Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity
Abstract
:1. History of Organ Transplantation
2. The Liver as an Immune-Privileged Organ
3. Impact of Liver Resident Cells on Liver Transplant Immunology
3.1. Impact of Hepatic Innate-like Lymphocytes on Liver Transplant Immunology
3.2. Impact of Hepatic DCs on Liver Transplant Immunology
3.3. Impact of Kupffer Cells, Hepatic Stellate Cellsand LSECs on Liver Transplant Immunology
| Inflammation/Rejection | Immune Regulation/Tolerance Induction |
|---|---|
| Liver allograft Peripheral NKG2D+ CD56dim CD16+ NK cells shift to the liver graft in the patients with EAD [30] | Liver allograft Donor liver-resident NKG2A+ CD56bright CD16+/− NK cells for the inhibition of CD8+ T cell-mediated rejection [32] |
| Inflamed liver Activation of iNKT cells during acute liver injury [39]; Proinflammatory cytokine production by sulfatide-reactive dNKT cells in patients with AIH [40] | Inflamed liver Prevention of liver inflammation by dNKT cells through the induction of anergy in iNKT cells [39] Liver allograft Tolerance induction by α-GalCer-activated iNKT cells [37] |
| Peripheral blood Lower value of the Vδ1+/Vδ2+ ratio as a predictive biomarker for rejection [45] | Liver allograft Elevation of Vδ1+γδ T cells in patients with stable liver allograft function [44] |
| Inflamed liver cDC1 for the stimulation of naïve CD4+ T cells and the induction of cytotoxic CD8+ T cells [49]; cDC2 for regulating type II immune responses to parasites, helminths and fungi [50,51] | Normal liver Immature state of cDCs (lower expression of MHC II and TLR4) with higher IL-10 production [53,54,55] Liver allograft PD-L1+pDCs-mediated reduction in infiltrating PD-1+ TIM-3+ CD8+ T cells and the induction of Treg [60,61] |
| Inflamed liver/Liver allograft Involvement of Kupffer cell activation in liver injury and liver transplant rejection [63,64,65,66] | Liver allograft Induction of FasL and IDO in Kupffer cells for the inhibition of alloreactive T cell response [71,72,73,74,75] |
| Liver allograft Involvement of hepatic stellate cell activation in long-term liver allograft fibrosis and chronic rejection [78,79] | Liver allograft Involvement of hepatic stellate cell activation in the induction of immune tolerance via Fas/FasL- or PD-L1-mediated apoptosis induction and MDSC induction [80,81,82,84] |
| Inflamed liver/Liver allograft Accumulation of neutrophils and macrophages and proinflammatory cytokine production in the liver by aging-associated LSEC dysfunction [91]; Rejection-induced severe damage of LSECs [92] | Normal liver LSEC-mediated induction of oral tolerance [87] Inflamed liver/Liver allograft LSEC-mediated clearance of scavenger cells, antibody-coated immune complexes and PAMPs/DAMPs [86]; Induction of allogeneic T cell tolerance [88,89,90] |
4. Mechanical Similarities and Differences between Liver Transplant Immunology and Fetomaternal Tolerance, Autoimmunity or Tumor Immunity
4.1. Liver Transplantation vs. Pregnancy
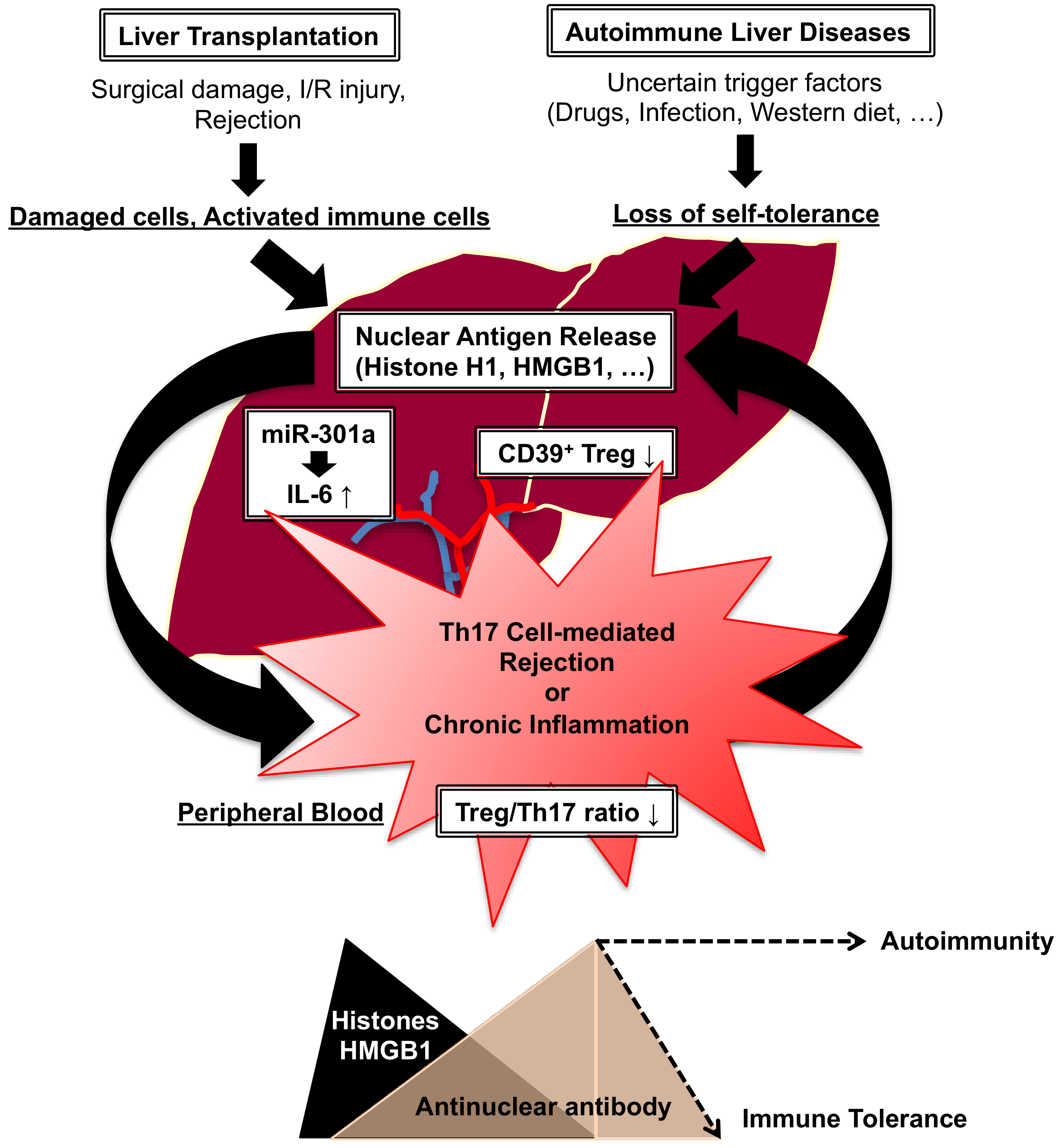
4.2. Liver Transplantation vs. Autoimmunity
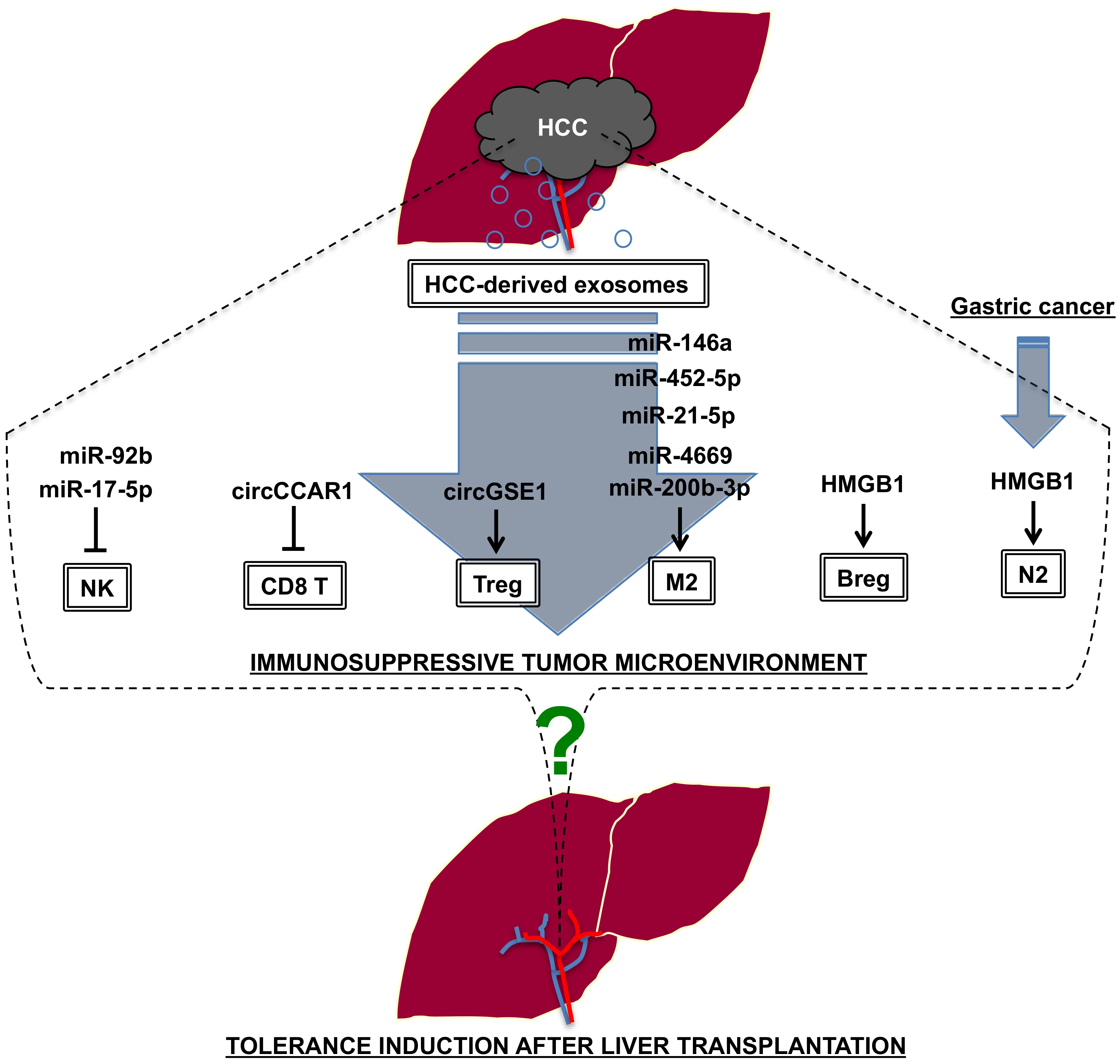
4.3. Liver Transplantation vs. Tumor Immunity
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nagy, J. A note on the early history of renal transplantation: Emerich (Imre) Ullmann. Am. J. Nephrol. 1999, 19, 346–349. [Google Scholar] [CrossRef]
- Druml, W. The beginning of organ transplantation: Emerich Ullmann (1861–1937). Wien. Klin. Wocheschr 2002, 114, 128–137. [Google Scholar]
- Druml, W.; Druml, C. Emerich Ullmann (1861–1937): Not only a pioneer of kidney transplantation. J. Nephrol. 2004, 17, 461–466. [Google Scholar]
- Morris, P.; Joseph, E. Murray (1919–2012). Nature 2013, 493, 164. [Google Scholar] [CrossRef]
- Starzl, T.E. Peter Brian Medawar: Father of transplantation. J. Am. Coll. Surg. 1995, 180, 332–336. [Google Scholar]
- Haeney, M. The immunological background to transplantation. J. Antimicrob. Chemother. 1995, 36 (Suppl. B), 1–9. [Google Scholar] [CrossRef]
- Borel, J.F.; Kis, Z.L.; Beveridge, T. The history of the discovery and development of Cyclosporin (Sandimmune). In The Search for Anti-Inflammatory Drugs Cas Histories from Concept to Clinic; Merluzzi, V.J., Adams, J., Eds.; Birkhäuser: Boston, MA, USA, 1995; pp. 27–63. [Google Scholar]
- Calne, R.Y.; White, D.J.; Thiru, S.; Evans, D.B.; McMaster, P.; Dunn, D.C.; Craddock, G.N.; Pentlow, B.D.; Rolles, K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978, 2, 1323–1327. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Miyata, S.; Inamura, N.; Nishiyama, M.; Yajima, T.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987, 40, 1256–1265. [Google Scholar] [CrossRef]
- Starzl, T.E.; Todo, S.; Fung, J.; Demetris, A.J.; Venkataramman, R.; Jain, A. FK506 for liver, kidney, and pancreas transplantation. Lancet 1989, 2, 1000–1004. [Google Scholar] [CrossRef]
- Todo, S.; Fung, J.J.; Starzl, T.E.; Tzakis, A.; Demetris, A.J.; Kormos, R.; Jain, A.; Alessiani, M.; Takaya, S.; Shapiro, R. Liver, kidney, and thoracic organ transplantation under FK506. Ann. Surg. 1990, 212, 295–305. [Google Scholar] [CrossRef]
- Tönshoff, B. Immunosuppressants in Organ Transplantation. Handb. Exp. Pharmacol. 2020, 261, 441–469. [Google Scholar]
