Unraveling the Molecular Mechanisms by Which the miR171b-SCL6 Module Regulates Maturation in Lilium
Abstract
1. Introduction
2. Results
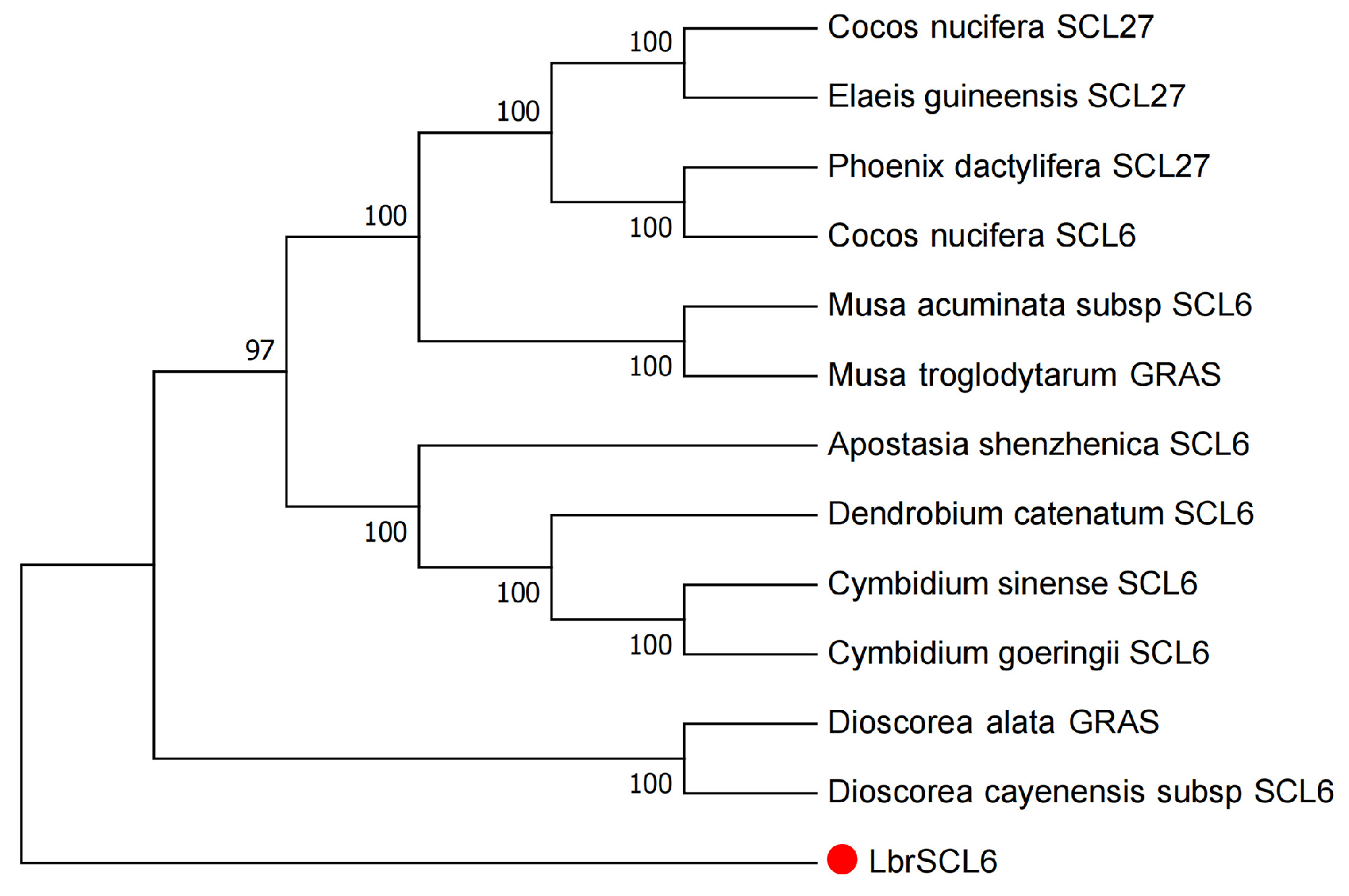
2.1. Bioinformatics Analysis of Lbr-miR171b and LbrSCL6
2.2. Validation of qRT-PCR for Lbr-miR171b and LbrSCL6
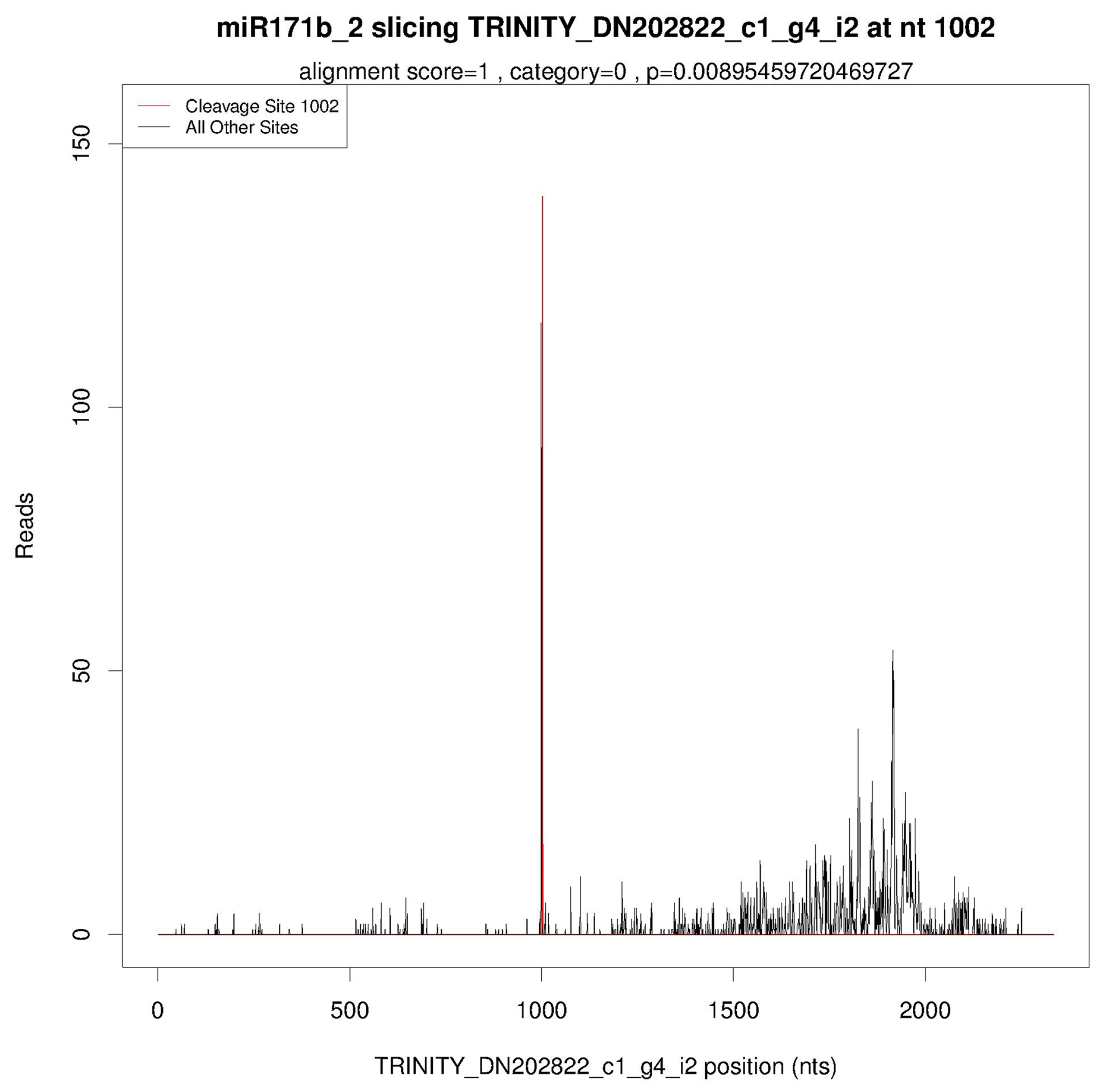
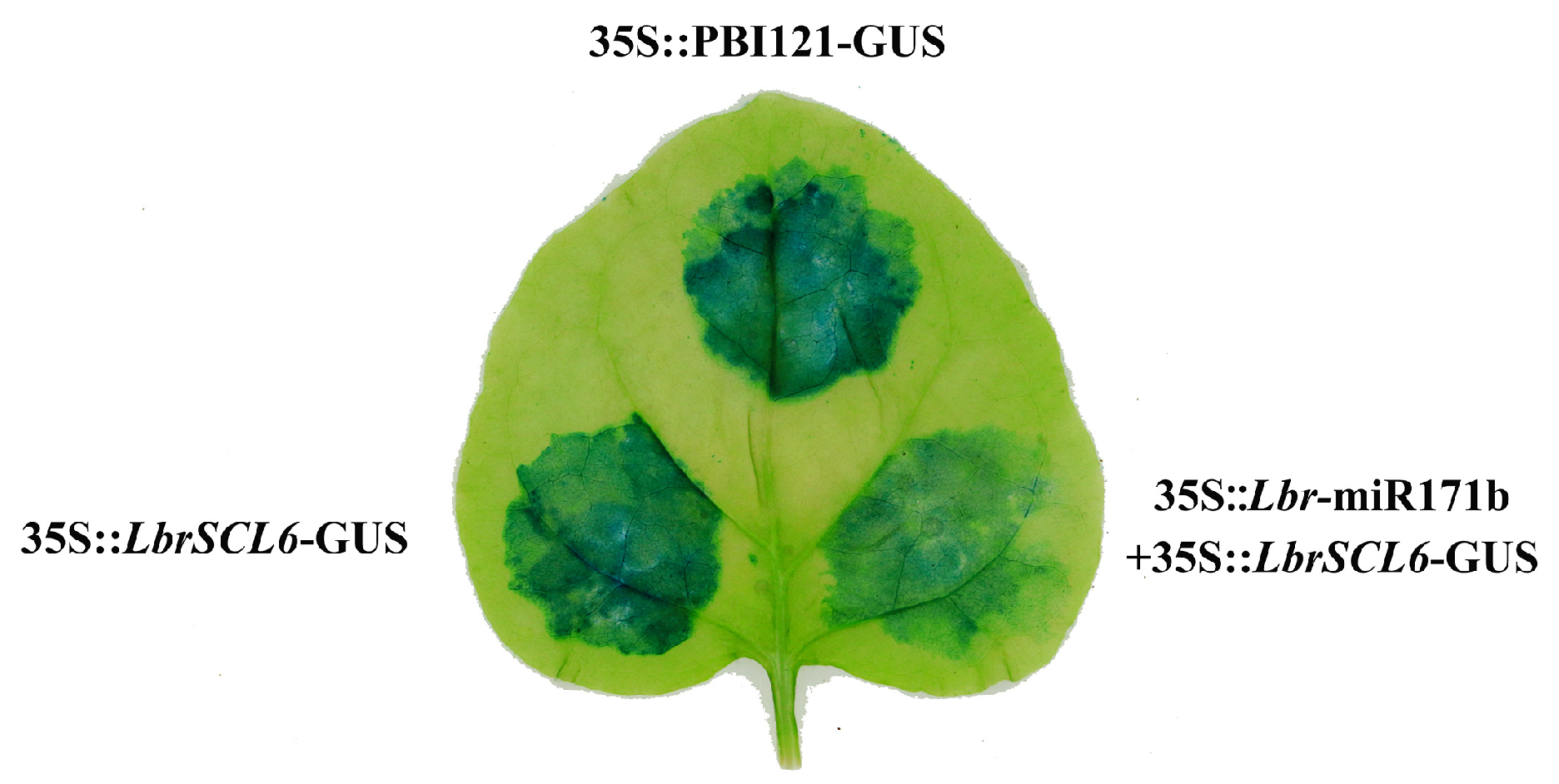
2.3. Identification of the Targeting Relationship between Lbr-miR171b and LbrSCL6
2.4. Subcellular Localization and Transcriptional Activation of LbrSCL6 and LbrWOX4
2.5. In Situ Hybridization of LbrSCL6
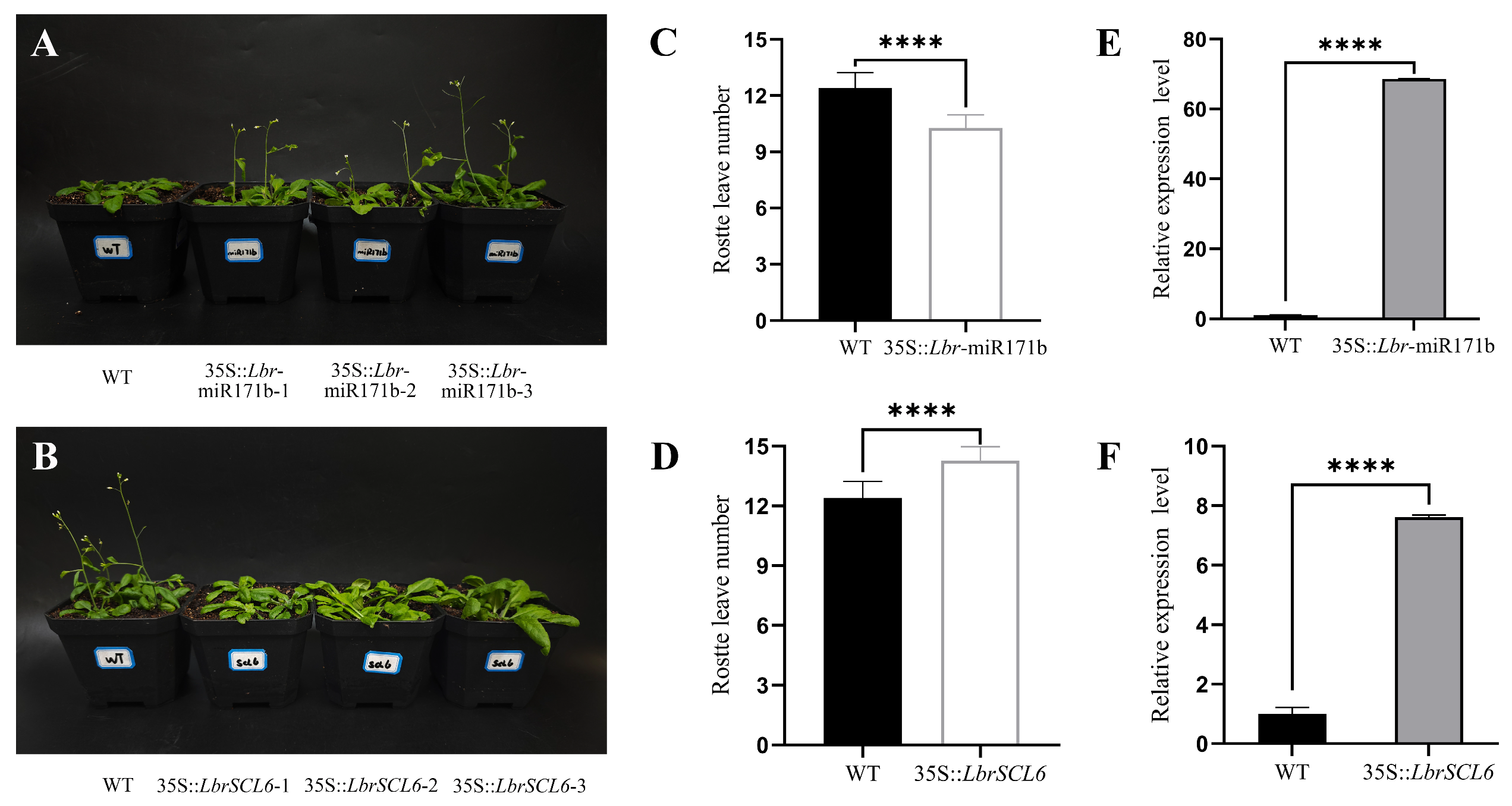
2.6. Transgenic Functional Validation of Lbr-miR171b and LbrSCL6
2.7. Bimolecular Fluorescence Complementation of LbrSCL6 and LbrWOX4
2.8. Yeast Two-Hybridization of LbrSCL6 and LbrWOX4
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Bioinformatics Analysis
4.3. qRT-PCR Analysis
4.4. Gene Cloning
4.5. Validation of the Targeting Relationship between Lbr-miR171b and LbrSCL6
4.6. Subcellular Localization and Transcriptional Activation
4.7. In Situ Hybridization
4.8. Transgenic Functional Verification
4.9. Bimolecular Fluorescence Complementation and Yeast Two-Hybrid Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Zhang, J.; Shao, S.; Yu, H.; Xiong, F.; Wang, Z. Morphological and physicochemical properties of bulb and bulbil starches from Lilium lancifolium. Starch-Starke 2015, 67, 448–458. [Google Scholar] [CrossRef]
- Li, X.Y.; Guo, F.; Ma, S.Y.; Zhu, M.Y.; Pan, W.H.; Bian, H.W. Regulation of flowering time via miR172-mediated APETALA2-like expression in ornamental gloxinia (Sinningia speciosa). J. Zhejiang Univ. Sci. B 2019, 20, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef]
