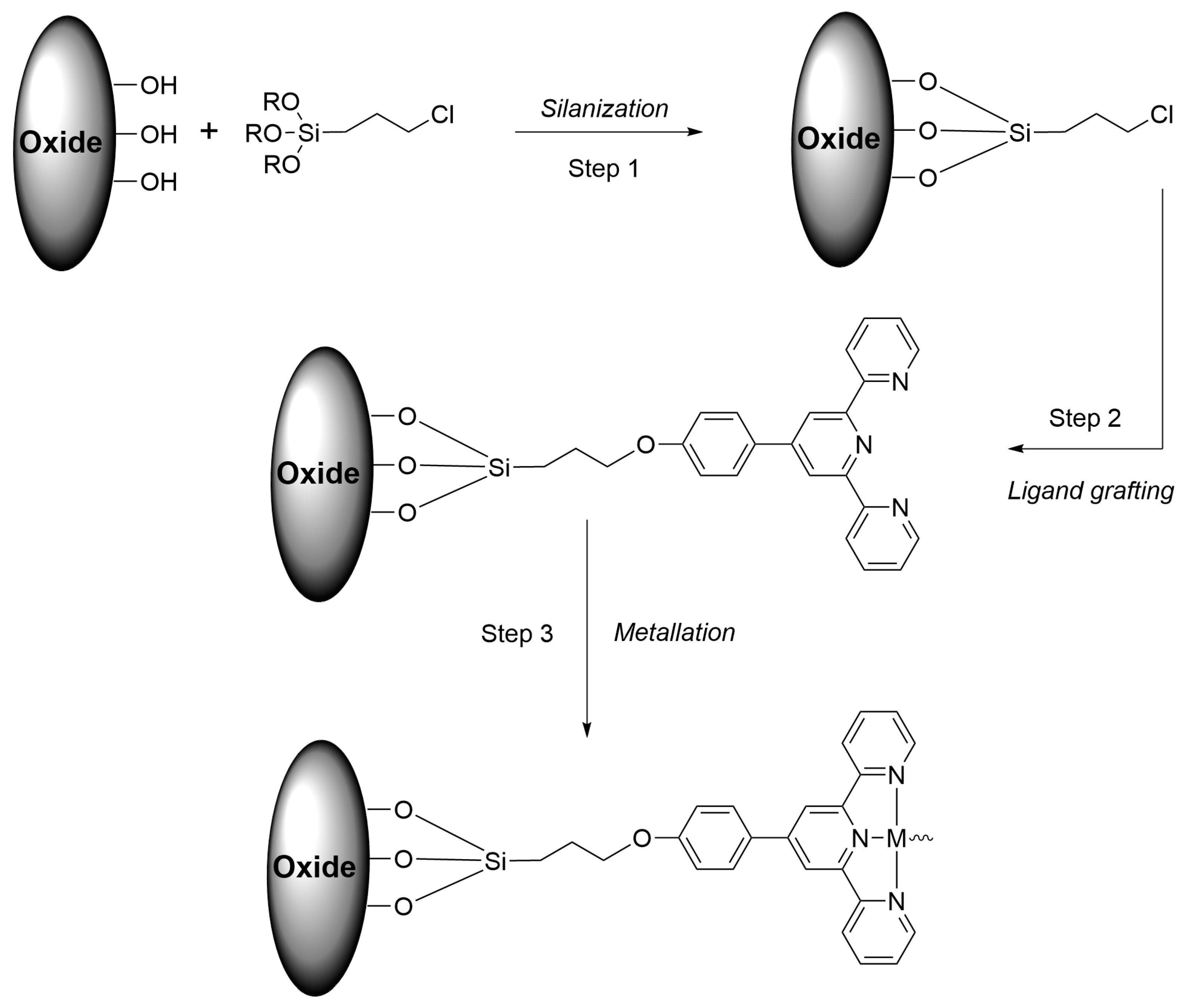
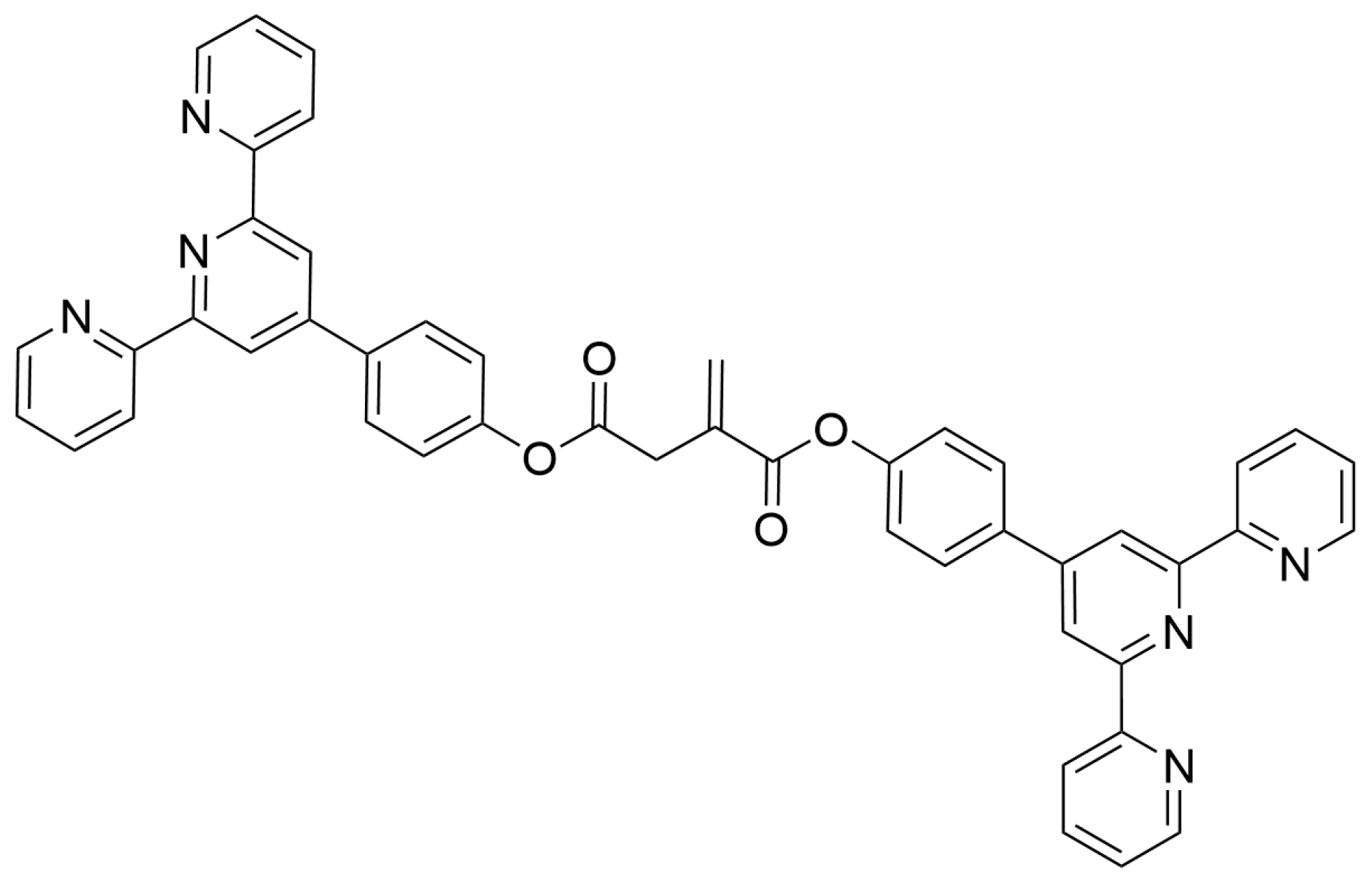
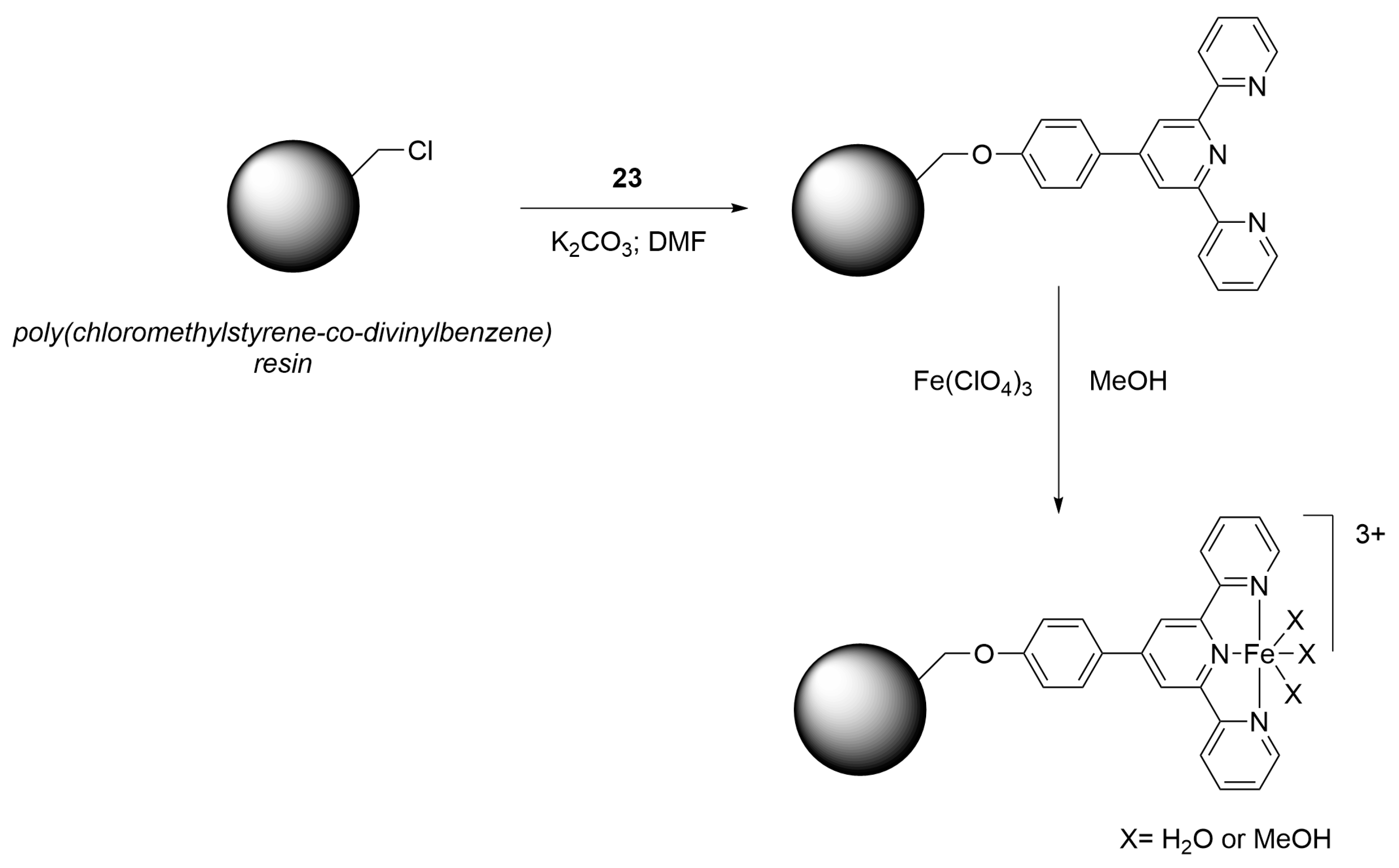
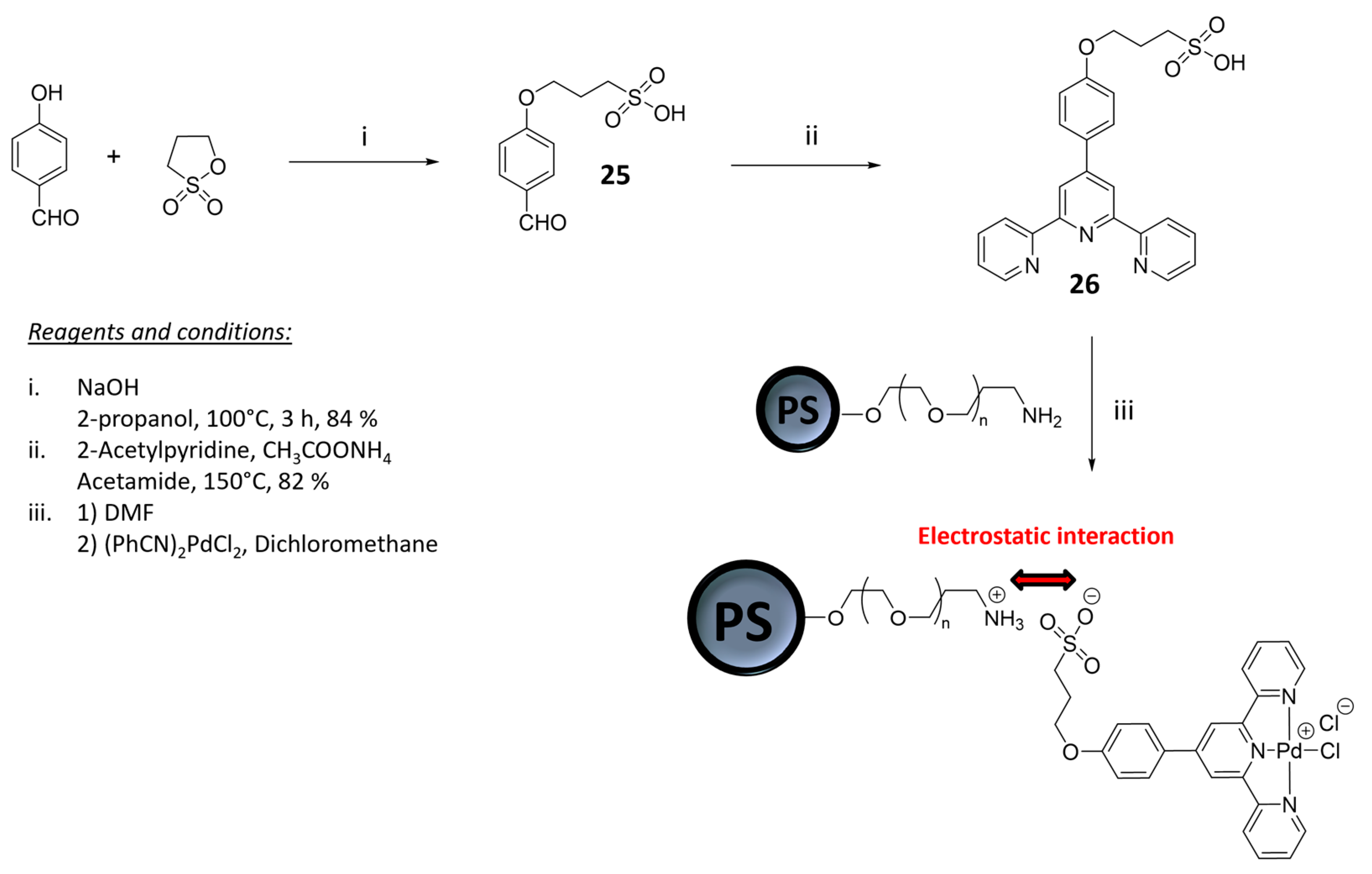

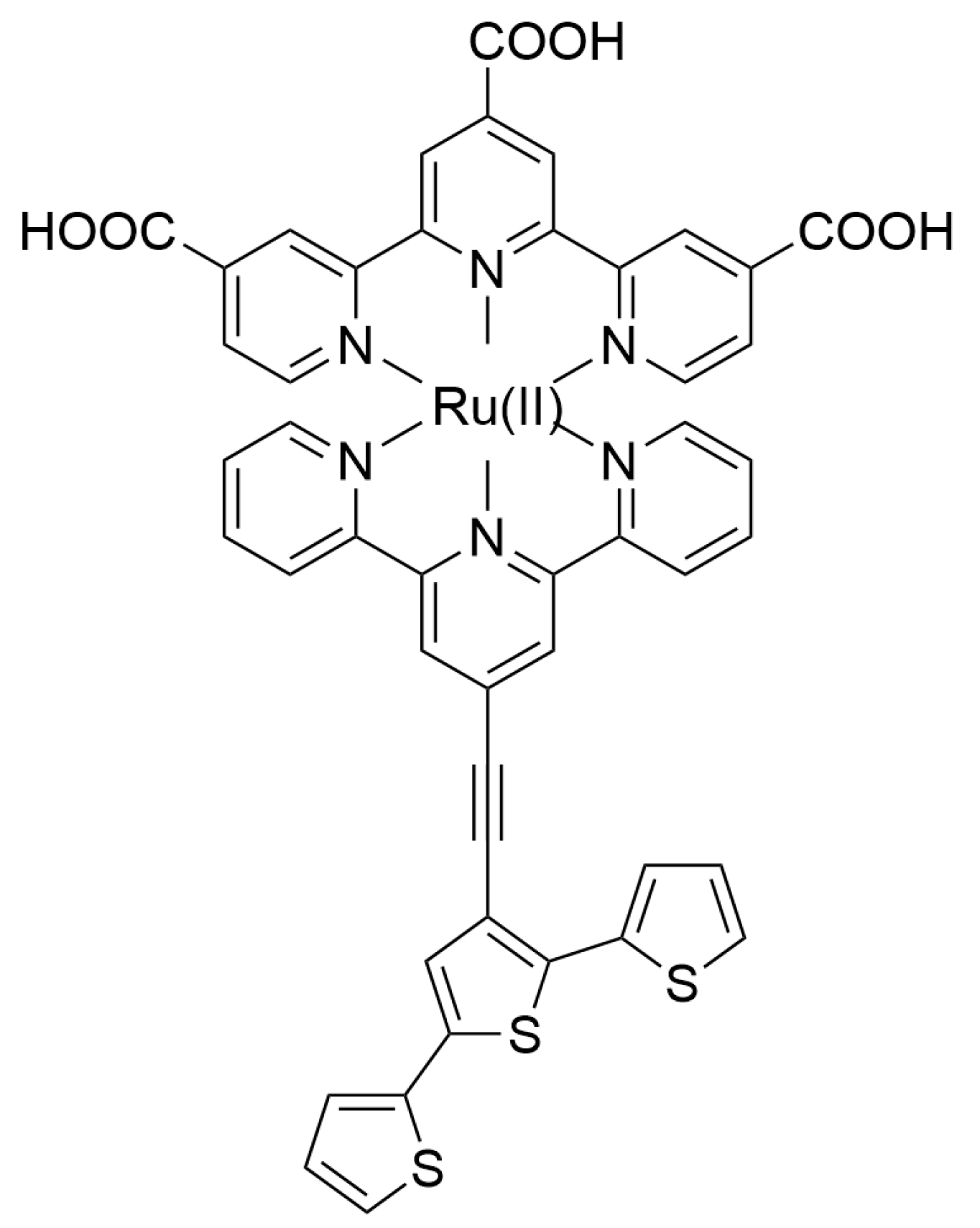