- Zheng, M.; Tian, Z. Liver-Mediated Adaptive Immune Tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef]
- Calne, R.Y.; White, H.J.; Yoffa, D.E.; Binns, R.M.; Maginn, R.R.; Herbertson, R.M.; Millard, P.R.; Molina, V.P.; Davis, D.R. Prolonged survival of liver transplants in the pig. Br. Med. J. 1967, 4, 645–648. [Google Scholar] [CrossRef]
- Calne, R.Y.; Sells, R.A.; Pena, J.R.; Davis, D.R.; Millard, P.R.; Hervertson, B.M.; Binns, R.M.; Davies, D.A. Induction of immunological tolerance by porcine liver allografts. Nature 1969, 223, 472–476. [Google Scholar] [CrossRef]
- Kamada, N.; Calne, R.Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979, 28, 47–50. [Google Scholar] [CrossRef]
- Kamada, N.; Calne, R.Y. A surgical experience with five hundred thirty liver transplants in the rat. Surgery 1983, 93 Pt 1, 64–69. [Google Scholar]
- Zimmermann, F.A.; Davies, H.S.; Knoll, P.P.; Gokel, J.M.; Schmidt, T. Orthotopic liver allografts in the rat. The influence of strain combination on the fate of the graft. Transplantation 1984, 37, 406–410. [Google Scholar] [CrossRef]
- Kamada, N.; Brons, G.; Davies, H.S. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation 1980, 29, 429–431. [Google Scholar] [CrossRef]
- Kamada, N.; Davies, H.S.; Roser, B. Reversal of transplantation immunity by liver grafting. Nature 1981, 292, 840–842. [Google Scholar] [CrossRef]
- Kamada, N. The immunology of experimental liver transplantation in the rat. Immunology 1985, 55, 369–389. [Google Scholar]
- Sun, J.; McCaughan, G.W.; Gallagher, N.D.; Sheil, A.G.; Bishop, G.A. Deletion of spontaneous liver allograft acceptance by donor irradiation. Transplantation 1995, 60, 233–236. [Google Scholar] [CrossRef]
- Tu, Y.; Arima, T.; Flye, M.W. Rejection of spontaneously accepted rat liver allografts with recipientinterleukin-2 treatment or donor irradiation. Transplantation 1997, 63, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43 (Suppl. 1), S54–S62. [Google Scholar] [CrossRef]
- Jiang, Y.; Que, W.; Zhu, P.; Li, X.K. The Role of Diverse Liver Cells in Liver Transplantation Tolerance. Front. Immunol. 2020, 11, 1203. [Google Scholar] [CrossRef]
- Halma, J.; Pierce, S.; McLennan, R.; Bradley, T.; Fischer, R. Natural killer cells in liver transplantation: Can we harness the power of the immune checkpoint to promote tolerance? Clin. Transl. Sci. 2022, 15, 1091–1103. [Google Scholar] [CrossRef]
- Harmon, C.; Sanchez-Fueyo, A.; O’Farrelly, C.; Houlihan, D.D. Natural Killer Cells and Liver Transplantation: Orchestrators of Rejection or Tolerance? Am. J. Transplant. 2016, 16, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, Y.; Zhou, T.; Gu, G.; Xia, Q. Innate Immune Cells in Immume Tolerance After Liver Transplantation. Front. Immunol. 2018, 9, 2401. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Fujiki, M.; Wang, M.; Piard-Ruster, K.; Wai, L.E.; Wei, L.; Martinez, O.M.; Krams, S.M. Identification of the rat NKG2D ligands, RAE1L and RRLT, and their role in allograft rejection. Eur. J. Immunol. 2010, 40, 1748–1757. [Google Scholar] [CrossRef]
- Lu, D.; Yang, X.; Pan, L.; Lian, Z.; Tan, W.; Zhuo, J.; Yang, M.; Lin, Z.; Wei, Q.; Chen, L.; et al. Dynamic immune cell profiling identified natural killer cell shift as the key event in early allograft dysfunction after liver transplantation. Cell Prolif. 2024, 57, e13568. [Google Scholar] [CrossRef]
- Yu, J.D.; Long, T.Z.; Li, G.L.; Lv, L.H.; Lin, H.M.; Huang, Y.H.; Chen, Y.J.; Wan, Y.L. Donor liver natural killer cells alleviate liver allograft acute rejection in rats. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 386–392. [Google Scholar] [CrossRef]
- Jameson, G.; Harmon, C.; Santiago, R.M.; Houlihan, D.D.; Gallagher, T.K.; Lynch, L.; Robinson, M.W.; O’Farrelly, C. Human Hepatic CD56bright NK Cells Display a Tissue-Resident Transcriptional Profile and Enhanced Ability to Kill Allogenic CD8+ TCells. Front. Immunol. 2022, 13, 921212. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.G. Natural killer T cells within the liver: Conductors of the hepatic immune orchestra. Dig. Dis. 2010, 28, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, M.; Song, S.; Zhi, Y.; Huan, C.; Lv, G. The role of natural killer T cells in liver transplantation. Front. Cell Dev. Biol. 2024, 11, 1274361. [Google Scholar] [CrossRef]
- Sag, D.; Krause, P.; Hedrick, C.C.; Kronenberg, M.; Wingender, G. IL-10-producing NKT10cells are a distinct regulatory invariant NKT cell subset. J. Clin. Investig. 2014, 124, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Fujino, M.; Li, X.K.; Kimura, H.; Nakayama, T.; Taniguchi, M.; Sugioka, A. Spontaneous tolerance involving natural killer T cells after hepatic grafting in mice. Transplant. Immunol. 2007, 18, 142–145. [Google Scholar] [CrossRef]
- Liu, Y.; Luan, X.; Li, J.; He, Y.; Li, M. The role of invariant NKT cells in liver transplant tolerance in rats. Transplant. Proc. 2012, 44, 1041–1044. [Google Scholar] [CrossRef]
- Singh, A.K.; Tripathi, P.; Cardell, S.L. Type II NKT Cells: An Elusive Population with Immunoregulatory Properties. Front. Immunol. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Halder, R.C.; Aguilera, C.; Maricic, I.; Kumar, V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Investig. 2007, 117, 2302–2312. [Google Scholar] [CrossRef]