- Poethig, R.S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar] [CrossRef] [PubMed]
- Scutt, C.P.; Theissen, G.; Ferrandiz, C. The evolution of plant development: Past, present and future: Preface. Ann. Bot. 2007, 100, 599–601. [Google Scholar] [CrossRef]
- Poethig, R.S. The past, present, and future of vegetative phase change. Plant Physiol. 2010, 154, 541–544. [Google Scholar] [CrossRef]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.Q. A new mechanism in plant engineering: The potential roles of microRNAs in molecular breeding for crop improvement. Biotechnol. Adv. 2010, 28, 301–307. [Google Scholar] [CrossRef]
- Fan, T.; Li, X.; Yang, W.; Xia, K.; Ouyang, J.; Zhang, M. Rice osa-miR171c mediates phase change from vegetative to reproductive development and shoot apical meristem maintenance by repressing four OsHAM transcription factors. PLoS ONE 2015, 10, e0125833. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, Y.; Shi, M.; Wu, X.; Wu, G. ABI5 acts downstream of miR159 to delay vegetative phase change in Arabidopsis. New Phytol. 2021, 231, 339–350. [Google Scholar] [CrossRef]
- Hofferek, V.; Mendrinna, A.; Gaude, N.; Krajinski, F.; Devers, E.A. MiR171h restricts root symbioses and shows like its target NSP2 a complex transcriptional regulation in Medicago truncatula. BMC Plant Biol. 2014, 14, 199. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Yang, Y.; Jia, N.; Wang, C.; Sun, H. miR171 and its target gene SCL6 contribute to embryogenic callus induction and torpedo-shaped embryo formation during somatic embryogenesis in two lily species. Plant Cell Tissue Organ Cult. 2017, 130, 591–600. [Google Scholar] [CrossRef]