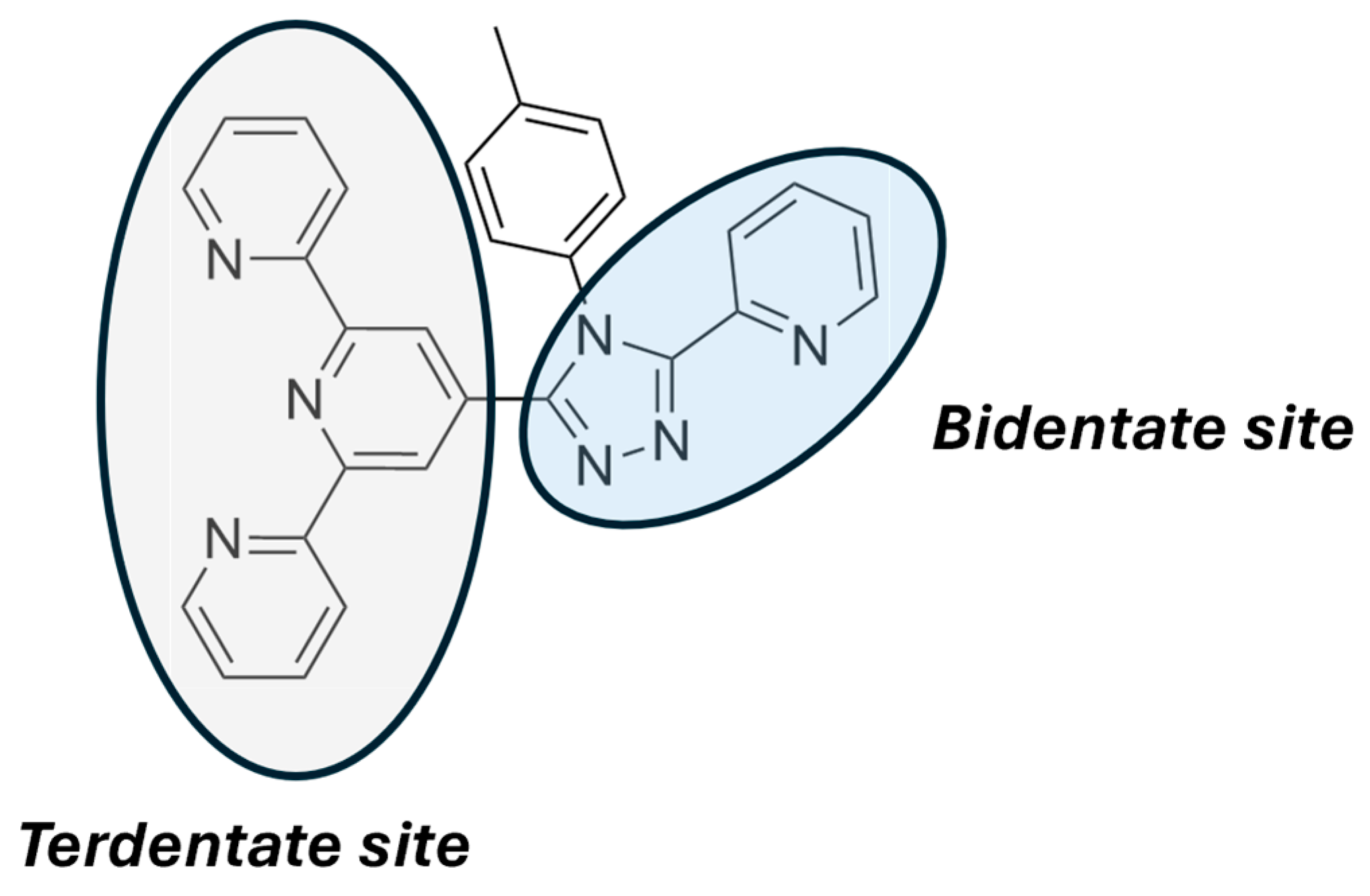
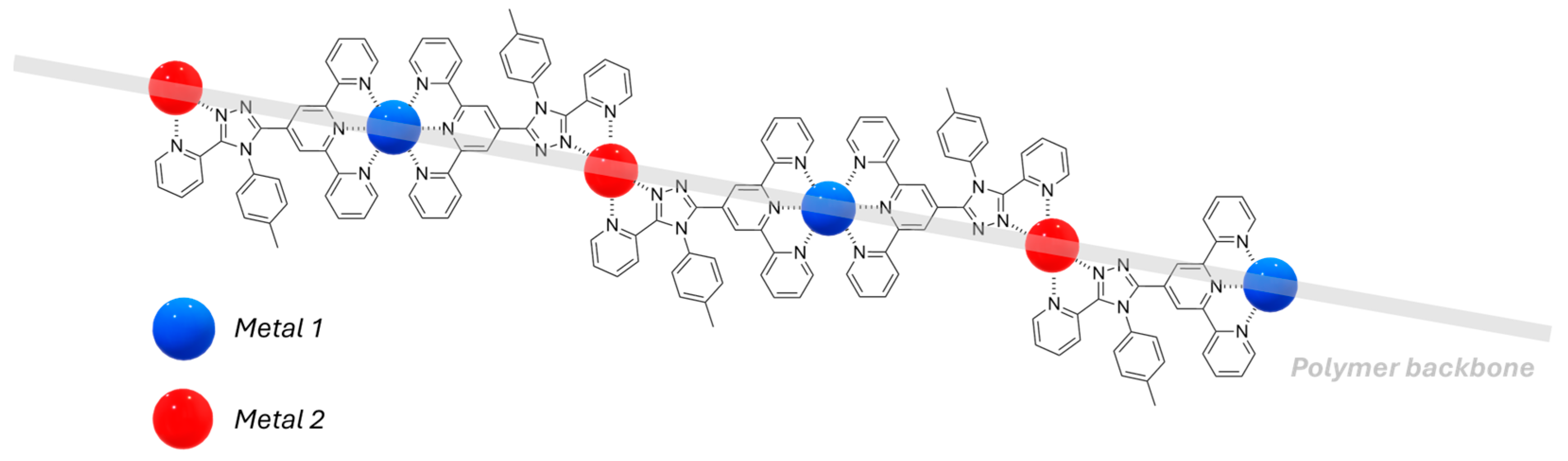

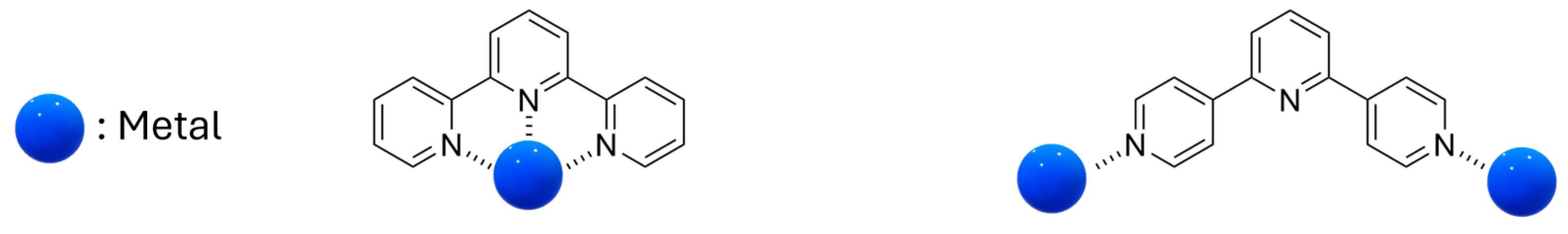
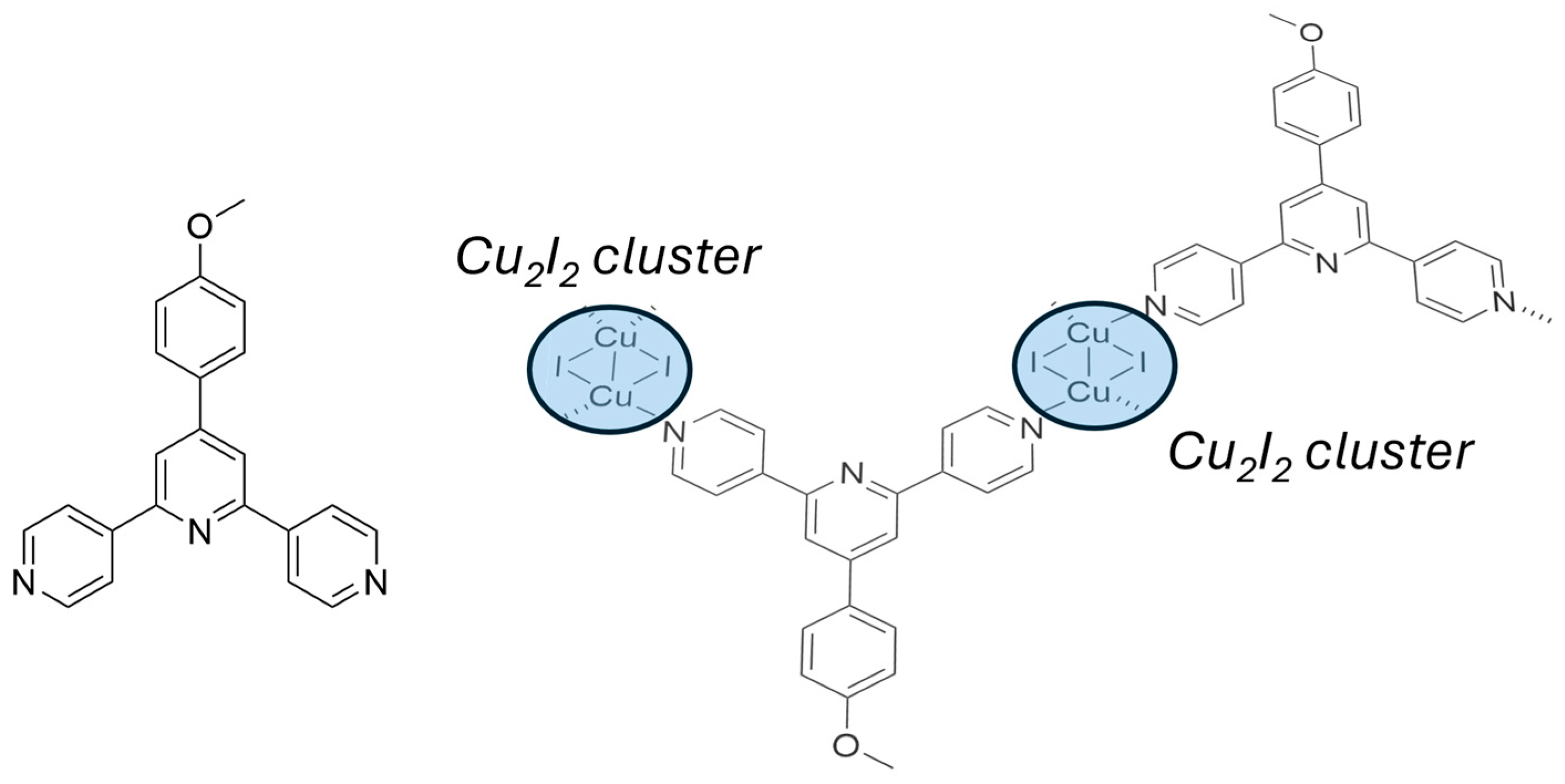
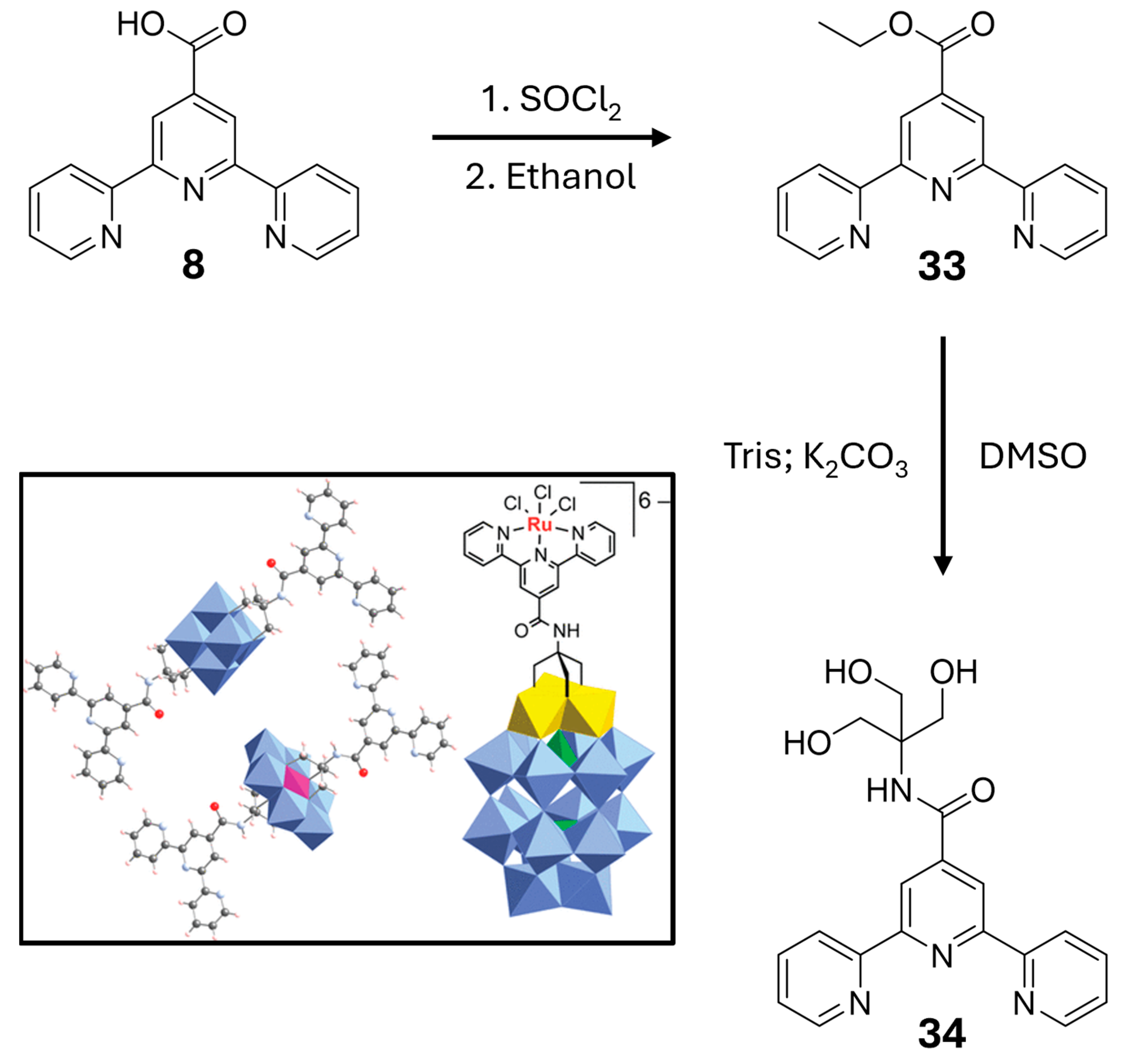