- Sebode, M.; Wigger, J.; Filpe, P.; Fischer, L.; Weidemann, S.; Krech, T.; Weiler-Normann, C.; Peiseler, M.; Hartl, J.; Tolosa, E.; et al. Inflammatory Phenotype of Intrahepatic Sulfatide-Reactive Type II NKT Cells in Humans with Autoimmune Hepatitis. Front. Immunol. 2019, 10, 1065. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Wu, F.T.; Pang, L.T.; Zhang, T.B.; Chen, Z. Role of γδT cells in liver diseases and its relationship with intestinal microbiota. World J. Gastroenterol. 2020, 26, 2559–2569. [Google Scholar] [CrossRef]
- Wu, D.; Wu, P.; Qiu, F.; Wei, Q.; Huang, J. Human γδT-cell subsets and their involvement in tumor immunity. Cell Mol. Immunol. 2017, 14, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.L.; Chu, K.J.; Li, M.; Duan, Y.X.; Yu, Y.X.; Kang, M.Q.; Fu, D.; Liao, R. Immune Regulatory Networks and Therapy of γδ T Cells in Liver Cancer: Recent Trends and Advancements. J. Clin. Transl. Hepatol. 2024, 12, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Ohe, H.; Nafady-Hego, H.; Uemoto, S.; Bishop, G.A.; Koshiba, T. Intragraft Vδ1 γδ T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation 2013, 95, 192–202. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Wang, Y.; Wang, H.; Zhang, M.; Sun, Y.; Su, H.; Jin, L.; Wang, F.; Shi, M. Characteristics of Vδ1(+) and Vδ2(+) γδ T cell subsets in acute liver allograft rejection. Transpl. Immunol. 2013, 29, 118–122. [Google Scholar] [CrossRef]
- Thomson, A.W.; Drakes, M.L.; Zahorchak, A.F.; O’Connell, P.J.; Steptoe, R.J.; Qian, S.; Lu, L. Hepatic dendritic cells: Immunobiology and role in liver transplantation. J. Leukoc. Biol. 1999, 66, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Lechler, R.; Ng, W.F.; Steinman, R.M. Dendritic cells in transplantation–friend or foe? Immunity 2001, 14, 357–368. [Google Scholar] [CrossRef]
- Du, X.; Li, M.; Huan, C.; Lv, G. Dendritic cells in liver transplantation immune response. Front. Cell Dev. Biol. 2023, 11, 1277743. [Google Scholar] [CrossRef]
- Pi, Y.; Li, Y.; Liang, R.; Xiao, J.; Leng, J.; Zhang, L. Generation of high cross-presentation ability human dendritic cells by combination of interleukin4, interferon β and GM-CSF. Cent. Eur. J. Immunol. 2022, 47, 125–138. [Google Scholar] [CrossRef]
- Anderson, D.A., 3rd; Dutertre, C.A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef]
- English, K.; Tan, S.Y.; Kwan, R.; Holz, L.E.; Sierro, F.; McGuffog, C.; Kaisho, T.; Heath, W.R.; MacDonald, K.A.; McCaughham, G.W.; et al. The liver contains distinct interconnected networks of CX3CR1+ macrophages, XCR1+ type 1 and CD301a+ type 2 conventional dendritic cells embedded within portal tracts. Immunol. Cell Biol. 2022, 100, 394–408. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, N.; Lu, Z.; Wu, W.; Wang, L.; Nakao, A.; Lotze, M.T.; Langer, C.E.; Fung, J.J.; Qian, S.; et al. In vivo expansion of two distinct dendritic cells in mouse livers and its impact on liver immune regulation. Liver Transpl. 2006, 12, 1850–1861. [Google Scholar] [CrossRef]
- Lu, L.; Woo, J.; Rao, A.S.; Li, Y.; Watkins, S.C.; Qian, S.; Starzl, T.E.; Dematris, A.J.; Thomson, A.W. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J. Exp. Med. 1994, 179, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Bosma, B.M.; Matselaar, H.J.; Mancgham, S.; Boor, P.P.; Kusters, J.G.; Kazemier, G.; Tilanus, H.W.; Kuipers, E.J.; Kwekkeboom, J. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 2006, 12, 384–393. [Google Scholar] [CrossRef]
- DeCreus, A.; Abe, M.; Lau, A.H.; Hackstein, H.; Raimondi, G.; Thomson, A.W. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic Tcells in response to endotoxin. J. Immunol. 2005, 174, 2037–2045. [Google Scholar] [CrossRef]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef]
- Wirtz, T.H.; Brandt, E.F.; Berres, M.L. Liver DCs in health and disease. Int. Rev. Cell Mol. Biol. 2019, 348, 263–299. [Google Scholar] [PubMed]
- Lu, L.; Bonham, C.A.; Liang, X.; Chen, Z.; Li, W.; Wang, L.; Watkins, S.C.; Nalesnik, M.A.; Schlissel, M.S.; Demestris, A.J.; et al. Liver-derived DEC205+B220+CD19- dendritic cells regulate T cell responses. J. Immunol. 2001, 166, 7042–7052. [Google Scholar] [CrossRef]
- Tokita, D.; Sumpter, T.L.; Raimondi, G.; Zahorchak, A.F.; Wang, Z.; Nakao, A.; Mazzariegos, G.V.; Abe, M.; Thomson, A.W. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J. Hepatol. 2008, 49, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Tokita, D.; Mazariegos, G.V.; Zahorchak, A.F.; Chien, N.; Abe, M.; Raimondi, G.; Thomson, A.W. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 2008, 85, 369–377. [Google Scholar] [CrossRef]
- Nakano, R.; Yoshida, O.; Kimura, S.; Nakao, T.; Yokota, S.; Ono, Y.; Minervini, M.I.; Geller, D.A.; Thomson, A.W. Donor plasmacytoid dendritic cells modulate effector and regulatory T cell responses in mouse spontaneous liver transplant tolerance. Am. J. Transplant. 2021, 21, 2040–2055. [Google Scholar] [CrossRef]
- Dizon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar]