- Zhu, X.; Leng, X.; Sun, X.; Mu, Q.; Wang, B.; Li, X.; Wang, C.; Fang, J. Discovery of conservation and diversification of miR171 genes by phylogenetic analysis based on global genomes. Plant Genome 2015, 8, plantgenome2014.10.0076. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Y.; He, X.; Zhu, Y.; Li, T.; Lin, X.; Mao, W.; Gishkori, Z.G.N.; Zhao, Z.; Zhang, J.; et al. The rice miR171b–SCL6-IIs module controls blast resistance, grain yield, and flowering. Crop J. 2022, 10, 117–127. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, C.; Zeng, W.; Xu, G.; Tan, X. Evolutionary and functional diversity of the poplar MIR171 genes. J. Plant Genet. Resour. 2014, 15, 313–319. [Google Scholar] [CrossRef]
- Wang, L.; Mai, Y.X.; Zhang, Y.C.; Luo, Q.; Yang, H.-Q. MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol. Plant 2010, 3, 794–806. [Google Scholar] [CrossRef]
- Llave, C.; Xie, Z.; Kasschau, K.D.; Carrington, J.C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 2002, 297, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Luo, X.; Wu, M.; Wei, L.; Fan, Z.; Zhu, Y. Genome-wide identification and expression of GRAS gene family members in cassava. BMC Plant Biol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Um, T.; Choi, J.; Park, T.; Chung, P.J.; Jung, S.E.; Shim, J.S.; Kim, Y.S.; Choi, I.Y.; Park, S.C.; Oh, S.J.; et al. Rice microRNA171f/SCL6 module enhances drought tolerance by regulation of flavonoid biosynthesis genes. Plant Direct 2022, 6, e374. [Google Scholar] [CrossRef] [PubMed]
- Curaba, J.; Talbot, M.; Li, Z.; Helliwell, C. Over-expression of microRNA171 affects phase transitions and floral meristem determinancy in barley. BMC Plant Biol. 2013, 13, 6. [Google Scholar] [CrossRef]