| Nature of Oxide | Complexed Metal (M) | Catalyzed Reaction | References |
|---|---|---|---|
| Fe3O4 | Pd | •Suzuki •Heck | [86] |
| Cu | •Huisgen •C-S coupling | [87] | |
| MnFe2O4 | Mo | •Epoxidation | [88] |
| Graphene Oxide | Pd | •Suzuki •Heck •C-N coupling •C-O coupling | [89] |
| SiO2 | Nb | •Phtalimides synthesis | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J. Functional Materials from Biomass-Derived Terpyridines: State of the Art and Few Possible Perspectives. Int. J. Mol. Sci. 2024, 25, 9126. https://doi.org/10.3390/ijms25169126
Husson J. Functional Materials from Biomass-Derived Terpyridines: State of the Art and Few Possible Perspectives. International Journal of Molecular Sciences. 2024; 25(16):9126. https://doi.org/10.3390/ijms25169126
Chicago/Turabian StyleHusson, Jérôme. 2024. "Functional Materials from Biomass-Derived Terpyridines: State of the Art and Few Possible Perspectives" International Journal of Molecular Sciences 25, no. 16: 9126. https://doi.org/10.3390/ijms25169126
APA StyleHusson, J. (2024). Functional Materials from Biomass-Derived Terpyridines: State of the Art and Few Possible Perspectives. International Journal of Molecular Sciences, 25(16), 9126. https://doi.org/10.3390/ijms25169126