- Cyrendorzhiev, D.D.; Kutina, S.N.; Zubakhin, A.A. Liver resistance to toxic effects of CCl(4) under conditions of gadolinium chloride depression of Kupffer cells. Bull. Exp. Bio Med. 2000, 129, 605–607. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kim, J.; Sharma, R.P. Fumonisin B1 hepatotoxicity in mice is attenuated by depletion of Kupffer cells by gadolinium chloride. Toxicology 2005, 207, 137–147. [Google Scholar] [CrossRef]
- Zandieh, A.; Payabvash, S.; Pasalar, P.; Morteza, A.; Zandieh, B.; Tavangar, S.M.; Dehpour, A.R. Gadolinium chloride, a Kupffer cell inhibitor, attenuates hepatic injury in a rat model of chronic cholestasis. Hum. Exp. Toxicol. 2011, 30, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Li, M.; Yang, Z.; Gong, J.; Zhang, W. Gadolinium chloride suppresses acute rejection and induces tolerance following rat liver transplantation by inhibiting Kupffer-cell activation. Exp. Ther. Med. 2014, 8, 1777–1782. [Google Scholar] [CrossRef]
- Sato, S.; Yabuki, K.; Haba, T.; Maekawa, T. Role of Kupffer cells in the induction of tolerance after liver transplantation. J. Surg. Res. 1996, 63, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ichida, T.; Watanabe, H.; Yamamoto, S.; Oya, H.; Nakatsuka, H.; Kobayashi, T.; Watanabe, T.; Kameyama, H.; Hatakeyama, K. Repeating intraportal donor-specific transfusion may induce tolerance following adult living-related donor livertransplantation. Hepatogastroenterology 2003, 50, 601–606. [Google Scholar]
- Shimizu, H.; Kataoka, M.; Kimura, F.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Miyazaki, M. Role of Kupffer cells in tolerance induction after portal venous administration of alloantigen. Hepatogastroenterology 2009, 56, 783–787. [Google Scholar]
- Zheng, W.; Yang, L.; Jiang, S.; Chen, M.; Li, J.; Liu, Z.; Wu, Z.; Gong, J.; Chen, Y. Role of Kupffer cells in tolerance induction after liver transplantation. Front. Cell Dev. Biol. 2023, 11, 1179077. [Google Scholar] [CrossRef]
- Sun, Z.; Wada, T.; Maemura, K.; Uchikura, K.; Hoshino, S.; Diehl, A.M.; Klein, A.S. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003, 9, 489–497. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liang, S.; Luan, X.; Long, F.; Chen, J.; Peng, Y.; Yan, L.; Gong, J. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008, 14, 823–836. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, C.L.; Nakano, T.; Goto, S.; Kao, Y.H.; Hsu, L.W.; Lai, C.Y.; Jawan, B.; Cheng, Y.F.; Tateno, C.; et al. Immunological role of indoleamine2,3-dioxygenase in rat liver allograft rejection and tolerance. J. Gastroenterol. Hepatol. 2008, 23 Pt 2, e243–e250. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.L.; Wang, Y.D.; Tian, Y.F.; Lai, Z.D.; Yan, L.N. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World J. Gastroenterol. 2010, 16, 636–640. [Google Scholar] [CrossRef]
- Luan, X.; Liao, W.; Lai, X.; He, Y.; Liu, Y.; Gong, J.; Li, J. Dynamic changes of indoleamine 2,3-dioxygenase of Kupffer cells in rat liver transplant rejection and tolerance. Transplant. Proc. 2012, 44, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Y.; Wu, X.; Xu, X.; Gong, J.; Chen, Y.; Gong, J. Rubicon promotes the M2 polarization of Kupffer cells via LC3-associated phagocytosis-mediated clearance to improve liver transplantation. Cell Immunol. 2022, 378, 104556. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef]
- Demirci, G.; Nashan, B.; Pichlmayr, R. Fibrosis in chronic rejection of human liver allografts: Expression patters of transforming growth factor-TGFbeta1 and TGF-beta3. Transplantation 1996, 62, 1776–1783. [Google Scholar] [CrossRef]
- Vij, M.; Rammohan, A.; Rela, M. Long-term liver allograft fibrosis: A review with emphasis on idiopathic post-transplant hepatitis and chronic antibody mediated rejection. World J. Hepatol. 2022, 14, 1541–1549. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Y.; Feng, X.; Jiang, J.; Chen, T.; Xie, H.; Zhou, L.; Zheng, S. Hepatic stellate cells promote immunotolerance following orthotopic liver transplantation in rats via induction of T cell apoptosis and regulation of Th2/Th3-like cell cytokine production. Exp. Ther. Med. 2013, 5, 165–169. [Google Scholar] [CrossRef]
- Yu, M.C.; Chen, C.H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Qian, S.; Fung, J.J.; Lin, F. Hepatic Stellate Cells Directly Inhibit B Cells via Programmed Death-Ligand 1. J. Immunol. 2016, 196, 1617–1625. [Google Scholar] [CrossRef]
- Osawa, Y.; Kawai, H.; Tsunoda, T.; Komatsu, H.; Okawara, M.; Tsutsui, Y.; Yoshida, Y.; Yoshikawa, S.; Mori, T.; Yamazoe, T.; et al. Cluster of Differentiation 44 Promotes Liver Fibrosis and Serves as a Biomarker in Congestive Hepatopathy. Hepatol. Commun. 2021, 5, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Höchst, B.; Schildberg, F.A.; Sauerborn, P.; Gäbel, Y.A.; Gevensleben, H.; Goltz, D.; Heukamp, L.C.; Türler, A.; Ballmaier, M.; Gieseke, F.; et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 2013, 59, 528–535. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front. Physiol. 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.; Ohl, J.; Wingender, G.; Berg, M.; Jüngerkes, F.; Schumak, B.; Djandji, D.; Scholz, K.; Klevenz, A.; Hegenbarth, S.; et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 2005, 35, 2970–2981. [Google Scholar] [CrossRef]
- Tokita, D.; Shishida, M.; Ohdan, H.; Onoe, T.; Hara, H.; Tanaka, Y.; Ishiyama, K.; Mitsuta, H.; Ide, K.; Arihiro, K.; et al. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J. Immunol. 2006, 177, 3615–3624. [Google Scholar] [CrossRef]