- Hai, B.; Qiu, Z.; He, Y.; Yuan, M.; Li, Y. Characterization and primary functional analysis of Pinus densata miR171. Biol. Plant. 2018, 62, 318–324. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Kondo, Y.; Fukuda, H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 2010, 22, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Engstrom, E.M.; Nimchuk, Z.L.; Pruneda-Paz, J.L.; Tarr, P.T.; Yan, A.; Kay, S.A.; Meyerowitz, E.M. Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 2015, 517, 377–380. [Google Scholar] [CrossRef]
- Tian, X.; Xie, J.; Yu, J. Physiological and transcriptomic responses of Lanzhou Lily (Lilium davidii, var. unicolor) to cold stress. PLoS ONE 2020, 15, e0227921. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Qiu, C.W.; Naz, S.; Cao, F.; Wu, F. Genome-wide discovery of miRNAs with differential expression patterns in responses to salinity in the two contrasting wheat cultivars. Int. J. Mol. Sci. 2021, 22, 12556. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, L.; Liu, X.; Jiang, J. Role of microRNA miR171 in plant development. PeerJ 2023, 11, e15632. [Google Scholar] [CrossRef] [PubMed]
- Asha, S.; Nisha, J.; Soniya, E.V. In silico characterisation and phylogenetic analysis of two evolutionarily conserved miRNAs (miR166 and miR171) from black pepper (Piper nigrum L.). Plant Mol. Biol. Rep. 2013, 31, 707–718. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, X.; Zhai, Y.; Wang, P.; Zhao, Q.; Li, J.; Lou, Q.; Chen, J. Cloning and functional analysis of microRNA171 in cucumber. Acta Hortic. Sin. 2019, 46, 864–876. [Google Scholar] [CrossRef]