- Ge, X.; Nowak, G.; Ericzon, B.G.; Sumitran-Holgersson, S. Liver sinusoidal endothelial cell function in rejected and spontaneously accepted rat liver allografts. Transpl. Int. 2008, 21, 49–56. [Google Scholar] [CrossRef]
- Banshodani, M.; Onoe, T.; Shishida, M.; Tahara, H.; Hashimoto, S.; Igarashi, Y.; Tanaka, Y.; Ohdan, H. Adaptive transfer of allogeneic liver sinusoidal endothelial cells specifically inhibits T-cell responses to cognate stimuli. Cell Transplant. 2013, 22, 1695–1708. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Slevin, E.; Harrison, K.; Li, T.; Zhang, Y.; Klaunig, J.E.; Wu, C.; Shetty, A.K.; Dong, X.C.; et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 2022, 36, e22125. [Google Scholar]
- Usui, M.; Kuriyama, N.; Kisawada, M.; Hamada, T.; Mizuno, S.; Sakurai, H.; Tabata, M.; Imai, H.; Okamoto, K.; Uemoto, S.; et al. Tissue factor expression demonstrates severe sinusoidal endothelial cell damage during rejection after living-donor livertransplantation. J. Hepatobiliary Pancreat. Surg. 2009, 16, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Oreshkova, T.; Dimitrov, R.; Mourdjeva, M. A cross-talk of decidual stromal cells, trophoblast, and immune cells: A prerequisite for the success of pregnancy. Am. J. Reprod. Immunol. 2012, 68, 366–373. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.J.; Lu, H.; Lei, S.T.; Ha, S.Y.; Lai, Z.Z.; Zheng, Z.M.; Ruan, L.Y.; He, Y.Y.; Li, D.J.; et al. Decidual stromal cells promote the differentiation of CD56bright CD16- NK cells by secreting IL-24 in early pregnancy. Am. J. Reprod. Immunol. 2019, 81, e13110. [Google Scholar] [CrossRef] [PubMed]
- Blaschitz, A.; Hutter, H.; Dohr, G. HLA Class I protein expression in the human placenta. Early Pregnancy 2001, 5, 67–69. [Google Scholar] [PubMed]
- Bhattarai, A.; Shah, S.; Dahal, K.; Neupane, R.; Thapa, S.; Neupane, N.; Barboza, J.J.; Shrestha, A.; Sah, R.; Apostolopoulos, V. Biomarker role of maternal soluble human leukocyte antigen G in pre-eclampsia: A meta-analysis. Immun. Inflamm. Dis. 2024, 12, e1254. [Google Scholar] [CrossRef] [PubMed]
- Creput, C.; LeFriec, G.; Bahri, R.; Amiot, L.; Charpentier, B.; Carosella, E.; Rouas-Freiss, N.; Durrbach, A. Detection of HLA-G in serum and graft biopsy associated with fewer acute rejections following combined liver-kidney transplantation: Possible implications for monitoring patients. Hum. Immunol. 2003, 64, 1033–1038. [Google Scholar] [CrossRef]
- Szereday, L.; Barakonyi, A.; Miko, E.; Varga, P.; Szekeres-Bartho, J. Gamma/deltaT-cell subsets, NKG2A expression and apoptosis of Vdelta2+ T cells in pregnant women with or without risk of premature pregnancy termination. Am. J. Reprod. Immunol. 2003, 50, 490–496. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Jiang, Y.; Tu, J.; Schust, D.J. Activation of decidual invariant natural killer T cells promotes lipopolysaccharide-induced preterm birth. Mol. Hum. Reprod. 2015, 21, 369–381. [Google Scholar] [CrossRef]
- Uemura, Y.; Suzuki, M.; Liu, T.Y.; Narita, Y.; Hirata, S.; Ohyama, H.; Ishihara, O.; Matsushita, S. Role of human non-invariant NKT lymphocytes in the maintenance of type 2 T helper environment during pregnancy. Int. Immunol. 2008, 20, 405–412. [Google Scholar] [CrossRef]
- Fang, W.N.; Shi, M.; Li, D.D.; Peng, J.P. The Balance between Conventional DCs and Plasmacytoid DCs Is Pivotal for Immunological Tolerance during Pregnancy in the Mouse. Sci. Rep. 2016, 6, 26984. [Google Scholar] [CrossRef]
- Kahler, D.J.; Mellor, A.L. T cell Regulatory Plasmacytoid Dendritic Cells Expressing Indoleamine 2,3 Dioxygenase. Handb. Exp. Pharmacol. 2009, 165–196. [Google Scholar]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Silvano, A.; Seravalli, V.; Strambi, N.; Tartarotti, E.; Tofani, L.; Calosi, L.; Parenti, A.; Di Tommaso, M. Tryptophan degradation enzymes and Angiotensin (1–7) expression inhuman placenta. J. Reprod. Immunol. 2022, 153, 103692. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes-E-Silva, A.C. The Anti-inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Al Zayadneh, E.M. Angiotensin-(1-7) and the Regulation of Anti-Fibrotic Signaling Pathways. J. Cell Signal. 2017, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Hirschfield, G.M. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases. Gut 2021, 70, 1989–2003. [Google Scholar] [CrossRef]
- Harputluoglu, M.; Caliskan, A.R.; Akbulut, S. Autoimmune hepatitis and liver transplantation: Indications, and recurrent and de novo autoimmune hepatitis. World, J. Transplant. 2022, 12, 59–64. [Google Scholar] [CrossRef]
- Ma, W.T.; Chang, C.; Garshwin, M.E.; Lian, Z.X. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J. Autoimmun. 2017, 83, 95–112. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, C.L.; Goto, S. Nuclear antigens and auto/alloantibody responses: Friend or foe in transplant immunology. Clin. Dev. Immunol. 2013, 2013, 267156. [Google Scholar] [CrossRef]
- Nakano, T.; Goto, S.; Lai, C.Y.; Hsu, L.W.; Kao, Y.H.; Lin, Y.C.; Kawamoto, S.; Chiang, K.C.; Ohmori, N.; Goto, T.; et al. Experimental and clinical significance of antinuclear antibodies in liver transplantation. Transplantation 2007, 83, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Goto, S.; Lai, C.Y.; Hsu, L.W.; Takaoka, Y.; Kawamoto, S.; Chiang, K.C.; Shimada, Y.; Ohmori, N.; Goto, T.; et al. Immunological aspects and therapeutic significance of an autoantibody against histone H1 in a rat model of concanavalin A-induced hepatitis. Immunology 2010, 129, 547–555. [Google Scholar] [CrossRef]
- Wang, C.; Nie, H.; Li, K.; Zhang, Y.X.; Yang, F.; Li, C.B.; Wang, C.F.; Gong, Q. Curcumin inhibits HMGB1 releasing and attenuates concanavalin A-induced hepatitis in mice. Eur. J. Pharmacol. 2012, 697, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, S.; Fang, D.; Fei, X.; Tan, Z.; Zheng, F.; Wang, W.; Fang, M. RAGE deficiency ameliorates autoimmune hepatitis involving inhibition of IL-6 production via suppressing protein Arid5a in mice. Clin. Exp. Med. 2023, 23, 2167–2179. [Google Scholar] [CrossRef]
- Penner, E.; Muller, S.; Zimmermann, D.; Van Regenmortel, M.H. High prevalence of antibodies to histones among patients with primary biliary cirrhosis. Clin. Exp. Immunol. 1987, 70, 47–52. [Google Scholar] [PubMed]
- Fida, S.; Myers, M.A.; Whittingham, S.; Rowley, M.J.; Ozaki, S.; Mackay, I.R. Autoantibodies to the transcriptional factor SOX13 in primary biliary cirrhosis compared with other diseases. J. Autoimmun. 2002, 19, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Julia, S.; Petr, S.; Karl-Heinz, W.; DanielNils, G.; Christian, R. Possible role of the HMGB1 and RAGE in inflammatory pathway in primary sclerosing cholangitis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101791. [Google Scholar] [CrossRef]
- Longhi, M.S.; Mieli-Vergani, G.; Vergani, D. Regulatory T cells in autoimmune hepatitis: An updated overview. J. Autoimmun. 2021, 119, 102619. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R.; Holder, B.S.; Cardone, J.; Ma, Y.; Robson, S.C.; Mieli-Vergani, G.; Verganin, D.; Longhi, M.S. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 2014, 59, 1007–1015. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, S.; Das, A.; Thapa, B.; Chawla, Y.K.; Minz, R.W. A molecular marker of disease activity in autoimmune liver diseases with histopathological correlation; Foxp3/RORγt ratio. APMIS 2015, 123, 935–944. [Google Scholar] [CrossRef]
- Wang, L.; Du, H.; Liu, Y.; Wang, L.; Ma, X.; Zhang, W. Chinese medicine bu xu yu recipe for the regulation of treg/th17 ratio imbalance in autoimmune hepatitis. Evid. Based Complement. Alternat Med. 2015, 2015, 461294. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, O.; Dou, L.; Kimura, S.; Yokota, S.; Isse, K.; Robson, S.C.; Geller, D.A.; Thomson, A.W. CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transplant. Immunol. 2015, 32, 76–83. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.L.; Wu, B.; Zhou, C.L.; Liu, W.; Gao, W. The T-helper cells 17 instead of Tregs play the key role in acute rejection after pediatric liver transplantation. Pediatr. Transplant. 2019, 23, e13363. [Google Scholar] [CrossRef]
- Assadiasl, S.; Toosi, M.N.; Mohebbi, B.; Ansaripour, B.; Soleimanifar, N.; Sadr, M.; Mojtahedi, H.; Mosharmovahed, B.; Fazeli, F.; Nicknam, M.H. Th17/Treg cell balance instable liver transplant recipients. Transplant. Immunol. 2022, 71, 101540. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.H.; Goto, S.; Lai, C.Y.; Tseng, H.P.; Hsu, L.W.; Chiu, K.W.; Lin, C.C.; Wang, C.C.; Cheng, Y.F.; et al. Hepatic miR-301a as a Liver Transplant Rejection Biomarkers? And Its Role for Interleukin-6 Production in Hepatocytes. OMICS 2017, 21, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, R.; Li, Q.; Zhang, G. MiR-301a-3p Advances IRAK1-Mediated Differentiation of Th17 Cells to Promote the Progression of Systemic Lupus Erythematosus via Targeting PELI1. J. Healthc. Eng. 2021, 2021, 2982924. [Google Scholar] [CrossRef] [PubMed]
- Kerkar, N.; Yanni, G. ‘De novo’ and ‘recurrent’ autoimmune hepatitis after liver transplantation: A comprehensive review. J. Autoimmun. 2016, 66, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N.B.; Schiano, T.D.; Fiel, M.I. Histologic and Clinical Outcomes of Patients Developing Post-Liver Transplant Plasma Cell-Rich Rejection. Am. J. Clin. Pathol. 2023, 160, 49–57. [Google Scholar] [CrossRef]
- Henson, J.B.; King, L.Y. Post-Transplant Management and Complications of Autoimmune Hepatitis, Primary Biliary Cholangitis, and Primary Sclerosing Cholangitis Including Disease Recurrence. Clin. Liver Dis. 2024, 28, 193–207. [Google Scholar] [CrossRef]
- Nakano, T.; Goto, S.; Lai, C.Y.; Hsu, L.W.; Tseng, H.P.; Chen, K.D.; Chiu, K.W.; Wang, C.C.; Cheng, Y.F.; Chen, C.L. Induction of antinuclear antibodies by de novo autoimmune hepatitis regulates alloimmune responses in rat liver transplantation. Clin. Dev. Immunol. 2013, 2013, 413928. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Abdullah, M.; Vidyasagar, K.; Alqahthani, S.A.; Broering, D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systemic Review of Real-World Evidence. Cancers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Sun, X.X.; Ma, X.; Chen, Z.N. microRNA-146a inhibits cancer metastasis by downregulating VEGF through dual pathways in hepatocellular carcinoma. Mol. Cancer 2015, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Callegari, E.; D’Abundo, L.; Guerriero, P.; Simioni, C.; Elamin, B.K.; Russo, M.; Cani, A.; Bassi, C.; Zagatti, B.; Giacomelli, L.; et al. miR-199a-3p Modulates MTOR and PAK4 Pathways and Inhibits Tumor Growth in a Hepatocellular Carcinoma Transgenic Mouse Model. Mol. Ther. Nucleic Acids 2018, 11, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.G.; Zheng, J.F.; Li, Q.; Bao, S.Y.; Yu, X.F.; Xu, P.; Liao, C.X. MicroRNA-181a-5p suppresses cell proliferation by targeting Egr1 and inhibiting Egr1/TGF-β/Smad pathway in hepatocellular carcinoma. Int. J. Biochem. Cell Biol. 2019, 106, 107–116. [Google Scholar] [CrossRef]