- Hu, G.; Ge, X.; Wang, P.; Chen, A.; Li, F.; Wu, J. The cotton miR171a-SCL6 module mediates plant resistance through regulating GhPR1 expression. Plant Physiol. Biochem. 2023, 202, 107995. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, X.; Cai, W.; Huang, W.; Zhou, X.; Luo, Q.; Yang, H.; Wang, J.; Huang, J. Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet. 2014, 10, e1004519. [Google Scholar] [CrossRef] [PubMed]
- Hakoshima, T. Structural basis of the specific interactions of GRAS family proteins. FEBS Lett. 2018, 592, 489–501. [Google Scholar] [CrossRef]
- He, G.; Cao, Y.; Wang, J.; Song, M.; Bi, M.; Tang, Y.; Xu, L.; Ming, J.; Yang, P. WUSCHEL-related homeobox genes cooperate with cytokinin to promote bulbil formation in Lilium lancifolium. Plant Physiol. 2022, 190, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Mayer, K.F.; Berger, J.; Jürgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, Y.J.; Muller, R.; Yumul, R.E.; Liu, C.; Pan, Y.; Cao, X.; Goodrich, J.; Chen, X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. Plant Cell 2011, 23, 3654–3670. [Google Scholar] [CrossRef]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef]
- Wang, Y.; He, R.; Peng, X.; Shen, S. The research progress of WOX transcription factor family. Pratacultural Sci. 2015, 9, 760. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, W.; Ji, D.; Wang, L.; Li, J.; Xiang, F. GUS activity for miR165a/166b, REV, and WUS/CLV3 in in vitro direct Arabidopsis thaliana shoot regeneration. Protoplasma 2013, 250, 1213–1218. [Google Scholar] [CrossRef]
- Engstrom, E.M.; Andersen, C.M.; Gumulak-Smith, J.; Hu, J.; Orlova, E.; Sozzani, R.; Bowman, J.L. Arabidopsis homologs of the petunia hairy meristem gene are required for maintenance of shoot and root indeterminacy. Plant Physiol. 2011, 155, 735–750. [Google Scholar] [CrossRef]