- Cao, F.; Yin, L.X. miR-122 enhances sensitivity of hepatocellular carcinoma to oxaliplatin via inhibiting MDR1 by targeting Wnt/β-catenin pathway. Exp. Mol. Pathol. 2019, 106, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Du, Q.; Cui, X.; Wan, P.; Kaltenmeier, C.; Luo, J.; Yan, B.; Yan, Y.; Geller, D.A. MicroRNA-301a (miR-301) is induced in hepatocellular carcinoma (HCC) and down-regulates the expression of interferon regulatory factor-1. Biochem. Biophys. Res. Commun. 2020, 524, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Wang, Z.; Huang, M.; Wu, X.; Hu, F.; Zhu, J.; Yu, Y.; Shen, H.; Wu, Y.; Xie, G.; et al. MiR-155 regulates M2 polarization of hepatitis B virus-infected tumor-associated macrophages which in turn regulates the malignant progression of hepatocellular carcinoma. J. Viral Hepat. 2023, 30, 417–426. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Tan, C.J.; Liao, B.Y.; Zhang, X.; Xu, M.; Dai, Z.; Qiu, S.J.; Huang, X.W.; Sun, J.; et al. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation 2013, 95, 991–999. [Google Scholar] [CrossRef]
- Rong, M.; He, R.; Dang, Y.; Chen, G. Expression and clinicopathological signature of miR-146a in hepatocellular carcinoma tissues. Ups. J. Med. Sci. 2014, 119, 19–24. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, W.; Chang, R.; Wu, K.; Wang, Z. miR-301a is a candidate oncogene that targets the homeobox gene Gax in human hepatocellular carcinoma. Dig. Dis. Sci 2012, 57, 1171–1180. [Google Scholar] [CrossRef]
- Mo, J.; Chen, Y.; Cheng, Y.; Hua, W.; He, W.; Chen, L. miR-199-3p may be an early warning marker for acute rejection after liver transplantation in rats. Cytokine 2021, 148, 155689. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dasgupta, D.; Ghosh, A.; Roychoudhury, S.; Kumar, D.; Gorain, M.; Butti, R.; Datta, S.; Agarwal, S.; Gupta, S.; et al. MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. 2017, 8, e2706. [Google Scholar] [CrossRef]
- Millán, O.; Ruiz, P.; Orts, L.; Ferré, P.; Crespo, G.; Santana, M.; Fortuna, V.; Quintairos, L.; Navasa, M.; Brunet, M. Monitoring of miR-181a-5p and miR-155-5p Plasmatic Expression as Prognostic Biomarkers for Acute and Subclinical Rejection in de novo adult liver Transplant Recipients. Front. Immunol. 2019, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Korhan, P.; Erdal, E.; Atabey, N. MiR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-Met. Biochem. Biophys. Res. Commun. 2014, 450, 1304–1312. [Google Scholar] [CrossRef]
- Fu, X.; Wen, H.; Jing, L.; Yang, Y.; Wang, W.; Liang, X.; Nan, K.; Yao, Y.; Tian, T. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Aktpathway. Cancer Sci. 2017, 108, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Millán, O.; Ríos, J.; Díaz, A.; Sastre, L.; Colmenero, J.; Crespo, G.; Brunet, M.; Navasa, M. MicroRNAs 155-5p, 122-5p, and 181a-5p Identify Patients with Graft Dysfunction Due to T Cell-Mediated Rejection after Liver Transplantation. Liver Transplant. 2020, 26, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Y.; Huang, J.C.; Wang, J.Y.; Pan, W.Y.; Zeng, J.H.; Pang, Y.Y.; Yang, H. Potential clinical value and putative biological function of miR-122-5p in hepatocellular carcinoma: A comprehensive study using microarray and RNA sequencing data. Oncol. Lett. 2018, 16, 6918–6929. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.H.; Wang, C.C.; Chen, P.J.; Tseng, H.P.; Huang, K.T.; Hu, T.H.; Li, L.C.; Goto, S.; Cheng, Y.F.; et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of postransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, T.; Li, J.; Lin, W.; Zheng, Q. Exosomal transfer of HCC-derived miR-17-5p downregulates NK cell function by targeting RUNX1-NKG2D axis. Int. Immunopharmacol. 2024, 136, 112361. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef]
- Huang, M.; Huang, X.; Huang, N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci. 2022, 113, 1968–1983. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Han, Q.; Xu, D.; Zheng, B.; Zhao, X.; Zhang, J. SALL4-mediated upregulation of exosomal miR-146a-5p drive T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 2019, 8, 1601479. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Zhao, Y.; Yan, C.; Wang, Y.; Zhu, J.; Li, L. Exosomal miR-452-5p Induce M2 Macrophage Polarization to Accelerate Hepatocellular Carcinoma Progression by Targeting TIMP3. J. Immunol. Res. 2022, 2022, 1032106. [Google Scholar]