- Engstrom, E.M. HAM proteins promote organ indeterminacy: But how? Plant Signal. Behav. 2012, 7, 227–234. [Google Scholar] [CrossRef]
- Desvergne, B.; Michalik, L.; Wahli, W. Transcriptional regulation of metabolism. Physiol. Rev. 2006, 86, 465–514. [Google Scholar] [CrossRef] [PubMed]
- Sparks, E.; Wachsman, G.; Benfey, P.N. Spatiotemporal signalling in plant development. Nat. Rev. Genet. 2013, 14, 631–644. [Google Scholar] [CrossRef]
- Jiang, J. Fluorescence in situ hybridization in plants: Recent developments and future applications. Chromosome Res. 2019, 27, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, G.; Song, H.; Chen, R.; Lan, T. The role of SBP-box/SPL genes in the formation and development of trichomes in plants. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2017, 46, 121–128. [Google Scholar] [CrossRef]
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a tomato miR171 target gene SIGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, B.; Chao, L.; Chen, D.; Cui, W.; Mao, Y.; Wang, L.; Chen, X. Interaction between two timing microRNAs controls trichome distribution in Arabidopsis. PLoS Genet. 2014, 10, e1004266. [Google Scholar] [CrossRef]
- Xing, J.; Zang, Q.; Ye, Z.; Qi, L.; Yang, L.; Li, W. Overexpression of Larch SCL6 inhibits transitions from vegetative meristem to inflorescence and flower meristem in Arabidopsis thaliana (L.). Plants 2024, 13, 1232. [Google Scholar] [CrossRef]
- Fields, S.; Song, O. A novel genetic system to detect protein–protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Wang, Z.; Long, C.; Fan, Z.; Zhang, L. Screening of OsCRK5-interacted proteins in rice using yeast two-hybrid system. Biotechnol. Bull. 2023, 39, 117125. [Google Scholar] [CrossRef]
- Ramachandran, V.; Chen, X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 2008, 321, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.; Giménez, M.J.; Palomino, C.; Avila, C.M. Assessment of candidate reference genes for expression studies in Vicia faba L. by real-time quantitative PCR. Mol. Breed. 2011, 28, 13–24. [Google Scholar] [CrossRef]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble cloning: A simple, versatile, and efficient system for standardized molecular cloning. Front. Bioeng. Biotechnol. 2020, 7, 460. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Song, M.; Wang, Y.; Lu, P.; Ge, W.; Zhang, K. Unraveling the Molecular Mechanisms by Which the miR171b-SCL6 Module Regulates Maturation in Lilium. Int. J. Mol. Sci. 2024, 25, 9156. https://doi.org/10.3390/ijms25179156
Li Q, Song M, Wang Y, Lu P, Ge W, Zhang K. Unraveling the Molecular Mechanisms by Which the miR171b-SCL6 Module Regulates Maturation in Lilium. International Journal of Molecular Sciences. 2024; 25(17):9156. https://doi.org/10.3390/ijms25179156
Chicago/Turabian StyleLi, Qing, Meiqi Song, Yachen Wang, Ping Lu, Wei Ge, and Kezhong Zhang. 2024. "Unraveling the Molecular Mechanisms by Which the miR171b-SCL6 Module Regulates Maturation in Lilium" International Journal of Molecular Sciences 25, no. 17: 9156. https://doi.org/10.3390/ijms25179156
APA StyleLi, Q., Song, M., Wang, Y., Lu, P., Ge, W., & Zhang, K. (2024). Unraveling the Molecular Mechanisms by Which the miR171b-SCL6 Module Regulates Maturation in Lilium. International Journal of Molecular Sciences, 25(17), 9156. https://doi.org/10.3390/ijms25179156