- Yu, H.; Pan, J.; Zheng, S.; Cai, D.; Luo, A.; Xia, Z.; Huang, J. Hepatocellular Carcinoma Cell-Derived Exosomal miR-21-5p Induces Macrophage M2 Polarization by Targeting RhoB. Int. J. Mol. Sci. 2023, 24, 4593. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Chen, C.L.; Chen, I.H.; Tseng, H.P.; Chiang, K.C.; Lai, C.Y.; Hsu, L.W.; Goto, S.; Lin, C.C.; Cheng, Y.F. Overexpression of miR-4669 Enhances Tumor Aggressiveness andGenerates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker. Int. J. Mol. Sci. 2023, 24, 7908. [Google Scholar] [CrossRef]
- Xu, Y.; Luan, G.; Liu, F.; Zhang, Y.; Li, Z.; Liu, Z.; Yang, T. Exosomal miR-200b-3p induce macrophage polarization by regulating transcriptional repressor ZEB1 in hepatocellular carcinoma. Hepatol. Int. 2023, 17, 889–903. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Q.; Cheng, Y.; Chen, X.; Wang, G.; Shi, M.; Zhang, T.; Cao, Y.; Pan, H.; Zhang, L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 2018, 6, 145. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, H.; Jiang, Y.; Yin, C.; Zhang, J. HCC-Derived Exosomes: Critical Player and Target for Cancer Immune Escape. Cells 2019, 8, 558. [Google Scholar] [CrossRef]
- Xiao, F.; Ai, G.; Yan, W.; Wan, X.; Luo, X.; Ning, Q. Intrahepatic recruitment of cytotoxic NK cells contributes to autoimmune hepatitis progression. Cell Immunol. 2018, 327, 13–20. [Google Scholar] [CrossRef]
- Gomaa, M.F.; Elkhouly, A.G.; Farghly, M.M.; Farid, L.A.; Awad, N.M. Uterine CD56dim and CD16+ Cells in Refractory Antiphospholipid Antibody-Related Pregnancy Loss and Chromosomally Intact Abortuses: A Case-Control Study. J. Hum. Reprod. Sci. 2017, 10, 18–23. [Google Scholar]
- Hu, J.; Wang, E.; Liu, L.; Wang, Q.; Xia, D.; Bai, W.; Tie, J.; Li, X.; Yuan, J.; Yang, S.; et al. Sorafenib may enhance antitumor efficacy in hepatocellular carcinoma patients by modulating the proportions and functions of natural killer cells. Investig. New Drugs 2020, 38, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Littera, R.; Perra, A.; Miglianti, M.; Piras, I.S.; Mocci, S.; Lai, S.; Melis, M.; Zolfino, T.; Balestrieri, C.; Conti, M.; et al. The double-sided of human leukocyte antigen-G molecules in type 1 autoimmune hepatitis. Front. Immunol. 2022, 13, 1007647. [Google Scholar] [CrossRef] [PubMed]
- Catamo, E.; Zupin, L.; Crovella, S.; Celsi, F.; Segat, L. Non-classical MHC-I human leukocyte antigen (HLA-G) in hepatotropic viral infections and in hepatocellular carcinoma. Hum. Immunol. 2014, 75, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Wang, H.; Chen, M.S.; Zhang, H.K.; Weng, D.S.; Zhou, J.; Huang, W.; Li, J.J.; Song, H.F.; Xia, J.C. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2008, 134, 1247–1253. [Google Scholar] [CrossRef]
- Fu, Y.P.; Yi, Y.; Cai, X.Y.; Sun, J.; Ni, X.C.; He, H.W.; Wang, J.X.; Lu, Z.F.; Huang, J.L.; Cao, Y.; et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br. J. Cancer 2016, 114, 767–776. [Google Scholar] [CrossRef]

| Donor (MHC) | Recipient (MHC) | Model | Reference |
|---|---|---|---|
| DA (RT1a) | PVG (RT1c) | Tolerance | [17] |
| PVG (RT1c) | DA (RT1a) | Tolerance | [18] |
| DA (RT1a) | LEW (RT1l) | Acute rejection | [18] |
| LEW (RT1l) | DA (RT1a) | Tolerance | [18] |
| BN (RT1n) | LEW (RT1l) | Tolerance (delayed rejection) | [18] |
| LEW (RT1l) | BN (RT1n) | Acute rejection | [18] |
| Rejection Marker | Expression | Functions in HCC | Reference |
|---|---|---|---|
| miR-146a (rat) [139] | Plasma, Liver tissue | Lower expression of miR-146a in HCC tissues (human) | [140] |
| Tumor suppressor microRNA, VEGF inhibition (human, cell line) | [133] | ||
| miR-301a (rat) [126] | Liver tissue | Upregulation of miR-301a in HCC tissues (human) | [141] |
| Onco-microRNA, IRF-1 inhibition (human, cell line) | [137] | ||
| miR-199a-3p (rat) [142] | Plasma, Liver tissue | Lower expression of miR-199a-3p in HCC tissues (human) | [143] |
| Tumor suppressor microRNA, mTOR, PAK4 inhibition (animal model) | [134] | ||
| miR-181a-5p (human) [144] | Plasma | Lower expression of miR-181a-5p in HCC tissues (human) | [145] |
| Tumor suppressor microRNA, Egr1 inhibition (human, cell line) | [135] | ||
| miR-155-5p (human) [144] | Plasma | Upregulation of miR-155-5p in HCC tissues (human) | [146] |
| Onco-microRNA, M2 macrophage polarization (human) | [138] | ||
| miR-122-5p (human) [147] | Plasma | Lower expression of miR-122-5p in HCC tissues (human) | [148] |
| Tumor suppressor microRNA, MDR1 inhibition (cell line, animal model) | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, T.; Goto, S.; Chen, C.-L. Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity. Int. J. Mol. Sci. 2024, 25, 9331. https://doi.org/10.3390/ijms25179331
Nakano T, Goto S, Chen C-L. Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity. International Journal of Molecular Sciences. 2024; 25(17):9331. https://doi.org/10.3390/ijms25179331
Chicago/Turabian StyleNakano, Toshiaki, Shigeru Goto, and Chao-Long Chen. 2024. "Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity" International Journal of Molecular Sciences 25, no. 17: 9331. https://doi.org/10.3390/ijms25179331
APA StyleNakano, T., Goto, S., & Chen, C.-L. (2024). Mechanisms of Tolerance Induction in Liver Transplantation: Lessons Learned from Fetomaternal Tolerance, Autoimmunity and Tumor Immunity. International Journal of Molecular Sciences, 25(17), 9331. https://doi.org/10.3390/ijms25179331






